Radioiodine (131I) is an established modality of definitive treatment of hyperthyroidism. In spite of the vast experience available, there are still several aspects to be clarified, such as whether fixed or calculated doses should be used. The aim of this study was to assess whether efficacy of this treatment could be improved by implementing a simple dosimetric calculation method including ultrasonographic estimation of thyroid volume and a single measurement of 24-h 131I thyroid uptake.
MethodsA prospective non-inferiority study was designed to compare two procedures to calculate radioiodine activity: the “semi-fixed” dose method (A), and the “calculated” dose method (B). The first consisted of activity escalation (185MBq steps) based on etiology of hyperthyroidism, 131I uptake, and treatment objective. The second method was based on the “dosimetric compromise” concept, considering 24-h uptake and thyroid volume as the only factors and using a standard half-life of 5.5 days. The target absorbed dose was 150Gy, but after a preliminary analysis (first 100 cases) it was increased to 200Gy in diffuse toxic goiters (DTGs).
ResultsA total of 212 patients were included. Method B was at least as effective in terms of final and functional outcome, with a trend to more success and less hypothyroidism. In addition, activities administered were significantly lower.
ConclusionIn radioiodine therapy of hyperthyroidism, a simple dosimetric method that provided results at least equal to those of a fixed dose-based method, with lower administered activities, could be implemented.
El radioyodo (131I) constituye una modalidad establecida de tratamiento definitivo del hipertiroidismo. A pesar de la vasta experiencia existente, persisten varios aspectos por clarificar, como qué tipo de dosis emplear, ¿fijas o calculadas? El objetivo del estudio fue determinar si se podría mejorar la eficacia de este tratamiento implementando un método simple de cálculo dosimétrico que incluyera la estimación ecográfica del volumen tiroideo y una medida única de captación de 131I (24h).
MétodosDiseñamos un estudio prospectivo de no inferioridad comparando entre dos modalidades de cálculo de la actividad de radioyodo: el método de dosis «semifijas» (A) y el de dosis «calculadas» (B). El primero consistió en escaladas de actividad (peldaños de 185MBq) teniendo en cuenta: etiología del hipertiroidismo, captación de 131I y objetivo terapéutico. El segundo se basó en el concepto de «compromiso dosimétrico», considerando como únicos factores la captación y el volumen tiroideos, empleando una vida media estándar de 5,5días. La dosis absorbida diana fue 150Gy, aunque tras un análisis preliminar (100 primeros casos) se aumentó a 200Gy en los bocios difusos tóxicos (BDT).
ResultadosSe incluyeron 212 pacientes. El métodoB resultó al menos igual de eficaz en cuanto al resultado final y funcional, con tendencia a más éxitos y menos hipotiroidismo. Además, las actividades administradas fueron significativamente menores.
ConclusiónEn la terapia con radioyodo del hipertiroidismo se pudo implementar un método dosimétrico sencillo que proporcionó resultados al menos iguales a los de un método basado en dosis fijas, con actividades administradas inferiores.
Hyperthyroidism is an endocrine disorder in which the most serious manifestation is tachyarrhythmia in the form of atrial fibrillation. It is important to rapidly deal with the symptoms and reduce the overproduction of thyroid hormones. In addition to the standard initial approach based on antithyroid drugs, a definitive solution to the problem is very often needed.
Radioiodine, or iodine-131 (131I), has been used since the 1940s for the treatment of both benign and malignant thyroid disorders,1–4 and is considered together with surgery to be a definitive treatment option for the main disorders causing hyperthyroidism, i.e., Graves-Basedow disease (GBD) and toxic nodular goiter.5,6 Its efficacy is very high and depends primarily on the type of disorder, thyroid gland size, and the activity of the administered 131I.
The main problem inherent to treatment is that a maximum cure rate inevitably leads to a higher incidence of hypothyroidism. The ideal objective is to restore euthyroidism, with the possible exception of “definitive” GBD treatment (the increased tendency toward autoimmune hyperthyroidism relapse may make “ablation” advisable).
Despite the extensive experience available, a number of aspects remain to be clarified. Should the remission of hyperthyroidism be prioritized with a single treatment, even at the expense of causing/triggering hypothyroidism? Should low or high activities be used? What factors influence the outcome? Can all of them be taken into account when planning radioiodine therapy? The salient question nowadays is what type of dose should be used: fixed or calculated?
The classical and still most common way of administering treatment is based on so-called “fixed doses”, comprising a range of activities which, as a result of the experience gained over the years, have come to be considered optimum. The British guides still advocate this simple approach.7 However, in recent years, and with the adoption of stricter regulations regarding radiation exposure8 and the basic principles of radioprotection (such as the ALARA [As Low As Reasonably Achievable] principle), most current guides recommend the use of “calculated doses”. The variables to be entered to calculate the activities required for “personalized” therapy are mainly the measurement of thyroid uptake(s) and target tissue size. Greater precision in the actual absorbed dose implies the need for an important number of pre- and post-therapeutic measurements of radioiodine uptake, which adversely affects the simplicity and functionality of treatment.
