To evaluate the safety and diagnostic performance of parathyroid hormone assay in fine-needle aspirate (PTH-FNA) in patients with primary hyperparathyroidism and suspicious parathyroid adenomas.
MethodologyA retrospective observational study was performed in 47 patients (57.7 ± 11.2 years of average age, 74% women) attending an endocrinology clinic for primary hyperparathyroidism (average calcemia: 11.6 ± 1.6 mg/dl and PTH: 276 ± 477 pg/mL) in which PTH-FNA was made. Sensibility, specificity, positive predictive value and negative predictive value were calculated in all surgical patients.
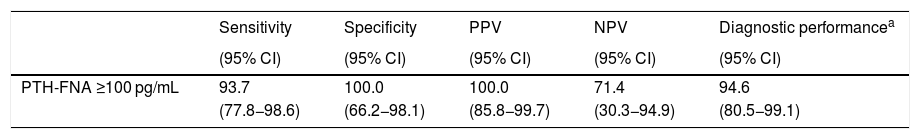
ResultsForty-seven lesions were punctured (mean adenoma maximum diameter: 1.8 ± 2.6 cm): negative image in the sestamibi scan (26 patients); the discordance between ultrasonography and the sestamibi scan (6 patients); possible intrathyroidal adenomas (4 patients); a positive sestamibi scan in 2 or more localizations (4 patients); ectopic adenoma (3 patients); persistent primary hyperparathyroidism (2 patients) and atypical adenomas (2 patients). Mean PTH-FNA was 2853 ± 3957 pg/mL and 68% were considered positive (PTH-FNA ≥ 100 pg/mL). No complications were detected during or after the puncture. Thirty-seven patients were operated on, 95% were cured and no parathyromatosis cases were detected. PTH-FNA ≥ 100 pg/mL as a diagnostic test had a sensitivity of 93.7%, a specificity of 100%, a positive predictive value of 100% and an negative predictive value of 71.4%.
ConclusionPTH-FNA is an easy and safe diagnostic test and has a high sensitivity and specificity for differentiating between parathyroid adenomas and other cervical masses in patients with primary hyperparathyroidism.
Evaluar la seguridad y el rendimiento diagnóstico de la medición de PTH en el lavado del aspirado (PTHa) de posibles adenomas de paratiroides en pacientes con hiperparatiroidismo primario.
MetodologíaEstudio observacional retrospectivo en 47 pacientes (74% mujeres; edad media: 57,7 ± 11,2 años) atendidos en consultas de endocrinología por hiperparatiroidismo primario (calcemia: 11,6 ± 1,6 mg/dl y PTH plasmática: 276 ± 477 pg/mL), a los cuales se les realiza PAAF para medir la PTHa. Se analiza la seguridad de la técnica y se calculan la sensibilidad, la especificidad y los valores predictivos positivo y negativo en los pacientes intervenidos.
ResultadosSe punzaron 47 lesiones (diámetro medio: 1,8 ± 2,6 cm) por: ausencia de lesiones en la gammagrafía (26 pacientes), discordancia entre gammagrafía y ecografía (6 pacientes), sospecha de adenomas intratiroideos (4 pacientes), positividad gammagráfica en más de una localización (4 pacientes), lesiones ectópicas (3 pacientes), enfermedad persistente (2 pacientes) y adenomas atípicos (2 pacientes). El nivel promedio de PTHa fue de 2.853 ± 3.957 pg/mL, considerándose positivo (PTHa ≥ 100 pg/mL) el 68% de los casos. No hubo complicaciones durante ni tras la punción. Se intervinieron 37 pacientes, curándose el 95%, y no se ha detectado ningún caso de paratiromatosis. La PTHa > 100 pg/mL presenta una sensibilidad del 93,7%, una especificidad del 100%, un valor predictivo positivo del 100% y un valor predictivo negativo del 71,4%.
ConclusiónEn pacientes con hiperparatiroidismo primario la medición de PTHa es una técnica diagnóstica sencilla, segura y con una elevada sensibilidad y especificidad que permite diferenciar entre adenomas paratiroideos y otras lesiones cervicales.
