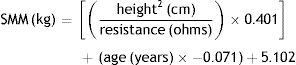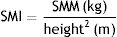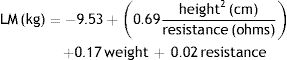Endocrine changes due to menopause have been associated to oxidative stress and muscle mass loss. The study objective was to determine the relationship between both variables in early postmenopause.
Material and methodsAn exploratory, cross-sectional study was conducted in 107 pre- and postmenopausal women (aged 40–57 years). Levels of serum lipid peroxides and uric acid and enzymes superoxide dismutase and glutathione peroxidase, as well as total plasma antioxidant capacity were measured as oxidative stress markers. Muscle mass using bioelectrical impedance and muscle strength using dynamometry were also measured. Muscle mass, skeletal muscle index, fat-free mass, and body mass index were calculated.
ResultsMore than 90% of participants were diagnosed with overweight or obesity. Postmenopausal women had lower values of muscle mass and strength markers, with a negative correlation between lipid peroxide level and skeletal muscle index (r=−0.326, p<0.05), and a positive correlation between uric acid and skeletal muscle index (r=0.295, p<0.05). A multivariate model including oxidative stress markers, age, and waist circumference showed lipid peroxide level to be the main contributor to explain the decrease in skeletal muscle mass in postmenopause, since for every 0.1μmol/l increase in lipid peroxide level, skeletal muscle index decreases by 3.03 units.
ConclusionOur findings suggest an association between increased oxidative stress and muscle mass loss in early postmenopause.
Los cambios endocrinológicos debidos a la menopausia se han asociado al estrés oxidativo y la pérdida de masa muscular. El objetivo fue determinar la relación entre ambas variables en la posmenopausia temprana.
Material y métodosEstudio transversal exploratorio con 107 mujeres pre- y posmenopáusicas (40-57 años). Como marcadores de estrés oxidativo se midieron los niveles de lipoperóxidos plasmáticos y ácido úrico sérico, las enzimas antioxidantes superóxido dismutasa y glutatión peroxidasa, y la capacidad plasmática antioxidante total. También se midió la masa muscular por impedancia bioeléctrica y la fuerza por dinamometría, y se calculó masa músculo-esquelética, índice de masa esquelética, masa libre de grasa e índice de masa corporal.
ResultadosMás del 90% de las participantes fueron diagnosticadas de sobrepeso u obesidad. En las mujeres posmenopáusicas los marcadores de masa y fuerza muscular eran más bajos, con correlación negativa entre el nivel de lipoperóxidos y el índice de masa esquelética (r= –0,326, p< 0,05), y positiva entre el ácido úrico (r=0,295, p< 0,05) y el mismo índice. En un modelo multivariante que incluye los marcadores de estrés oxidativo, edad y circunferencia de cintura, se encontró que el nivel de lipoperóxidos es el que más contribuye a explicar la disminución de la masa esquelética en la posmenopausia; por cada aumento de 0,1μmol/l de lipoperóxidos hay un decremento del índice de masa esquelética de 3,03 unidades.
ConclusiónNuestros hallazgos sugieren una asociación entre el aumento del estrés oxidativo y la pérdida de masa muscular en la posmenopausia temprana.