Our working hypothesis was that the efficacy of radioiodine treatment of hyperthyroidism could be improved by the implementation of a simple dosimetric calculation method including the ultrasound estimation of thyroid gland volume and a single measurement of the thyroid uptake of 131I at 24h. The ultimate aim was to implement an individual calculation method of 131I activity at least as effective as the protocol we had been using in the past: a weighted fixed-dose variant which we call “semi-fixed” dosing.
We sought to prospectively compare both methods in terms of the success rate (non-hyperthyroid status) and functional outcome (the proportion of hypothyroidism cases); assess whether success is achieved with lower 131I activities (lesser total irradiation of the patient); and study the influence of the type of hyperthyroid disease upon the results obtained.
Material and methodsIn March 2010 we started a prospective, comparative study of two modalities for estimating radioiodine activity in the treatment of hyperthyroidism. We designed a prospective non-inferiority trial in which the first 50 consecutive hyperthyroid patients referred for treatment with 131I would receive the first “semi-fixed” dose method (method A), and the next 50 patients would receive the dosimetric or “calculated” dose method (method B). The idea was to perform a preliminary analysis of the first 100 patients to verify non-inferiority and calibrate the need for adjustments to method B.
PatientsAll patients seen between March 2010 and May 2014 were included in the study. The patients were required to be able to complete the initial study and not to meet any exclusion criteria.
We did not include euthyroid patients referred for radioiodine therapy to reduce gland volume. The absolute contraindications in the European Association of Nuclear Medicine (EANM) guidelines (pregnancy and lactation) also constituted exclusion criteria.
However, we did include patients with thyroid ophthalmological disease, in all cases receiving corticosteroid prophylactic treatment, after consultation with their reference physician.
We instructed antithyroid medication to be suspended 5 days before the uptake test, advised restricting the use of other drugs that could interfere with radioiodine incorporation to the thyroid gland, and recommended a low-iodine diet.
On the first day of attendance at the clinic, the patient signed the informed consent document, and we then proceeded with the necessary complementary tests. On the second day, the 131I uptake measurement procedure was completed. The patient was given an appointment for treatment, and was recommended to continue without antithyroid medication (and on a diet) if treatment was to be imminent, or to restart the medication followed by suspension for at least 5 days if the patient could not be treated the following week. Assignment and/or calculation of the activities were also carried out.
Complementary testsThyroid scintigraphyWe injected 185MBq (5mCi) of 99mTc-pertechnetate, and imaging centered on the cervicothoracic region was acquired in anterior projection after 10–15min. We only used scintigraphy for visual analysis, but not for uptake calculations or estimates of gland size or functioning thyroid tissue.
The results of the test could modify the therapeutic approach in two circumstances:
- –
An image corresponding to a “hot” nodule with autonomous behavior in patients referred to as multinodular goiter cases. These were classified as “autonomous toxic nodule” (ATN).
- –
“Cold” nodules not previously studied. These patients were sent to the reference physician for ultrasound evaluation and possible ultrasound-guided fine needle aspiration biopsy (FNAB) if there was a solid component or suspicious image.9
On Day 1, an oral dose of 3.7MBq (100μCi) of 131I, dissolved in water, was administered. A calculation was made on day2. The CAPTUS 2000 probe (Capintec®) was used to perform the necessary measurements, with the following pre-established principal parameters: radioisotope 131I, in liquid form, counts of 300seconds, and a distance of 30cm.
Thyroid ultrasoundThyroid ultrasound was performed with the patient in dorsal decubitus and with hyperextension of the neck. An ultrasound system with a high-frequency linear probe (7–15MHz) was used, accompanied by soluble transducer gel. We determined the volume of each lobe and of the whole thyroid gland in milliliters, performing longitudinal and transverse scans, using the Brunn formula,10 which considers the volume of the isthmus to be negligible. Each lobe is assumed to be an ellipsoid, whereby:
Lobe volume (ml)=transverse×longitudinal×anteroposterior diameter (cm)×π/6
Total volume=right volume+left volume
Assignment of activitiesMethod ABased on activity escalations in steps of 185MBq (5mCi). The key features are:
- •
Dosing criteria (activities):
- ∘
Toxic diffuse goiter (TDG): 185/370/550MBq (5/10/15mCi).
- ∘
ATN: 370/550/740MBq (10/15/20mCi).
- ∘
Toxic multinodular goiter (TMG): 550/740/925MBq (15/20/25mCi).
- •
These activity levels within each disorder were assigned based on the calculated thyroid uptake:
- ∘
>50%: first level (lowest activity).
- ∘
25–50%: second level (intermediate activity).
- ∘
<25%: third level (greatest activity).
- •
In addition, we considered the therapeutic objective: one step above the previous scheme was made (+185MBq), provided that the clinical criteria caused efficacy in a single dose to outweigh the side effect of hypothyroidism.
- •
Thus, the minimum activity we administered was 185MBq (5mCi), while the maximum activity was 1.11GBq (30mCi).