Primary hyperparathyroidism (PHPT) is a common endocrine disorder characterised by excessive secretion of parathyroid hormone (PTH) from one or more of the parathyroid glands. Currently, the most common form of presentation (80%) is asymptomatic, with mild or intermittent hypercalcaemia and an absence of classic signs and symptoms such as lithiasis, nephrocalcinosis or osteitis fibrosa cystica. The biochemical diagnosis of PHPT is generally simple and is established by detecting hypercalcaemia (of variable magnitude) and elevated or inappropriately normal PTH concentrations, frequently associated with hypophosphataemia, hypercalciuria and a moderate increase in bone remodelling markers.1–3
Although imaging techniques should not be used to rule out or confirm the diagnosis of PHPT or to establish the indication for surgery, most of the clinical practice guidelines currently recommend some type of imaging before operating,4–6 to help enable minimally-invasive parathyroidectomy and detect thyroid nodules, found in 15–60% of patients with PHPT.7–9 At present, the best available location techniques are: parathyroid scintigraphy with Technetium (Tc)-99 m sestamibi (MIBI) scan (associated or not with SPECT), with a diagnostic sensitivity of around 80%; and cervical ultrasound, with very variable sensitivity ranging from 55% to 88%, depending on the experience of the examiner, the size of the adenoma, the frequency of ectopic lesions, the coexistence of multinodular goiter and the presence of lymph nodes or other cervical lesions which can be confused with parathyroid adenomas.1,9,10 In fact, the combination of the two techniques is the strategy that provides the best results.6,9–11 However, a sestamibi scan can give a false positive result in the presence of thyroid nodules and false negative in cases of small parathyroid adenomas, necrosis in the adenoma, or the coexistence of autoimmune thyroid disease.4,6,9,12 In a recent study carried out in our area, 26% of the patients studied in endocrinology clinics for PHPT had no lesions detected on the sestamibi scan.9
In patients with conflicting imaging studies or with negative sestamibi scan and positive parathyroid ultrasound, a number of authors have proposed performing a fine needle aspiration (FNA) for PTH assay in the fine needle aspirate (PTH-FNA), as this is a simple, safe, inexpensive and highly specific diagnostic technique to confirm the location of parathyroid adenomas prior to surgery, and it distinguishes between parathyroid lesions and other types of lesion.6,13–21 However, the information currently available on the safety and diagnostic efficacy of this technique is based on a limited number of studies with few patients, all of which were performed outside of Spain.13–23 The main aim of this study was therefore to evaluate the safety and diagnostic performance of PTH-FNA in our series of patients with PHPT.
Material and methodsRetrospective observational study in which we assessed the diagnostic tests performed on 195 patients with biochemical criteria for PHPT seen in an endocrinology clinic from January 2013 to January 2020, selecting the cases in which FNA was performed for PTH-FNA because of suspected parathyroid adenomas. The study was approved by the Cadiz Independent Ethics Committee and no informed consent was required to access the research information, although all the patients signed the corresponding informed consent forms before undergoing FNA or parathyroid surgery.
Analysis of laboratory tests and imaging studiesThe levels of calcium, phosphorus, total proteins, urea, creatinine, PTH, 25 OH vitamin D and calciuria were determined in all patients. The serum calcium concentration was adjusted for the plasma protein level. The levels of PTH in plasma and in the PTH-FNA were measured by in-vitro immunological analysis by electrochemiluminescence on an Elecsys® E170 automated analyser (Roche Diagnostics) (reference plasma levels: 15–65 pg/ml).
In cases of confirmed PHPT, an ultrasound of the neck for mapping purposes was performed in endocrinology clinics using Sonosite MicroMaxx® and Hitachi Aloka® F37 ultrasound machines with 10−18 MHz transducers, in order to locate lesions suggestive of parathyroid adenomas. These lesions usually show up as well defined, hypoechoic, oval, polygonal or triangular nodules located posterior to the upper or lower pole of both lobes of the thyroid gland.9,10 If not found in these locations, the ultrasound examination was extended to the rest of the neck as far as the superior mediastinum (areas iv, vi and vii). Regardless of the ultrasound result, in patients who were candidates for parathyroidectomy, a sestamibi scan was requested (associated or not with SPECT), with 15 mCi of Tc-99 m-MIBI injected intravenously to obtain early (15 min) and late (2−3 h) neck and chest images.