Menopause is caused by the loss of ovarian follicular activity, with a resultant decrease in the secretion of sex steroids (mainly estrogens), and affects different tissues and produces a series of disorders.1 According to the Consensus of the Stages of Reproductive Aging Workshop (STRAW), postmenopause starts from the last menstrual bleeding, and is referred to as early postmenopause during the first four years after this event.2
In postmenopause, lean mass is replaced by fat throughout the body, though mainly in the abdominal area, increasing body weight and loss of muscle mass (MM); sarcopenic obesity may therefore result.1,3
Sarcopenia is characterized by a generalized and gradual decrease in skeletal muscle mass (SMM) and strength (MS), accompanied by fat gain, that intensify with advancing age.4,5 It has been suggested that women experience an accelerated decrease in MM and MS at the time of menopause that may be associated with the observed estrogen deficiency.6 Muscle loss during aging is attributable to an imbalance between muscle protein synthesis, its degradation, and increased catabolic factors such as oxidative stress (OS), inflammation and mitochondrial dysfunction. The interaction of these parameters in turn induces apoptosis through different signaling pathways. In women, estrogen reduction appears to lead to an increase in proinflammatory cytokines that accelerate this OS-induced loss.7
Oxidative stress is a biochemical imbalance favored by an excess of reactive oxygen species (ROS) and free radicals that oxidize biomolecules, in which the physiological antioxidant systems prove ineffective,8 and which increases with advancing age. In women, the different functional changes caused by estrogen deficiency play an important role in increasing OS, very probably because these hormones can function as antioxidants via different mechanisms.9,10
At muscle level, estrogen deficiency has been shown to lead to the accumulation of oxidative damage in tissues, contributing to the loss of tissue homeostasis, and causing increased free radical production and cell damage that could induce apoptosis, a key mechanism for the development of sarcopenia.11 However, muscle and joint function studies in postmenopause have focused on clinical symptoms such as pain, attributable to the hormonal changes found in this period of life.12 Likewise, few studies are available on the loss of MM and the prevalence of sarcopenia in postmenopause, since the existing studies are mainly concerned with women over 60 years of age.13 Moreover, research into the relationship between the loss of MM and OS is mainly restricted to animal models.14,15 Therefore, the objective of this study was to determine the relationship between OS and the reduction of MM in women with early postmenopause.
Material and methodsStudy design and participantsWith regard to recruitment, in 2015 and 2016 residents in the eastern zone of Mexico City were invited to participate in the project “Menopause and Oxidative Stress”. A total of 132 women were enrolled. Those who were still menstruating were candidates for inclusion in the premenopausal group, while those with spontaneous amenorrhea for at least 12 months and/or serum estradiol levels <25pg/ml and follicle-stimulating hormone (FSH) levels >50mU/ml were candidates for inclusion in the postmenopausal group. Of the total candidates, 19 did not wish to participate, two were under 40 years of age, and four were over 57 years of age. A convenience sample was therefore established comprising 107 women aged 40–57 years, with a view to conducting an exploratory cross-sectional study. Two groups were established: one involving 51 premenopausal women and another with 56 postmenopausal women, with a mean duration of postmenopause of 2.7±1.5 years. Both groups included participants with no cardiovascular, renal or liver disorders, cancer or a history of depression. The women were of average socioeconomic level, with no prior hormone therapy or use of antioxidant supplements or any other drugs in the previous 6 months. All of the participants signed the informed consent approved by the Ethics Committee of the Facultad de Estudios Superiores Zaragoza, UNAM (agreement 28/04/SO/3.4.1).
Measurement of muscle mass and functionAll participants underwent bioelectrical impedance analysis (Quantum III, RJL Systems; MI, USA). Resistance was measured in ohms, and total fat and percentage fat were recorded. Their whole body measurements were taken between the wrist and right ankle while they were in the supine position and after fasting for 8h. Muscle strength was determined using a hydraulic dynamometer (Jamar; IL, USA). Body weight was recorded with the participants in underwear, under fasting conditions, and after voiding, using a Torino scale (Tecnológica Mexicana, TLM; Mexico) calibrated before each measurement. Height (in mm) was measured with an aluminum stadiometer. The body mass index (BMI) was calculated by dividing weight (kg) by height (meters and mm) squared. Waist circumference was measured using a 0.5-cm graded measuring tape surrounding the waist at the level of the navel and without applying pressure on the skin. All measurements were made by trained and supervised technical staff in order to avoid bias.
Skeletal muscle mass was calculated using the bioelectrical impedance data in the equation proposed by Janssen et al.16 for women:
Absolute muscle mass was calculated using the skeletal mass index (SMI), standardizing SMM according to body height-squared16:
Lean mass (LM) was estimated using the equation proposed by Sun et al.17:
Health statusHealth status was assessed by a gynecologist on a problem-oriented basis from the abbreviated clinical records, and from the blood count and measurements of glucose, cholesterol, triglycerides, HDL-cholesterol and LDL-cholesterol. These results were interpreted against the reference values for the Mexican population.18 Confirmation of premenopausal/postmenopausal status was based on the measurement of estradiol levels by radioimmunoassay (Siemens; PA, USA), and FSH by chemiluminescence (Siemens), with an intra-assay precision of 3.1% and 7.4%, respectively, and an analytical sensitivity for estradiol of 5pg/ml.