- ∘
We assumed the concept of “dosimetric compromise”11: the target volume is not the autonomous tissue (which is difficult to determine precisely) but the entire gland. In an attempt to prioritize simplicity, we adopted the uptake of 131I at 24h and thyroid volume as the only factors. The activity to be administered was calculated using the Marinelli algorithm12,13:
Activity of 131I (MBq)=(target dose×thyroid volume)/(0.04×uptake 24h 131I×T1/2)
- •
The target dose was 150Gy for at least the first 50 patients, with the possibility of having to change the dose in the second phase of the study depending on the disorder causing hyperthyroidism.
- •
Thyroid volume was measured by ultrasound, because it is simple and inexpensive, with better correlation to magnetic resonance imaging (the gold standard).
- •
In addition, in making a single determination of 131I uptake, it was necessary to use a standard half-life for radioiodine (5.5 days).
For comparison purposes and due to radiological protection issues, we decided to adopt the same activity limits as in methodA: minimum activity 185MBq and maximum activity 1.11GBq. This means that if the calculated activity was <185MBq, we assigned 185MBq, and if the calculated activity was >1.11GBq, we assigned 1.11GBq.
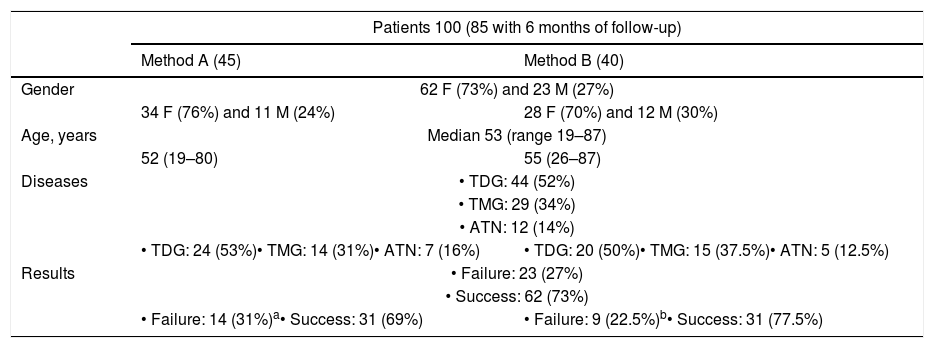
Based on the preliminary analysis of the first 100 treated patients (50 per method) (Table 1), we decided to increase the target dose to 200Gy in TDG.14
Characteristics of the population corresponding to the preliminary analysis.
| Patients 100 (85 with 6 months of follow-up) | ||
|---|---|---|
| Method A (45) | Method B (40) | |
| Gender | 62 F (73%) and 23 M (27%) | |
| 34 F (76%) and 11 M (24%) | 28 F (70%) and 12 M (30%) | |
| Age, years | Median 53 (range 19–87) | |
| 52 (19–80) | 55 (26–87) | |
| Diseases | • TDG: 44 (52%) | |
| • TMG: 29 (34%) | ||
| • ATN: 12 (14%) | ||
| • TDG: 24 (53%)• TMG: 14 (31%)• ATN: 7 (16%) | • TDG: 20 (50%)• TMG: 15 (37.5%)• ATN: 5 (12.5%) | |
| Results | • Failure: 23 (27%) | |
| • Success: 62 (73%) | ||
| • Failure: 14 (31%)a• Success: 31 (69%) | • Failure: 9 (22.5%)b• Success: 31 (77.5%) | |
On the day of treatment, the patients were required to arrive under fasting conditions (at least 4h). A pregnancy test was made in women of childbearing potential. The patients were admitted for 24h as agreed with the Radiological Protection Department.
At discharge, the patients were instructed regarding their medication and follow-up. We recommended the reintroduction of antithyroid medication after 5 days, following a descending regimen over three weeks (reducing the dose on a weekly basis).
Follow-up was based on the thyroid profile test findings 3, 6 and 12 months after radioiodine treatment. In the event of clinical signs of hypothyroidism or hyperthyroidism manifesting before the dates established for the tests and consultation, we recommended that the latter be performed earlier.
“Success” was taken to be the absence of hyperthyroidism, while “failure” corresponded to persistent (including subclinical) hyperthyroidism.
Statistical analysisUse was made of the RStudio version 0.98.1091 package, with comparison of means analyses. The primary endpoint (success or failure) was a dichotomous variable with a non-normal distribution; the Wilcoxon test and Mann–Whitney U-test were therefore applied.
In order to determine whether, regarding the type of dose, a lesser amount of medication was administered in the case of method B versus method A, the study variable (administered activity) was a continuous quantitative variable; a different statistical test was therefore used for the comparison of means. Prior comparison of variances and normal data distribution testing (the Kolmogorov–Smirnov test) were performed with a view to applying the Student t-test (if the variances were the same) or the Welch test (if the variances were different).
Statistical significance was considered for p<0.05.
ResultsBetween 3 March 2010 and 26 May 2014, a total of 221 hyperthyroid patients were treated with radioiodine in our Department. We excluded 9 patients in which fixed doses were used because the measurement of uptake could not be carried out (due to patient unavailability). The final study population therefore consisted of 212 patients. The final and functional outcomes could only be assessed in 179 patients (in 33 cases the outcomes could not be established due to insufficient or lost follow-up).