FNA and measurement of PTH-FNAThe indication of FNA for PTH-FNA in patients with biochemical criteria suggesting PHPT was established in the following cases: (1) negative sestamibi scan and ultrasound image indicative of parathyroid adenoma; (2) conflicting results in the imaging tests: sestamibi scan positive in more than one location or with a location other than that detected by ultrasound; (3) suspected intrathyroid parathyroid adenoma: compatible ultrasound image and negative or positive sestamibi scan on the same side as the intrathyroid lesion, but not seen in the extrathyroid ultrasound; (4) ectopic adenomas: lesions compatible on ultrasound with parathyroid adenomas separated from the thyroid gland by at least 2 cm and located in areas iv, vi and vii, and (5) ultrasound image of “atypical” parathyroid adenoma: lesions with cystic degeneration or possible calcifications. The ultrasound-guided FNA was performed by the same endocrinologist in the clinic, with the patient lying down with the neck extended, using a 23 G needle attached to a 20 ml syringe (without connecting tube or aspiration gun). In a single step, the needle was introduced into the suspect parathyroid lesion shown on the image with gentle movements until it was verified that there was material in the shaft of the needle, otherwise negative pressure was applied until material was obtained. The material was diluted in 1 cc of normal saline and put in a biochemistry tube without heparin, then immediately sent to the laboratory for PTH-FNA. Although there are no standardised cut-off points of normality for parathyroid lesions,24 PTH-FNA levels of 100 pg/mL or above were considered positive in our study.
Indication for surgery and cureIn all cases the indication for parathyroid surgery was assessed on an individual basis, including as general criteria: age <50, blood calcium >1 mg/dl from the upper limit of normal, presence of associated complications (renal lithiasis, osteoporosis, glomerular filtration <60 ml/min), or the patient's preference for surgery. The parathyroid surgical approach was established prior to surgery, based on the results of the imaging studies, with minimally invasive keyhole surgery being indicated in the case of single adenomas and a broader surgical approach where thyroidectomy was indicated due to the presence of associated nodular thyroid disease. PTH assay was not performed intraoperatively in any of the cases. Biochemical cure was defined, following the recommendations of the American Association of Endocrine Surgeons, by return to normal levels of corrected serum calcium at least six months after parathyroidectomy.6
Side effectsWe registered all cases of side effects associated with FNA (inflammation, local infection or bruising) and, in the patients who had surgery, we investigated the possible cases of local fibrosis or parathyromatosis, defined as the presence of nodules of hyperfunctioning parathyroid tissue spread around the neck or mediastinum suspected to be caused by seeding of parathyroid cells during the FNA or surgery, for which patients often require reoperation.24,25
Statistical analysisStatistical analysis was performed using SPSS® version 12.0 for Windows®. The Shapiro-Wilk test was used to determine whether or not the continuous variables were distributed normally. The results for the continuous variables were expressed as mean ± standard deviation and the qualitative variables as absolute numbers and percentages.
For the assessment of the PTH-FNA as a diagnostic test, lesions with PTH-FNA ≥100 pg/mL, located, surgically removed and confirmed histologically, were considered true positives; lesions with PTH-FNA ≥100 pg/mL, not located by surgery and confirmed histologically in another location, were considered false positives; lesions with PTH-FNA <100 pg/mL, not located and removed in another location, and confirmed histologically, were considered true negatives; and lesions with PTH-FNA <100 pg/mL, located and removed by surgery, and confirmed histologically, were considered false negatives. The positive predictive value was calculated using the formula: true positives/(true positives + false positives), and the negative predictive value by the formula: true negatives/(true negatives + false negatives). The 95% confidence interval was assessed for all estimates.
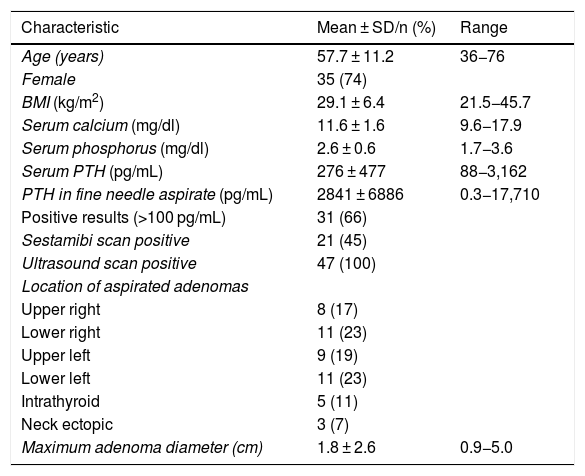
ResultsIn the eight years of activity analysed since the introduction of ultrasound scans in endocrinology clinics, 195 patients with biochemical criteria for PHPT have been treated, with FNA of suspected parathyroid-origin lesions for PTH-FNA in 47 patients (24% of the total). As shown in Table 1, the majority were female (74.5%), middle-aged (mean age: 57.7 ± 11.2 years; median: 58 years) with blood calcium levels of 11.6 ± 1.6 mg/dl (median: 11.2 mg/dl) and mean plasma PTH levels of 276 ± 447 pg/dl (median: 151 pg/mL). The lesions on which FNA was performed had a mean maximum diameter of 1.8 ± 2.6 cm (median: 1.5 cm) and were distributed evenly among the four parathyroid glands, although five intrathyroid lesions and three ectopic neck lesions were aspirated. The mean PTH-FNA was 2853 ± 3957 pg/mL (median: 2454 pg/mL), and 68% of the cases (32 patients) were considered positive for a lesion of parathyroid origin (PTH-FNA ≥100 pg/mL). There were no cases of inflammation, local pain or infection after the FNA.
