Struma ovarii is the presence of thyroid tissue as the predominant component in an ovarian teratoma. It accounts for 0.3%–1% of ovarian tumors and for 2%–4% of ovarian teratomas.1,2 A diagnosis of struma ovarii requires that thyroid tissue represents at least 50% of the teratoma. Its clinical presentation is very variable, ranging from benign asymptomatic tumors to tumors with distant metastases. Approximately 5%–10% of cases are malignant tumors, and 5% of these present with metastasis at the time of diagnosis.3–6
As in thyroid gland tumors, the most common histological types are papillary and follicular carcinomas. More aggressive variants are extremely rare.7
The management of malignant struma ovarii is controversial.8–10 In metastatic disease, aggressive gynecological surgery is required, and treatment should be completed with 131I, as in differentiated thyroid carcinoma (DTC), for which prior total thyroidectomy is required.6 The coexistence of malignant struma ovarii with DTC in the thyroid gland has been reported occasionally.9 Subsequent suppressive treatment with levothyroxine is recommended. Because of the risk of relapse in the long-term, as in DTC, follow-up should include regular measurement of basal and stimulated thyroglobulin levels and thyroglobulin antibodies, and imaging tests if local relapse or metastasis is suspected.8,10
The cases of two patients diagnosed with metastatic malignant struma ovarii presenting as a suspect ovarian tumor are reported below.
The first patient, a 57-year-old woman, complained of fever and abdominal pain and distension. A computed tomography (CT) showed a solid-cystic mass in the left ovary.
Laboratory tests revealed elevated tumor markers: CA-125: 1510U/mL (normal range [NR]: 2–24); CA 15.3: 58.3U/mL (NR: 9–42). An ovarian malignancy was suspected, and a laparoscopic bilateral adnexectomy was performed, which did not reveal gross peritoneal implants. A histological examination found a malignant struma ovarii in the left adnexum; it was a follicular variant of papillary carcinoma, 10cm in size, with foci of poorly differentiated carcinoma infiltrating the capsule and invading the blood vessels. In the right ovary there was a 2.5mm poorly differentiated carcinoma, probably of thyroid origin.
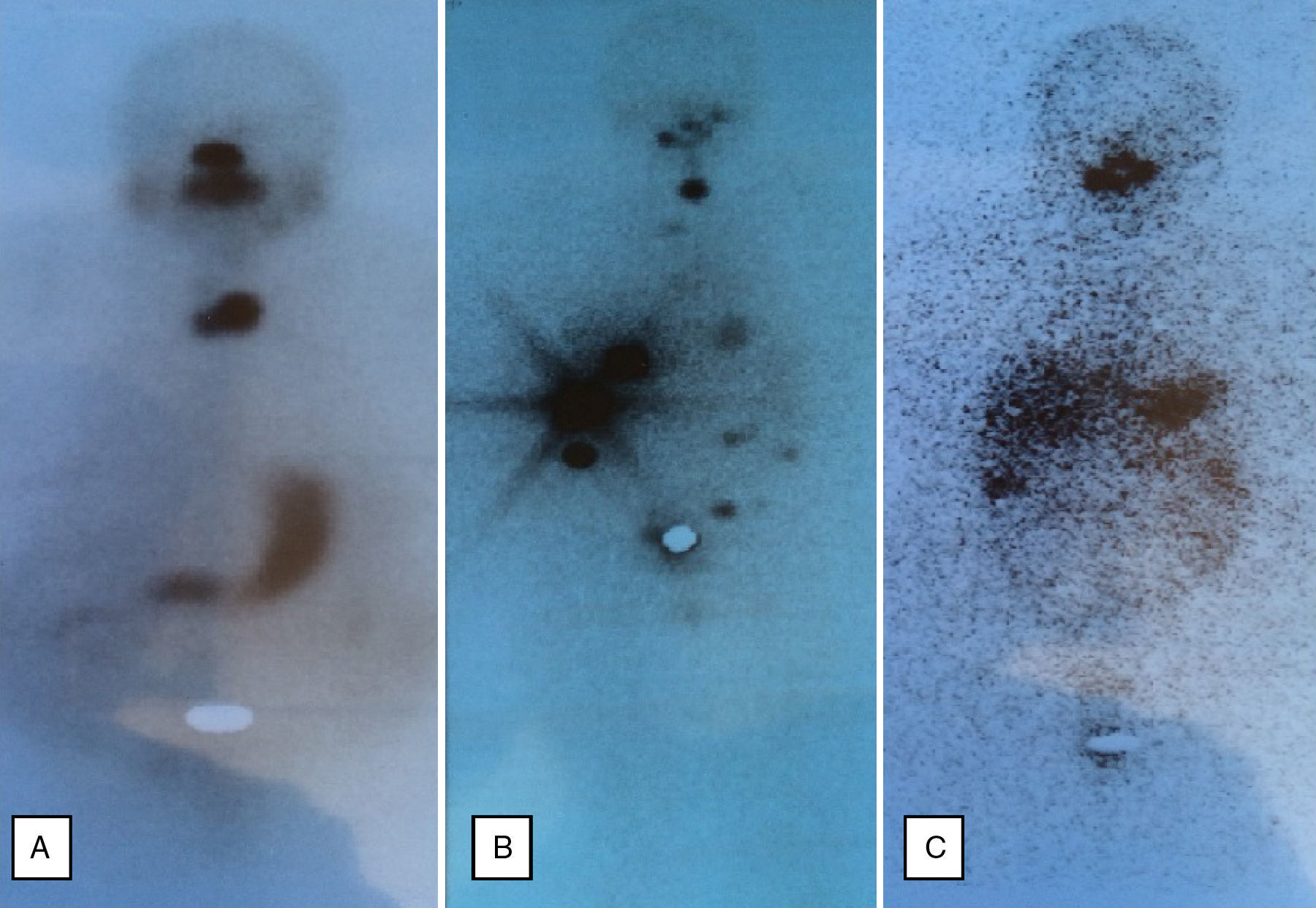
Based on this diagnosis, total thyroidectomy was performed. A histological examination revealed multinodular hyperplasia without evidence of malignancy. The patient was subsequently treated with 105mCi of 131I. A subsequent whole body scan showed a cervical deposit related to a thyroid remnant (Fig. 1A). Treatment was started with levothyroxine 175μg/day to keep TSH levels suppressed. After surgery, CA-125 dropped to 89.4U/mL; CA 15.3, thyroglobulin, and thyroglobulin antibodies were undetectable.
However, the patient returned four months later for ascites, and relapsing disease was detected. A CT scan showed peritoneal implants and multiple liver metastases. Since this was a poorly differentiated carcinoma refractory to standard treatment, chemotherapy with cisplatin and adriamycin was started. Progress was unfavorable, and the patient died 18 months after diagnosis.