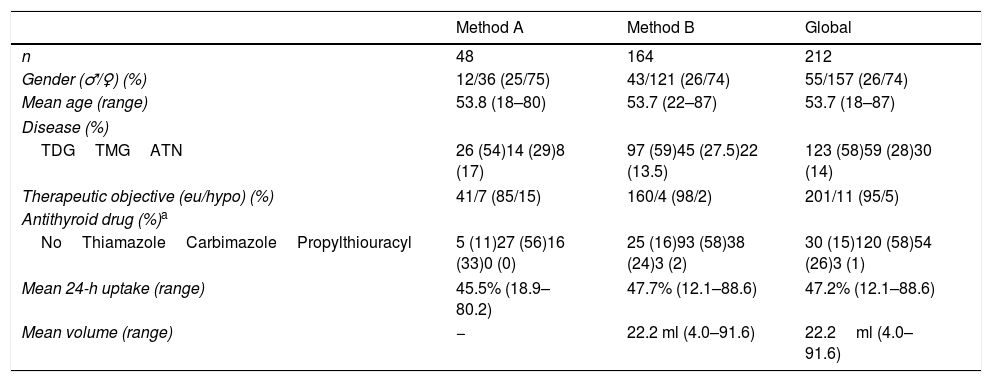
The main characteristics of the study population are shown in Table 2. No major differences were observed between the patients treated with either method.
Principal patient characteristics.
| Method A | Method B | Global | |
|---|---|---|---|
| n | 48 | 164 | 212 |
| Gender (♂/♀) (%) | 12/36 (25/75) | 43/121 (26/74) | 55/157 (26/74) |
| Mean age (range) | 53.8 (18–80) | 53.7 (22–87) | 53.7 (18–87) |
| Disease (%) | |||
| TDGTMGATN | 26 (54)14 (29)8 (17) | 97 (59)45 (27.5)22 (13.5) | 123 (58)59 (28)30 (14) |
| Therapeutic objective (eu/hypo) (%) | 41/7 (85/15) | 160/4 (98/2) | 201/11 (95/5) |
| Antithyroid drug (%)a | |||
| NoThiamazoleCarbimazolePropylthiouracyl | 5 (11)27 (56)16 (33)0 (0) | 25 (16)93 (58)38 (24)3 (2) | 30 (15)120 (58)54 (26)3 (1) |
| Mean 24-h uptake (range) | 45.5% (18.9–80.2) | 47.7% (12.1–88.6) | 47.2% (12.1–88.6) |
| Mean volume (range) | − | 22.2 ml (4.0–91.6) | 22.2ml (4.0–91.6) |
TDG: toxic diffuse goiter; TMG: toxic multinodular goiter; ATN: toxic adenoma.
Women represented almost 3/4 of the total, and the mean age was 53.7 years (range: 18–87). The most common disease was GBD, while multinodular goiter was the predominant disorder in nodular disease. Hypothyroidism was the therapeutic target in only 11 patients (5%).
The uptake of 131I at 24h and thyroid volume estimated by ultrasound were two variables required for activity calculation in the dosimetric method. In addition, uptake was also required for the assignment of activities in method A. Thus, data were available for the first in all patients (n=212), but for the second only in those corresponding to method B (n=163). With regard to thyroid gland size, despite some extreme or outlier values (4–91.6ml), the glands were generally not large in our population (22.25±15.92ml) (Table 2).
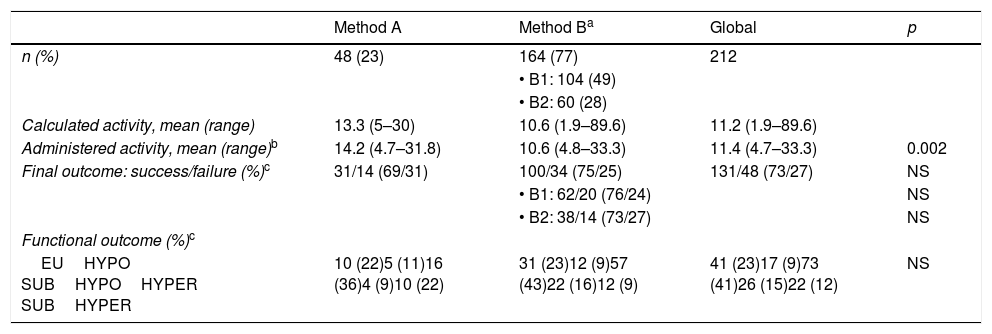
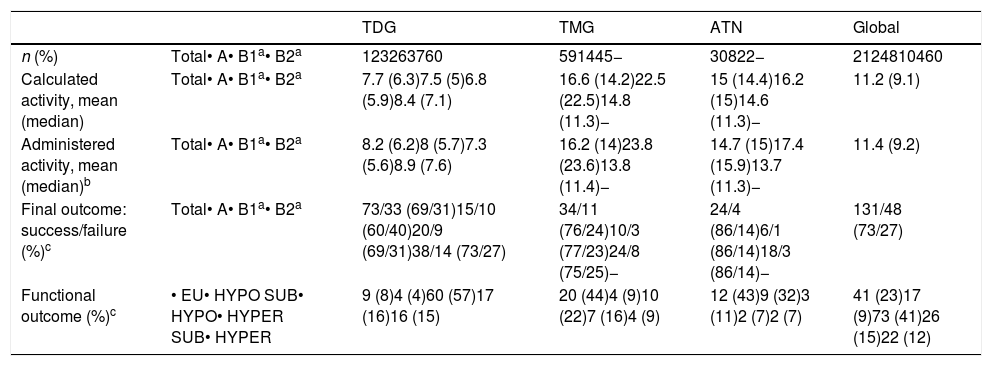
Results according to calculation methodOf the 212 patients, 48 received treatment according to method A and the remainder (n=164) according to methodB; of these, 104 (49% of the total) had a target thyroid absorbed dose of 150Gy (B1) and 60 (28%; all with GBD) had a dose of 200Gy (B2). Table 3 shows the calculated and actually administered activities and the results obtained with each method.