Clinical, biochemical and imaging study characteristics of patients with hyperparathyroidism undergoing FNA for PTH-FNA (n = 47).
| Characteristic | Mean ± SD/n (%) | Range |
|---|---|---|
| Age (years) | 57.7 ± 11.2 | 36−76 |
| Female | 35 (74) | |
| BMI (kg/m2) | 29.1 ± 6.4 | 21.5−45.7 |
| Serum calcium (mg/dl) | 11.6 ± 1.6 | 9.6−17.9 |
| Serum phosphorus (mg/dl) | 2.6 ± 0.6 | 1.7−3.6 |
| Serum PTH (pg/mL) | 276 ± 477 | 88−3,162 |
| PTH in fine needle aspirate (pg/mL) | 2841 ± 6886 | 0.3−17,710 |
| Positive results (>100 pg/mL) | 31 (66) | |
| Sestamibi scan positive | 21 (45) | |
| Ultrasound scan positive | 47 (100) | |
| Location of aspirated adenomas | ||
| Upper right | 8 (17) | |
| Lower right | 11 (23) | |
| Upper left | 9 (19) | |
| Lower left | 11 (23) | |
| Intrathyroid | 5 (11) | |
| Neck ectopic | 3 (7) | |
| Maximum adenoma diameter (cm) | 1.8 ± 2.6 | 0.9−5.0 |
BMI: body mass index; FNA, fine needle aspiration; PTH: parathyroid hormone; PTH-FNA: parathyroid hormone assay in fine needle aspirate; SD: standard deviation.
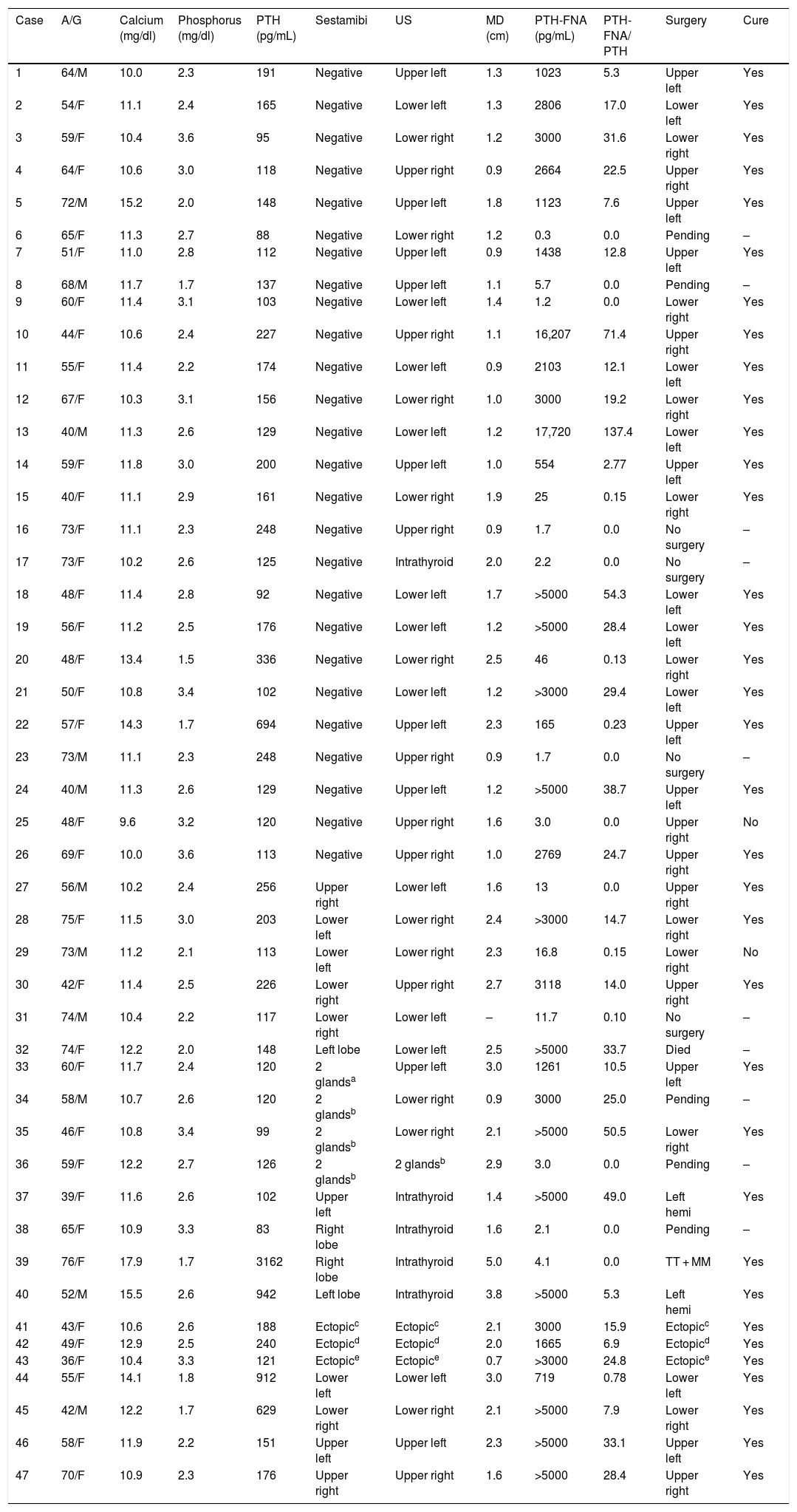
Table 2 shows a summary of the clinical and analytical characteristics, imaging tests and post-intervention results of the patients studied. The main indication criterion for FNA for PTH-FNA was the absence of lesions on the sestamibi scan (26 patients; 52% of the total) with clearly positive results (mean PTH-FNA: 2703 ± 4673 pg/mL) in 17 of these patients (65%). Fourteen patients (30% of the total) underwent FNA due to conflicting data on lesion location from the sestamibi and ultrasound scans (6 cases), suspected intrathyroid adenomas on ultrasound images (4 cases), or sestamibi scan positive in more than one location (4 cases), with the PTH-FNA being positive in eight patients (57%). Lastly, seven patients (15% of the total) underwent FNA whose sestamibi and ultrasound scan results coincided; three were ectopic lesions, two were patients with persistent disease after prior surgery, and two had parathyroid adenomas with atypical ultrasound features, with positive PTH-FNA in all seven cases.
Summary of clinical, hormone and imaging study characteristics (n = 47).
| Case | A/G | Calcium (mg/dl) | Phosphorus (mg/dl) | PTH (pg/mL) | Sestamibi | US | MD (cm) | PTH-FNA (pg/mL) | PTH-FNA/ PTH | Surgery | Cure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64/M | 10.0 | 2.3 | 191 | Negative | Upper left | 1.3 | 1023 | 5.3 | Upper left | Yes |