Measurement of oxidative stressBlood samples were collected in vacuum tubes containing heparin as an anticoagulant and without anticoagulant (Becton-Dickinson; Mexico), between 7 and 9 a.m. after a fasting period of at least 8h.
Plasma lipoperoxide (LPO) levels were measured by quantifying thiobarbituric acid reactive substances (TBARS),19 a method validated in our laboratory, with an intra-assay precision of 6.0%. The artificial formation of TBARS in the samples was prevented by the addition of 10μl of butyryl-hydroxytoluene 2mM in 95% ethanol immediately after plasma separation.
Red cell superoxide dismutase enzyme activity was measured using the xanthine oxidase method, and glutathione peroxidase through the oxidation of glutathione. In addition, total plasma antioxidant capacity was assessed by measurement of the generation kinetics of 2,2-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS+). Testing was performed using commercial kits (Randox Laboratories, Ltd., Crumlin Co., UK). The methods were previously validated, with intra-assay precisions of 3.8%, 4.6%, and 4.3%, respectively. All measurements were performed on a Shimadzu UV-1601 UV–vis spectrophotometer (Kyoto, Japan).
Samples without anticoagulant were measured for uric acid and albumin levels using a Cobas C111 analyzer (Roche Diagnostics; Basel, Switzerland), with intra-assay coefficients of variation of <5%. The antioxidant gap was calculated.9,20
On a complementary basis, a structured questionnaire on pro-oxidant factors was administered. This addressed the following: smoking (≥2cigarettes/day), consumption of alcohol and beverages with caffeine (≥2 drinks or cups/day), sedentary lifestyle (<30min exercise/day) and insomnia (≤6h sleep/day).
Statistical analysisA decrease in MM was taken into account if the patient presented a SMI <6.42kg/m2, while a decrease in MS was defined by hand pressure <20kg, according to the European Consensus on Sarcopenia.4
The mean and standard deviation were calculated for quantitative variables exhibiting a normal distribution, while the median and range were calculated in the presence of a non-normal distribution. Frequencies and percentages were determined for categorical variables. Quantitative variables were compared using the Student t-test for independent groups or the Mann–Whitney U-test, as required according to data distribution. Categorical variables in turn were compared using the χ2 Pearson chi-squared test. To establish associations, simple linear regression was performed between MM and MS markers as dependent variables and OS markers as independent variables (total and stratified according to menopausal status). Multiple linear regression models were constructed using the saturated method. The following were entered in the final model as independent variables: all OS markers, age because of its association with MM loss, and waist circumference as an indicator of central adiposity because it is associated with OS. The SMI was entered as a dependent variable. The other muscle markers showed no significant association in the multiple models. The SPSS version 20.0 statistical package (IBM SPSS Statistics, Armonk, NY, USA) was used throughout. Statistical significance was considered for p<0.05.
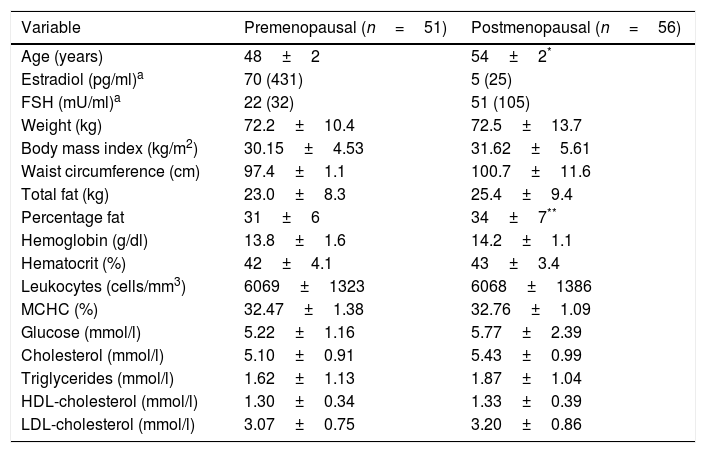
ResultsPatient characteristicsThe study groups were similar in terms of the anthropometric and biochemical-hematological measurements; only age, estradiol, FSH and percentage fat differed (p<0.05) (Table 1). Forty-six of the premenopausal women (90%) and 52 of the postmenopausal women (93%) were overweight or obese (BMI≥25.00kg/m2).