Results according to the calculation method.
| Method A | Method Ba | Global | p | |
|---|---|---|---|---|
| n (%) | 48 (23) | 164 (77) | 212 | |
| • B1: 104 (49) | ||||
| • B2: 60 (28) | ||||
| Calculated activity, mean (range) | 13.3 (5–30) | 10.6 (1.9–89.6) | 11.2 (1.9–89.6) | |
| Administered activity, mean (range)b | 14.2 (4.7–31.8) | 10.6 (4.8–33.3) | 11.4 (4.7–33.3) | 0.002 |
| Final outcome: success/failure (%)c | 31/14 (69/31) | 100/34 (75/25) | 131/48 (73/27) | NS |
| • B1: 62/20 (76/24) | NS | |||
| • B2: 38/14 (73/27) | NS | |||
| Functional outcome (%)c | ||||
| EUHYPO SUBHYPOHYPER SUBHYPER | 10 (22)5 (11)16 (36)4 (9)10 (22) | 31 (23)12 (9)57 (43)22 (16)12 (9) | 41 (23)17 (9)73 (41)26 (15)22 (12) | NS |
The calculated activity was 11.21±9.17mCi (range: 1.9–89.6mCi). The mean activity administered with methodA was 14.16mCi, versus 10.55mCi with methodB. After using the Kolmogorov-Smirnov test to confirm that the variable “administered activity” exhibited a normal distribution, the Student t-test for the equality of means was applied, obtaining p=0.002. Thus, the activity administered with method B was significantly lower than that administered with method A.
Success was recorded in 131 of the 179 patients evaluated (73%) and failure (persistent hyperthyroidism) in 48 (27%). Table 3 shows percentage success to be greater with method B than with method A, though without reaching statistical significance.
Table 3 also reflects the high percentage of resulting hypothyroidism, independently of the dose assignment method: the figure proved higher than the percentage of euthyroidism, though with the inclusion of cases of subclinical hypothyroidism. Within the treatment failure group, overt hyperthyroidism predominated among the patients treated with method A, while subclinical hyperthyroidism predominated among the patients treated with method B. No significant differences were found from the functional perspective.
Results according to causal diseaseOf the total patients included, 58% had TDG, 28% TMG and 14% ATN. Table 4 shows the activities of 131I and the treatment outcomes in each type of disease.
Outcomes according to disease conditions.
| TDG | TMG | ATN | Global | ||
|---|---|---|---|---|---|
| n (%) | Total• A• B1a• B2a | 123263760 | 591445− | 30822− | 2124810460 |
| Calculated activity, mean (median) | Total• A• B1a• B2a | 7.7 (6.3)7.5 (5)6.8 (5.9)8.4 (7.1) | 16.6 (14.2)22.5 (22.5)14.8 (11.3)− | 15 (14.4)16.2 (15)14.6 (11.3)− | 11.2 (9.1) |
| Administered activity, mean (median)b | Total• A• B1a• B2a | 8.2 (6.2)8 (5.7)7.3 (5.6)8.9 (7.6) | 16.2 (14)23.8 (23.6)13.8 (11.4)− | 14.7 (15)17.4 (15.9)13.7 (11.3)− | 11.4 (9.2) |
| Final outcome: success/failure (%)c | Total• A• B1a• B2a | 73/33 (69/31)15/10 (60/40)20/9 (69/31)38/14 (73/27) | 34/11 (76/24)10/3 (77/23)24/8 (75/25)− | 24/4 (86/14)6/1 (86/14)18/3 (86/14)− | 131/48 (73/27) |
| Functional outcome (%)c | • EU• HYPO SUB• HYPO• HYPER SUB• HYPER | 9 (8)4 (4)60 (57)17 (16)16 (15) | 20 (44)4 (9)10 (22)7 (16)4 (9) | 12 (43)9 (32)3 (11)2 (7)2 (7) | 41 (23)17 (9)73 (41)26 (15)22 (12) |
Final and functional outcome applies to the 179 patients with sufficient follow-up. TDG: diffuse toxic goiter; TMG: toxic multinodular goiter; EU: euthyroidism; HYPO SUB: subclinical hypothyroidism; HYPO: hypothyroidism; HYPER SUB: subclinical hyperthyroidism; HYPER: hyperthyroidism; ATN: toxic adenoma.