| 2 | 54/F | 11.1 | 2.4 | 165 | Negative | Lower left | 1.3 | 2806 | 17.0 | Lower left | Yes |
| 3 | 59/F | 10.4 | 3.6 | 95 | Negative | Lower right | 1.2 | 3000 | 31.6 | Lower right | Yes |
| 4 | 64/F | 10.6 | 3.0 | 118 | Negative | Upper right | 0.9 | 2664 | 22.5 | Upper right | Yes |
| 5 | 72/M | 15.2 | 2.0 | 148 | Negative | Upper left | 1.8 | 1123 | 7.6 | Upper left | Yes |
| 6 | 65/F | 11.3 | 2.7 | 88 | Negative | Lower right | 1.2 | 0.3 | 0.0 | Pending | – |
| 7 | 51/F | 11.0 | 2.8 | 112 | Negative | Upper left | 0.9 | 1438 | 12.8 | Upper left | Yes |
| 8 | 68/M | 11.7 | 1.7 | 137 | Negative | Upper left | 1.1 | 5.7 | 0.0 | Pending | – |
| 9 | 60/F | 11.4 | 3.1 | 103 | Negative | Lower left | 1.4 | 1.2 | 0.0 | Lower right | Yes |
| 10 | 44/F | 10.6 | 2.4 | 227 | Negative | Upper right | 1.1 | 16,207 | 71.4 | Upper right | Yes |
| 11 | 55/F | 11.4 | 2.2 | 174 | Negative | Lower left | 0.9 | 2103 | 12.1 | Lower left | Yes |
| 12 | 67/F | 10.3 | 3.1 | 156 | Negative | Lower right | 1.0 | 3000 | 19.2 | Lower right | Yes |
| 13 | 40/M | 11.3 | 2.6 | 129 | Negative | Lower left | 1.2 | 17,720 | 137.4 | Lower left | Yes |
| 14 | 59/F | 11.8 | 3.0 | 200 | Negative | Upper left | 1.0 | 554 | 2.77 | Upper left | Yes |
| 15 | 40/F | 11.1 | 2.9 | 161 | Negative | Lower right | 1.9 | 25 | 0.15 | Lower right | Yes |
| 16 | 73/F | 11.1 | 2.3 | 248 | Negative | Upper right | 0.9 | 1.7 | 0.0 | No surgery | – |
| 17 | 73/F | 10.2 | 2.6 | 125 | Negative | Intrathyroid | 2.0 | 2.2 | 0.0 | No surgery | – |
| 18 | 48/F | 11.4 | 2.8 | 92 | Negative | Lower left | 1.7 | >5000 | 54.3 | Lower left | Yes |
| 19 | 56/F | 11.2 | 2.5 | 176 | Negative | Lower left | 1.2 | >5000 | 28.4 | Lower left | Yes |
| 20 | 48/F | 13.4 | 1.5 | 336 | Negative | Lower right | 2.5 | 46 | 0.13 | Lower right | Yes |
| 21 | 50/F | 10.8 | 3.4 | 102 | Negative | Lower left | 1.2 | >3000 | 29.4 | Lower left | Yes |
| 22 | 57/F | 14.3 | 1.7 | 694 | Negative | Upper left | 2.3 | 165 | 0.23 | Upper left | Yes |
| 23 | 73/M | 11.1 | 2.3 | 248 | Negative | Upper right | 0.9 | 1.7 | 0.0 | No surgery | – |
| 24 | 40/M | 11.3 | 2.6 | 129 | Negative | Upper left | 1.2 | >5000 | 38.7 | Upper left | Yes |
| 25 | 48/F | 9.6 | 3.2 | 120 | Negative | Upper right | 1.6 | 3.0 | 0.0 | Upper right | No |