Anthropometric measures and biochemical-hematological profile of the study groups.
| Variable | Premenopausal (n=51) | Postmenopausal (n=56) |
|---|---|---|
| Age (years) | 48±2 | 54±2* |
| Estradiol (pg/ml)a | 70 (431) | 5 (25) |
| FSH (mU/ml)a | 22 (32) | 51 (105) |
| Weight (kg) | 72.2±10.4 | 72.5±13.7 |
| Body mass index (kg/m2) | 30.15±4.53 | 31.62±5.61 |
| Waist circumference (cm) | 97.4±1.1 | 100.7±11.6 |
| Total fat (kg) | 23.0±8.3 | 25.4±9.4 |
| Percentage fat | 31±6 | 34±7** |
| Hemoglobin (g/dl) | 13.8±1.6 | 14.2±1.1 |
| Hematocrit (%) | 42±4.1 | 43±3.4 |
| Leukocytes (cells/mm3) | 6069±1323 | 6068±1386 |
| MCHC (%) | 32.47±1.38 | 32.76±1.09 |
| Glucose (mmol/l) | 5.22±1.16 | 5.77±2.39 |
| Cholesterol (mmol/l) | 5.10±0.91 | 5.43±0.99 |
| Triglycerides (mmol/l) | 1.62±1.13 | 1.87±1.04 |
| HDL-cholesterol (mmol/l) | 1.30±0.34 | 1.33±0.39 |
| LDL-cholesterol (mmol/l) | 3.07±0.75 | 3.20±0.86 |
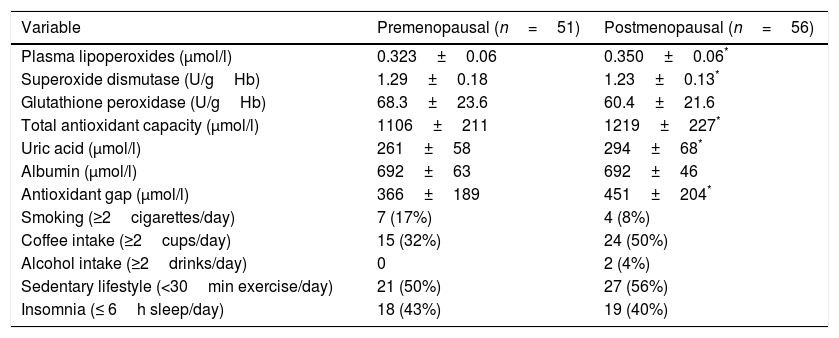
Of the different OS markers, LPO and the plasma antioxidant markers were found to be higher in postmenopausal women, while superoxide dismutase (SOD) activity was lower in this group. No differences between the groups were recorded in terms of the prooxidant factors (Table 2).
Measurements of oxidative stress markers and prooxidant factors of the study groups.
| Variable | Premenopausal (n=51) | Postmenopausal (n=56) |
|---|---|---|
| Plasma lipoperoxides (μmol/l) | 0.323±0.06 | 0.350±0.06* |
| Superoxide dismutase (U/gHb) | 1.29±0.18 | 1.23±0.13* |
| Glutathione peroxidase (U/gHb) | 68.3±23.6 | 60.4±21.6 |
| Total antioxidant capacity (μmol/l) | 1106±211 | 1219±227* |
| Uric acid (μmol/l) | 261±58 | 294±68* |
| Albumin (μmol/l) | 692±63 | 692±46 |
| Antioxidant gap (μmol/l) | 366±189 | 451±204* |
| Smoking (≥2cigarettes/day) | 7 (17%) | 4 (8%) |
| Coffee intake (≥2cups/day) | 15 (32%) | 24 (50%) |
| Alcohol intake (≥2drinks/day) | 0 | 2 (4%) |
| Sedentary lifestyle (<30min exercise/day) | 21 (50%) | 27 (56%) |
| Insomnia (≤ 6h sleep/day) | 18 (43%) | 19 (40%) |
Student t-test for independent groups.