The mean administered activities were 8.2, 16.2 and 14.7mCi in TDG, TMG and ATN, respectively. The greatest differences between the administered activities according to method A or B corresponded to nodular goiters, particularly TMG (mean 23.8 versus 13.8mCi, method A versus method B).
The greatest success rate corresponded to the group of patients with ATN (86%), while failure was more common in TDG than in the other disorders (31% versus 24% in TMG and 14% in ATN).
A higher proportion of resulting hypothyroidism was likewise observed among the patients with GBD: 57% versus 8% “pure” euthyroidism (with no pharmacological requirements). Euthyroid status was more common in nodular goiters (44% in TMG and 43% in ATN). The disease with the lowest proportion of hypothyroidism requiring hormone replacement therapy was ATN (11%).
In TDG, the percentage of failures with method A reached 40%, while with method B the failure rate was 31% for a target absorbed dose of 150Gy (B1), and 27% for 200Gy (B2). In TMG, the success rate was very similar with both methods (77% and 75% for method A versus method B), and was even higher in the case of ATN (86% for both methods).
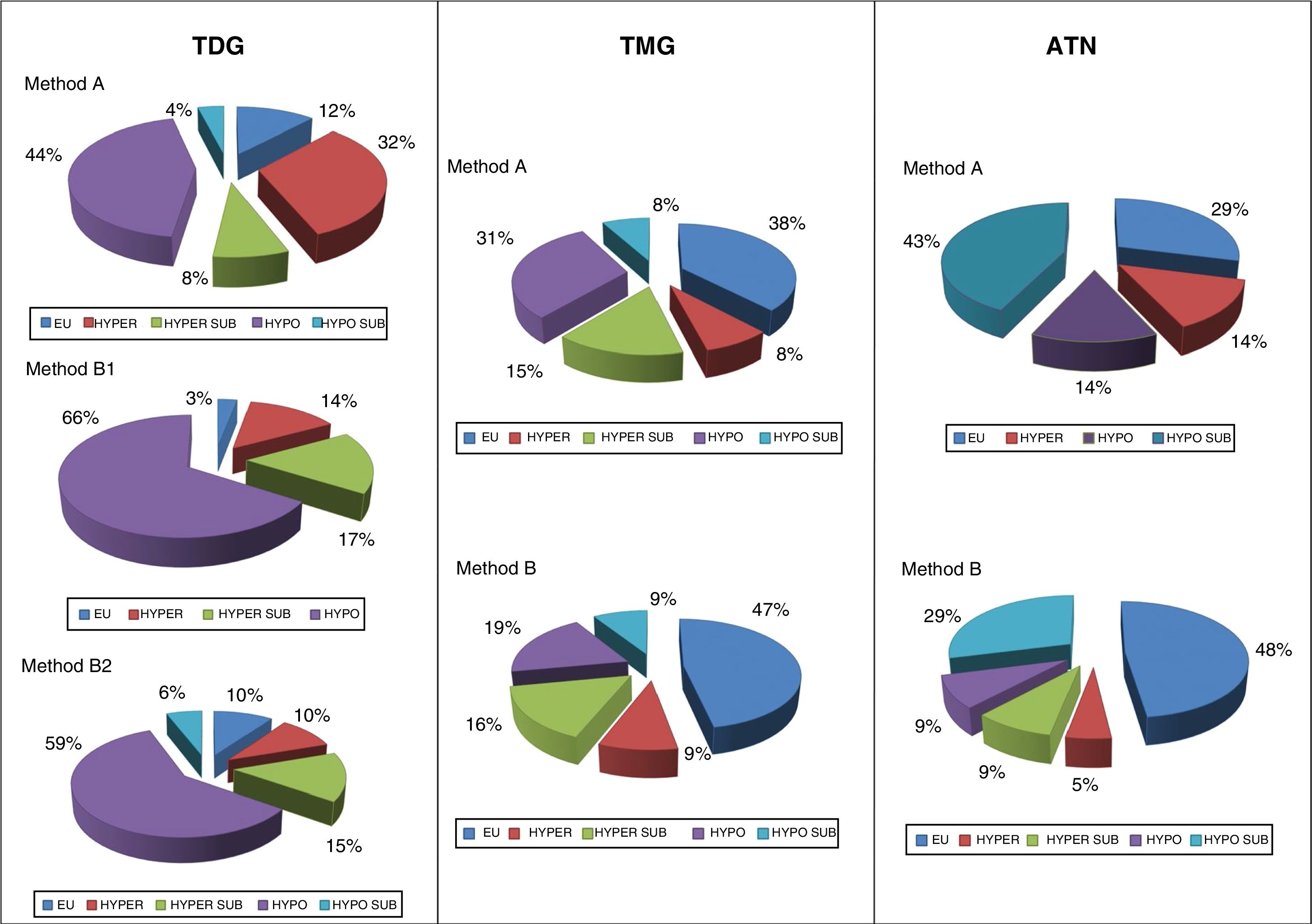
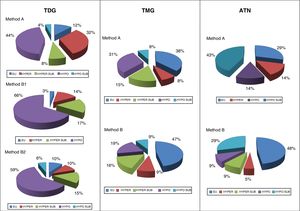
With regard to functional outcome, the greater percentage success rate with method B in TDG was seen to occur at the expense of a parallel increase in resulting hypothyroidism. However, on adjusting the target dose to 200Gy, this trend was slowed. By contrast, in nodular goiters, the hypothyroidism/euthyroidism balance was inverted, with a significant improvement of the functional outcomes using the calculated doses method, and an euthyroidism rate of close to 50% in both TMG and ATN being reached. Even in ATN (with an identical percentage of successes using both methods), a clear increase was seen in the final euthyroidism rate (48% versus 29%) (Fig. 1).