| 26 | 69/F | 10.0 | 3.6 | 113 | Negative | Upper right | 1.0 | 2769 | 24.7 | Upper right | Yes |
| 27 | 56/M | 10.2 | 2.4 | 256 | Upper right | Lower left | 1.6 | 13 | 0.0 | Upper right | Yes |
| 28 | 75/F | 11.5 | 3.0 | 203 | Lower left | Lower right | 2.4 | >3000 | 14.7 | Lower right | Yes |
| 29 | 73/M | 11.2 | 2.1 | 113 | Lower left | Lower right | 2.3 | 16.8 | 0.15 | Lower right | No |
| 30 | 42/F | 11.4 | 2.5 | 226 | Lower right | Upper right | 2.7 | 3118 | 14.0 | Upper right | Yes |
| 31 | 74/M | 10.4 | 2.2 | 117 | Lower right | Lower left | – | 11.7 | 0.10 | No surgery | – |
| 32 | 74/F | 12.2 | 2.0 | 148 | Left lobe | Lower left | 2.5 | >5000 | 33.7 | Died | – |
| 33 | 60/F | 11.7 | 2.4 | 120 | 2 glandsa | Upper left | 3.0 | 1261 | 10.5 | Upper left | Yes |
| 34 | 58/M | 10.7 | 2.6 | 120 | 2 glandsb | Lower right | 0.9 | 3000 | 25.0 | Pending | – |
| 35 | 46/F | 10.8 | 3.4 | 99 | 2 glandsb | Lower right | 2.1 | >5000 | 50.5 | Lower right | Yes |
| 36 | 59/F | 12.2 | 2.7 | 126 | 2 glandsb | 2 glandsb | 2.9 | 3.0 | 0.0 | Pending | – |
| 37 | 39/F | 11.6 | 2.6 | 102 | Upper left | Intrathyroid | 1.4 | >5000 | 49.0 | Left hemi | Yes |
| 38 | 65/F | 10.9 | 3.3 | 83 | Right lobe | Intrathyroid | 1.6 | 2.1 | 0.0 | Pending | – |
| 39 | 76/F | 17.9 | 1.7 | 3162 | Right lobe | Intrathyroid | 5.0 | 4.1 | 0.0 | TT + MM | Yes |
| 40 | 52/M | 15.5 | 2.6 | 942 | Left lobe | Intrathyroid | 3.8 | >5000 | 5.3 | Left hemi | Yes |
| 41 | 43/F | 10.6 | 2.6 | 188 | Ectopicc | Ectopicc | 2.1 | 3000 | 15.9 | Ectopicc | Yes |
| 42 | 49/F | 12.9 | 2.5 | 240 | Ectopicd | Ectopicd | 2.0 | 1665 | 6.9 | Ectopicd | Yes |
| 43 | 36/F | 10.4 | 3.3 | 121 | Ectopice | Ectopice | 0.7 | >3000 | 24.8 | Ectopice | Yes |
| 44 | 55/F | 14.1 | 1.8 | 912 | Lower left | Lower left | 3.0 | 719 | 0.78 | Lower left | Yes |
| 45 | 42/M | 12.2 | 1.7 | 629 | Lower right | Lower right | 2.1 | >5000 | 7.9 | Lower right | Yes |
| 46 | 58/F | 11.9 | 2.2 | 151 | Upper left | Upper left | 2.3 | >5000 | 33.1 | Upper left | Yes |
| 47 | 70/F | 10.9 | 2.3 | 176 | Upper right | Upper right | 1.6 | >5000 | 28.4 | Upper right | Yes |
A: age; F: female; FNA-PTH: parathyroid hormone in fine needle aspirate; G: gender; Hemi: hemithyroidectomy; M: male; MD: maximum diameter; MM: mediastinal mass; PTH: parathyroid hormone; TT: total thyroidectomy; US: ultrasound.