The second patient, aged 48 years, complained of abdominal pain and distension. A CT showed a tumor in the right adnexum, abundant intra-abdominal free fluid, and multiple peritoneal implants. Laboratory tests showed a CA-125 level of 1351U/mL (NR: 0–35U/mL).
An exploratory laparoscopy was performed, and several biopsy samples were taken. A histological examination showed mature thyroid tissue. As metastatic malignant struma ovarii was suspected, total hysterectomy, bilateral adnexectomy, implant resection, and total thyroidectomy were performed at a single surgery. Preoperative thyroid function was normal.
On the right ovary and in the implants, a 10-cm malignant struma ovarii was seen: nodular hyperplasia with areas of follicular variant of papillary carcinoma. A histological examination of the thyroid gland showed thyroid atrophy with fibrosis and severe lymphocytic thyroiditis. The patient was subsequently treated with 57mCi of 131I. A whole body scan after 131I showed uptake by thyroid remnant and six peritoneal deposits (Fig. 1B).
Treatment was started with levothyroxine 100μg/day to maintain the suppression of TSH. After six months, the basal thyroglobulin level was 28μg/L (NR: <0.2) and thyroglobulin antibodies were elevated (81.52IU/mL; NR: 0–4.2). The patient was given an additional dose of 56.7mCi of 131I. A subsequent scan showed the persistence of only two peritoneal implants (Fig. 1C).
Twelve months after the second dose of 131I, basal and stimulated thyroglobulin levels were undetectable (<0.2μg/L), and thyroglobulin antibodies were negative. CA-125 levels normalized during follow-up.
Three years after diagnosis, the patient remains on regular follow-up and meets the criteria for cure (undetectable basal and stimulated thyroglobulin levels, no uptake on the body scan, no abnormal findings in abdominal CT).
These cases are particularly interesting because both patients had a very different course following the diagnosis of metastatic malignant struma ovarii. In the first patient, the presence of areas of poorly differentiated carcinoma and capsular and vascular infiltration explains the unfavorable course that led to the patient's death. Recent studies suggest that the presence of a poorly differentiated component is associated with a poorer prognosis7, and would also explain the absence of uptake in the body scan after treatment with 131I and the absence of elevated thyroglobulin levels. The second patient had a well-differentiated tumor with a good response to standard treatment currently meeting the criteria for cure. Neither of the patients had a synchronous thyroid malignancy.
Given the rarity of the disease, there are few studies of metastatic malignant struma ovarii. As our study shows, tumor histology is fundamental for making the most adequate clinical decisions during the course of the disease.
Please cite this article as: Ernaga Lorea A, Hernández Morhain MC, Anda Apiñániz E, Lapeña Calavia S, Eguílaz Esparza N. Estruma ovárico maligno metastásico: 2 escenarios de una misma enfermedad. Endocrinol Diabetes Nutr. 2017;64:121–122.