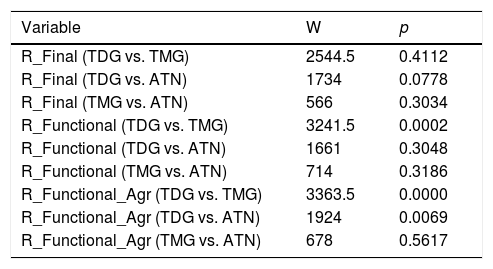
On comparing the outcomes according to the causal disease, the data failed to offer sufficient evidence to assume that success depended on the type of disease. In the case of TDG versus ATN, the p-value was very close to being rejected, but was found to be >0.05. On comparing the functional outcomes, the pooled variable (R_FXL_AGR) showed differences between TDG and the rest (Table 5).
W-M-W test for the comparison of means of R_Final, R_Functional and R_Functional_Agr divided by disease conditions.
| Variable | W | p |
|---|---|---|
| R_Final (TDG vs. TMG) | 2544.5 | 0.4112 |
| R_Final (TDG vs. ATN) | 1734 | 0.0778 |
| R_Final (TMG vs. ATN) | 566 | 0.3034 |
| R_Functional (TDG vs. TMG) | 3241.5 | 0.0002 |
| R_Functional (TDG vs. ATN) | 1661 | 0.3048 |
| R_Functional (TMG vs. ATN) | 714 | 0.3186 |
| R_Functional_Agr (TDG vs. TMG) | 3363.5 | 0.0000 |
| R_Functional_Agr (TDG vs. ATN) | 1924 | 0.0069 |
| R_Functional_Agr (TMG vs. ATN) | 678 | 0.5617 |
R_Final: final result (outcome) (success/failure); R_Functional: functional result (outcome) (euthyroidism/hypothyroidism/subclinical hypothyroidism/hyperthyroidism/subclinical hyperthyroidism). R_Functional_Agr: pooled (Agr) functional result (outcome) (subclinical cases are integrated into their corresponding category).
TDG: toxic diffuse goiter; TMG: toxic multinodular goiter; ATN: toxic adenoma.
Among the 179 treated patients with sufficient follow-up, the hyperthyroidism cure rate was 73%. On comparing the results obtained with the two methods, a trend favorable to the dosimetric method versus method A was observed (75% versus 69%) (Table 3), though the difference failed to reach statistical significance. Those success rates are within the limits to be expected from the literature. Bonnema and Hegedüs15 reported cure rates at one year ranging from 50% to 90%.
Bernard et al.16 published the results of a French national survey on nuclear medicine practices regarding radioiodine treatment for hyperthyroidism. Although these authors did not collect data on cure rates, they did mention that these vary considerably between studies and are dependent both on the definition of the outcome and on the knowledge and integration of several factors that influence the outcome, many of which are not taken into account in the dosimetric formulas. Another interesting finding concerns the most common therapeutic approach at that time in France, where fixed doses were most often used (in 60% of all cases of GBD and 72.5% of all nodular goiters).
In our study, approximately 50% of the patients developed hypothyroidism (Table 3), though no significant differences were observed between the two methods. Our results agree with those found in the literature, though hypothyroidism derived from 131I therapy is still subject to greater variability and its significance is the subject of debate. In this regard, the EANM guidelines9 state that “the main adverse effect of radioiodine treatment is hypothyroidism, and its incidence continues to grow over the course of follow-up”. The guidelines indicate that pre-treatment prediction is not possible using the current variables, even though the incidence is higher in GBD than in TMG, and low in ATN. In fact, with longer follow-up periods, permanent hypothyroidism appears to be inevitable in post-radioiodine therapy GBD.15
Bernard et al.16 recorded that 33% of the referring physicians considered euthyroidism to be a successful outcome, as compared to 26% whose objective was hypothyroidism. They also considered whether hypothyroidism was a complication or a negative outcome. In line with Shapiro,17 they concluded that it was a post-radioiodine therapy endpoint that could be easily detected and resolved.
A maximum cure rate is known to be invariably associated with an increased incidence of hypothyroidism.18 In the 2007 editorial discussion between Van Isselt et al.19 and Sisson et al.20 on fixed or calculated doses, one of the points of discrepancy was the therapeutic objective (euthyroidism or hypothyroidism). In this respect, Van Isselt did not advocate seeking hypothyroidism as an initial outcome. Both groups agreed that the use of calculated doses would make it possible to better comply with the ALARA principle.
Excellent results regarding the healing of hyperthyroidism with a single dose, as in the study published by Leow et al.,21 should not be achieved at the expense of increasing the radiation dose.