To date, 37 have patients undergone parathyroidectomy; one patient died during follow-up, five patients are awaiting intervention and four patients were ruled out for surgery. In the 30 patients with PTH-FNA ≥100 pg/mL, both surgery and histology confirmed the presence of parathyroid tissue, and all patients were cured. Three patients with PTH-FNA <100 pg/mL had surgery and adenomas were removed from locations other than the FNA site, and they were cured. Four patients with PTH-FNA <100 pg/mL had surgery in which the aspirated lesion was removed; two were cured and two continue to show the biochemical criteria for PHPT. Table 3 shows how the PTH-FNA performed as a diagnostic technique in the 37 patients operated on, with the figures indicating high sensitivity (93.7%) and specificity (100%). There were no reports of local fibrosis or inflammation complicating surgical removal, and after an average follow-up of 1.7 ± 1.5 years (0.3–4.9 years), no cases of parathyromatosis have been detected.
Diagnostic performance of the measurement of PTH-FNA in operated patients (n = 37).
| Sensitivity | Specificity | PPV | NPV | Diagnostic performancea | |
|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| PTH-FNA ≥100 pg/mL | 93.7 | 100.0 | 100.0 | 71.4 | 94.6 |
| (77.8−98.6) | (66.2−98.1) | (85.8−99.7) | (30.3−94.9) | (80.5−99.1) |
95% CI: 95% confidence interval; NPV: negative predictive value; PPV: positive predictive value; PTH-FNA: parathyroid hormone in fine needle aspirate.
First described in 1983,25 using FNA to measure PTH in suspected parathyroid lesions is a diagnostic method for locating parathyroid tissue, with a high specificity in distinguishing between parathyroid glands and other tissues13–23 and no false positives when considering PTH-FNA levels ≥100 pg/mL14,19 or simply PTH-FNA levels higher than plasma levels.17,18,20–22 However, the method has the drawbacks of a lack of standardisation, not only of the technique (steps and dilution) and the diagnostic test (PTH-FNA or PTH-FNA/PTH plasma ratio), but also of the cut-off points for a result to be considered as positive.23,24 Firstly, although there is no firm consensus, most authors recommend one or two aspirations and dilutions of the sample with 1 cc of normal saline, and in terms of the diagnostic test, there seem to be no differences in the diagnostic performance between PTH-FNA and the PTH-FNA/PTHp ratio.23,24 However, the biggest discrepancies arise in the cut-off points, which range from 20 to 103 pg/mL23,24 for PTH-FNA and ≥1 to ≥2 for the PTH-FNA/PTHp ratio.23,24 In our experience, in most cases the PTH-FNA levels were clearly high (above 1000 pg/mL) and there was little doubt. However, one case considered negative with PTH-FNA of 46 pg/mL was cured after the removal of the aspirated gland, which raises the possibility of reducing the cut-off point for positivity of PTH-FNA in our population, as some authors recommend.13,26 Another point is that it is necessary when using this technique to take into account that a high thyroglobulin value in the aspirate does not exclude the possibility of parathyroid tissue in the aspirated nodule. Moreover, the diagnostic yield of cytology ranges from 17% to 53%17,19,21, as on the one hand a high number of non-diagnostic aspirations are documented and, on the other, the cytomorphology of parathyroid proliferations is very difficult to differentiate from thyroid proliferations, and although immunohistochemistry may be useful, it is not always possible due to unsuitability of the material.17
In our series, the sensitivity of the PTH-FNA was 93.7% (95% CI: 77.7–98.6), in line with other studies which report sensitivity in the range of 83%–100%.15–21 A recent systematic review and meta-analysis of nine clinical studies, which included 222 lesions aspirated for PTH-FNA, reported a combined sensitivity of 95% (95% CI: 91–98%), with a positive predictive value of 97% (95% CI: 93–100%), and no reports of major complications related to the procedure.23 However, the performance of this technique depends directly on the number of false negatives caused by technical failures (such as not actually aspirating the lesion), aspirating cystic areas of parathyroid adenomas or considering as negative cases with PTH-FNA from 40 to 100 pg/mL.15,23,26 It is therefore dependent on both the examiner’s expertise and the patient selection criteria, with sensitivity decreasing in the case of inexperienced examiners or protocols which extend the criteria for the indication of FNA to all patients with possible parathyroid adenomas detected by ultrasound.