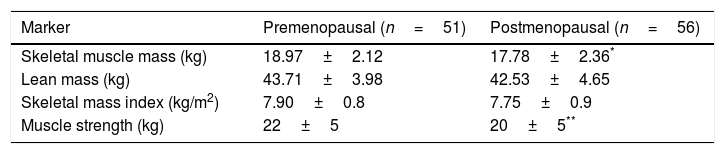
Four postmenopausal women (7%) had sarcopenia and obesity, while MM proved normal in premenopausal women. Twenty-five of the postmenopausal women (45%) and 11 premenopausal women (22%) lost MS (p<0.05). Among the muscle markers, SMM and MS were significantly lower in postmenopausal women (Table 3), and a negative correlation was found between SMM and age (r=−0.245; p<0.05).
Muscle mass and function markers of the study groups.
| Marker | Premenopausal (n=51) | Postmenopausal (n=56) |
|---|---|---|
| Skeletal muscle mass (kg) | 18.97±2.12 | 17.78±2.36* |
| Lean mass (kg) | 43.71±3.98 | 42.53±4.65 |
| Skeletal mass index (kg/m2) | 7.90±0.8 | 7.75±0.9 |
| Muscle strength (kg) | 22±5 | 20±5** |
Student t-test for independent groups.
A negative correlation was observed between LPO levels and SMI in postmenopausal women (r=−0.326, r2=0.11; p<0.05), with a positive correlation between uric acid and SMI (r=0.295, r2=0.09; p<0.05).
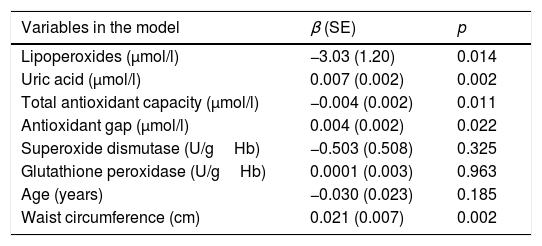
In the multivariate model corresponding to this same group, LPO was the parameter that accounted most for the SMI value, exhibiting a negative and independent association even after age and waist circumference were added to the model. Uric acid remained the most closely correlated antioxidant, in addition to the non-enzymatic antioxidants, which showed a slight correlation to SMI. No association was found for the enzymatic antioxidants. Accordingly, for every 0.1μmol/l increase in LPO, an increase in SMI of 3.03 units was recorded, while for each 1μmol/l increase in uric acid the SMI increased by 0.007 units. With regard to the pro-oxidant factors, only waist circumference was found to be associated (Table 4).
βNon-standardized beta-coefficient values of the oxidative stress markers, together with age and waist circumference as pro-oxidant factors, in the multivariate regression model for skeletal mass index in postmenopause.
| Variables in the model | β (SE) | p |
|---|---|---|
| Lipoperoxides (μmol/l) | −3.03 (1.20) | 0.014 |
| Uric acid (μmol/l) | 0.007 (0.002) | 0.002 |
| Total antioxidant capacity (μmol/l) | −0.004 (0.002) | 0.011 |
| Antioxidant gap (μmol/l) | 0.004 (0.002) | 0.022 |
| Superoxide dismutase (U/gHb) | −0.503 (0.508) | 0.325 |
| Glutathione peroxidase (U/gHb) | 0.0001 (0.003) | 0.963 |
| Age (years) | −0.030 (0.023) | 0.185 |
| Waist circumference (cm) | 0.021 (0.007) | 0.002 |
R: 0.516; R2: 0.267.
p<0.0001.