The activities administered based on calculated doses were significantly lower than those of the semi-fixed doses (Table 3). In other words, with non-significantly poorer cure and hypothyroidism rates (even with a trend toward a higher success rate), the activities administered, and thus patient radiation exposure, were lower with method B. These results therefore support the use of the dosimetric method, since it is more in line with the ALARA principle and European Union Directive 2013/59/EURATOM.8
In 2004, Jönsson and Mattsson22 conducted a comparative study of 187 patients with GBD treated with various protocols. The patients in whom no pre-treatment 131I uptake measurements were made were mostly seen to have received unnecessarily high activity (the average excess being 2.5-fold and, in some cases, up to 8-fold).
Two systematic reviews and meta-analyses are available in the literature, comparing estimated (fixed dose) versus calculated activity in the treatment of hyperthyroidism,23,24 the second study being limited to the context of toxic nodular goiter.24 De Rooij et al.23 analyzed 8 studies (3 randomized and 5 non-randomized trials), while Rokni et al.24 analyzed 7 studies (2 randomized and 5 non-randomized trials), with four of the selected studies being common to both. In both reviews and meta-analyses, the main limiting factor was the considerable heterogeneity: different formulas for calculating 131I activity, different ways of assigning estimated activities (fixed), different ways of determining thyroid volume (in some cases through palpation), different definitions of “low” and “high” doses, etc.
According to de Rooij et al.,23 both treatment methods proved equally successful. as in our study, while the meta-analysis published by Rokni et al.24 found the response rate to be higher with the calculated doses, with no relevant increase in hypothyroidism. The authors therefore concluded that such doses were preferable. Neither study found significant differences in the amount of 131I administered in the two groups.
The success rates were 69%, 76% and 86% for TDG, TMG and ATN, respectively (Table 4). It is well known that the outcomes vary according to the underlying causal condition, with a broader range of cure rates reported in TDG than in nodular diseases, and that the single-dose success rates are usually lower. In this regard, the treatment objective in GBD is determinant (a concept we took into account in method A). There are two conflicting concepts in this regard10: “function-oriented” treatment25 versus “ablative dose” therapy.26 However, from the start of radioiodine treatment for GBD, most patients were seen to become hypothyroid over the long term, in contrast to patients with toxic nodular goiters.27
The reported range of cure rates for hyperthyroidism due to GBD is broader than in nodular goiters (50–90% versus 75–95%), and permanent hypothyroidism is also more common in the former.15 Many factors that explain why the outcomes of radioiodine therapy in TDG are less predictable are inherent to the disease itself28: TDG is an autoimmune disorder in which hyperthyroidism is caused by antibodies acting against the thyroid follicular cells,29 and in which radioiodine turnover is usually accelerated (due to increased vascularization and increased metabolic activity of the thyroid follicular cells).30 By contrast, the lower frequency of hypothyroidism in TMG and ATN is due, at least in part, to reduced 131I uptake in the partially suppressed paranodular thyroid tissue.
As seen in Table 5, although there were no statistically significant differences in the final outcome (success/failure) between TDG and nodular goiters, there were statistically significant differences in the functional outcome: the incidence of hypothyroidism was significantly higher in TDG, in line with the data reported in the literature.
ConclusionsIn the treatment of hyperthyroidism with radioiodine, our proposed simple method for calculating activities was at least as successful as that previously used, based on a range of fixed activities. Although not significant, a favorable trend was observed, with a higher cure rate.
Similar observations apply to the functional outcome, where no significant differences were recorded, though a trend in favor of method B was observed, at least in nodular goiters (with a lesser hypothyroidism rate).
These results were achieved by administering significantly lower activities to the patients, thus resulting in lesser radiation exposure.
Overall, the results obtained did not demonstrate a significant dependence upon the underlying causal disorder. Nevertheless, both TMG and ATN showed a trend toward better final functional outcomes.
AuthorshipFrancisco José Pena-Pardo is the main author, responsible for study conception and design, data acquisition, analysis and interpretation, and the drafting of the manuscript. Mr. López-Serrano contributed to the statistical analysis of the data. Dr. García-Cases collaborated in the study design and in electronic data recording. Dr.s Redal-Peña and Crespo-Jara participated in data acquisition, and Dr.s García-Vicente and Martínez-Almagro Andreo participated in the critical review of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The present study was carried out at Hospital Quironsalud Torrevieja, and would not have been possible without the nurses of the Department of Nuclear Medicine (Mónica Berná-Campos, Encarnación Salinas-Sánchez, Pedro Jorge Contreras-Sánchez, Stefano Bonetti, M. Carmen Ortuño-Meseguer, M. Carmen Balboa-Almira, Nuria Armengol-Hernández, Noelia García-Amat and Santos Maciá-Berna) Thanks are due for their great professionalism and disinterested work in the collection of data.
Please cite this article as: Pena Pardo FJ, López Serrano R, García Cases FJ, Redal Peña MC, Crespo-Jara A, García Vicente AM, et al. Estudio prospectivo comparativo de dos métodos de cálculo individual de la actividad de 131I en el tratamiento del hipertiroidismo. Endocrinol Diabetes Nutr. 2020;67:568–577.