The main limitation of this technique is its dependence on the ultrasound identification of suspected parathyroid adenomas. Recent systematic reviews report that the sensitivity and positive predictive value for ultrasound detection of parathyroid adenomas range from 70% to 88%,27,28 while in the series recently reported by our working group, sensitivity was 85% and positive predictive value 95%.9 Particularly interesting are the cases with a negative sestamibi scan, with a prevalence ranging from 10% to 38%, of patients with biochemical criteria for PHPT, in whom suspected parathyroid adenomas are identified by ultrasound in 51% to 77%.9,29,30 These patients would be potential candidates for FNA for PTH-FNA in order to distinguish these lesions from thyroid nodules, lymphadenopathy or other neck lesions.12,21 In these cases, one possible limitation is the difficulty in accessing some lesions for aspiration due to their location or small size.9,15,20–23 In our series, 52% (26 out of 50 cases) of patients with sestamibi scans negative for PHPT underwent FNA.9 The procedure was not carried out in 13 patients (26%) due to technical difficulty, and in another 11 patients (22%) because their ultrasound scans were negative.
As regards safety, in our series we detected no complications associated with the aspiration during either the procedure, surgery or follow-up. The lack of local adverse effects has also been reported in the vast majority of the case series of patients undergoing FNA for PTH-FNA.13,23 Only Banks et al. at the Mayo Clinic21 reported that three out of 74 patients had inflammatory changes or haematomas in the surgical site, resulting in the minimally invasive surgical technique being modified to a standard technique with the consequent lengthening of operating time. The authors suggest that the complications associated with this technique may be related to the experience of the examiner, the gauge of the needle used and the number of aspirations21, so it seems reasonable to propose a single aspiration with a fine needle (23–25 G), as we did with our patients. Last of all, cases of parathyromatosis associated with this technique are rare,31–34 although the lack of studies with a sufficient number of patients followed up for a prolonged period of time makes it impossible for conclusions to be drawn in this regard.
In conclusion, in our setting, FNA for PTH-FNA is a simple, safe diagnostic technique with high sensitivity and specificity enabling differentiation between parathyroid adenomas and other neck lesions (thyroid nodules, lymphadenopathy) in patients with biochemically confirmed PHPT. At present, this diagnostic technique is not generally recommended in patients with PHP1,6 and should be avoided in cases of suspected parathyroid cancer.35 It is, however, indicated in patients with PHPT who are candidates for parathyroidectomy with a sestamibi scan (or other additional imaging technique) that is negative or with findings that are inconsistent with those detected by ultrasound. Nonetheless, clarification is still needed on whether or not the combination of ultrasound and PTH-FNA on suspected parathyroid adenomas could be used as first-line technique for presurgical location in PHPT, consigning sestamibi scans (with or without SPECT) exclusively to negative or uncertain cases. This will have to be determined in future studies.
FundingThis study has been partially funded by unconditional research grants awarded by Laboratorios Menarini, S.A. and the Sociedad Andaluza de Endocrinología, Diabetes y Nutrición (SAEDYN) [Andalusian Society of Endocrinology, Diabetes and Nutrition].
Conflicts of interestThe authors have no conflicts of interest in relation to the objectives of or the results presented in this article.
Please cite this article as: Carral F, Jiménez AI, Tomé M, Alvarez J, Díez A, García C, et al. Seguridad y rendimiento diagnóstico de la medición de PTH en el lavado del aspirado de lesiones sospechosas de adenomas de paratiroides. Endocrinol Diabetes Nutr. 2021;68:481–488.