During menopause transition, estrogen depletion contributes to bone mass loss and the redistribution of subcutaneous fat toward the visceral zone. This process is characterized by lesser gluteal-femoral fat accumulation, with increased abdominal visceral lipogenesis and a decreased progesterone – glucocorticoid competitive effect.3,6 As a result, overweight/obesity is very common in women at this stage of life. This was confirmed in our study by the very high prevalence of overweight/obesity in both groups, consistent with the data reported in North American and Spanish women.21,22
This drop in estrogen levels has a direct impact upon the muscle tissue. Some studies indicate that MM starts to decrease at about 45 years of age, coinciding in women with the perimenopausal period, with a decrease in MM of 1–2% per year and a decrease in MS of 1.5% per year between 50 and 60 years of age. In our study,23,24 SMM decreased significantly after menopause, with a postmenopausal sarcopenia rate of 7% and a loss of MS in 45% of the women. In this regard, a prevalence of postmenopausal sarcopenia of 14–26% has been reported in women between 50 and 70 years of age.25,26 The lower prevalence found in our study was due to the fact that the women were younger and in the early postmenopausal phase. Of note is the observation that the prevalence of loss in MS was greater than that of the decrease in MM, which suggests that muscle damage starts with a loss of function in premenopause, and increases in postmenopause.
As commented earlier, menopause transition is associated with an increase in OS. In this regard, our research group reported that menopause is a risk factor for OS, probably due to decreased estrogen levels,9 which is consistent with the increase in LPO and the decrease in enzymatic antioxidants seen in the present study. A relationship was also found between changes in MM and OS in postmenopause, an association has been analyzed in animal models,14,15 but in humans only in women over 60 years of age.7 This underscores the importance of our study.
In this regard, a negative correlation was found between SMI and LPO levels in both the simple and multivariate regression models. A relevant observation in the multivariate regression model was that the extracellular antioxidant markers contributed only minimally; oxidative stress therefore occurs with no efficient antioxidant response due to the lowered MM, independently of age and waist circumference. In this sense, it has been proposed that OS is one of the main factors related to muscle deficiency in the course of aging, with increased oxidative damage to lipids, proteins and DNA.27,28 This was shown in our study, where each 0.1μmol/l increase in LPO was found to be associated with a decrease in SMI of 3.03 units. In fact, the level of lipid peroxidation in human skeletal muscle depends on muscle fiber composition and muscle function,29 suggesting that increased ROS levels are harmful for the maintenance of skeletal muscle size since they have an impact upon the signaling pathways implicated in muscle hypertrophy.30 Muscle fiber size is known to be regulated by the balance between protein synthesis and catabolism, and it has been suggested that OS favors muscle proteolysis in three ways: by promoting the gene expression of important proteins implicated in several proteolytic systems; by increasing cytosolic free calcium leading to the activation of calpain and caspase-3; and by modifying the myofibrilar proteins by an increase in their susceptibility to proteolytic catabolism,31 thereby causing the loss of MM.
The positive relationship between uric acid and SMI observed in our study is consistent with the data published elsewhere.32–34 Studies conducted in Asian populations have reported a positive association between SMI and MS and serum uric acid concentrations, underscoring their protective role against OS due to their capacity to clear ROS,32 especially in women.34
Lastly, the limitations of our study are its cross-sectional design and small sample size. However, the consistent association observed in the different mathematical models between the changes in MM and OS after menopause suggests that there is an increase in OS associated with the loss of MM in women with early postmenopause. This represents a finding not previously reported, and one which needs to be confirmed by longitudinal studies.
Financial supportThis study was supported by the PAPIIT-UNAM program (identifying code: IN224115).
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Zacarías-Flores M, Sánchez-Rodríguez MA, García-Anaya OD, Correa-Muñoz E, Mendoza-Núñez VM. Relación entre el estrés oxidativo y la pérdida de masa muscular en la posmenopausia temprana: estudio exploratorio. Endocrinol Diabetes Nutr. 2018;65:328–334.