Treatment of type 2 diabetes mellitus (T2DM) is complex and is intended to decrease morbidity and mortality. Management should therefore include adequate diabetes education, lifestyle changes, drug treatment to achieve early blood glucose control and reduction of cardiovascular (CV) risk factors, early detection and treatment of complications, and assessment of associated comorbidities.
The objective was to prepare a document including all aspects required for a comprehensive approach to T2DM.
ParticipantsMembers of the Diabetes Mellitus Working Group of the Spanish Society of Endocrinology.
MethodsThe available evidence regarding each aspect of diabetes management (blood glucose control goals, diet and exercise, drug treatment, risk factor management and control, detection of complications, and management of frail patients) was reviewed. Recommendations were formulated based on the grades of evidence stated in the 2018 Standards of Medical Care in Diabetes. Recommendations were discussed and agreed by the working group members.
ConclusionsThis document is intended to provide evidence-based practical recommendations for comprehensive management of T2DM by clinical endocrinologists.
El tratamiento de la diabetes tipo 2 (DM2) es complejo y su propósito es reducir la morbimortalidad, por lo que su manejo tiene que incluir: un control glucémico individualizado precoz (mediante una adecuada educación diabetológica, modificaciones del estilo de vida y tratamiento farmacológico), el control de los factores de riesgo cardiovascular (CV), la detección y tratamiento precoz de las complicaciones y la evaluación de las comorbilidades asociadas. El objetivo fue elaborar un documento para unificar los aspectos necesarios para el abordaje integral de las personas con DM2.
ParticipantesMiembros del Grupo de trabajo de Diabetes Mellitus de la Sociedad Española de Endocrinología y Nutrición.
MétodosSe realizó una revisión de la evidencia disponible relativa a cada aspecto del manejo de la diabetes: objetivos de control glucémico, dieta y ejercicio, tratamiento farmacológico, tratamiento y control de factores de riesgo, detección de complicaciones y manejo del paciente frágil con DM2. Las recomendaciones se formularon según los grados de evidencia recogidos en los Standards of Medical Care in Diabetes 2018. Tras la formulación de las recomendaciones el documento fue consensuado por los miembros del Grupo de trabajo de Diabetes Mellitus de la Sociedad Española de Endocrinología y Nutrición.
ConclusionesEl objetivo de este documento es proporcionar, desde el punto de vista del endocrinólogo clínico, unas recomendaciones prácticas basadas en la evidencia acerca de todos los aspectos necesarios para el abordaje integral de la DM2.
The management of type 2 diabetes mellitus (DM2) is complex, and seeks to decrease patient morbidity and mortality. Early individualized blood glucose control without hypoglycemic episodes is required through adequate diabetes education, changes in lifestyle, and drug treatment. Moreover, global control of cardiovascular (CV) risk factors is required, with adequate detection and treatment of the complications of the disease, both the classical microvascular complications (retinopathy, nephropathy) and other important complications such as non-alcoholic fatty liver disease (NAFLD), heart failure or sleep apnea–hypopnea syndrome. This article adopts a different approach to those of previous guidelines, seeking to offer a practical document which focuses on somewhat more novel and sometimes controversial aspects regarding the management of type 2 diabetes. In addition, the availability of new therapies for diabetes allows for a better individualization of treatment based on the particular clinical characteristics of each patient. Within this context, the Diabetes Mellitus Working Group of the Spanish Society of Endocrinology and Nutrition (Sociedad Española de Endocrinología y Nutrición [SEEN]) decided to prepare this document with the aim of unifying the aspects needed for a comprehensive approach to DM2, addressed to all professionals involved in the treatment of the disease.
The purpose of this document is to offer practical, evidence-based recommendations on all aspects needed for the comprehensive management of DM2, from the viewpoint of the clinical endocrinologist.
Material and methodsA review was made of the available evidence regarding each aspect of diabetes care. The recommendations were made according to the levels of evidence contained in the Standards of Medical Care in Diabetes 20181 (Table 1). After the recommendations had been established, the final document was accepted by the members of the Diabetes Mellitus Working Group of the SEEN. The recommendations were posted on the SEEN website (www.seen.es) on 5 May 2018 and are freely accessible.
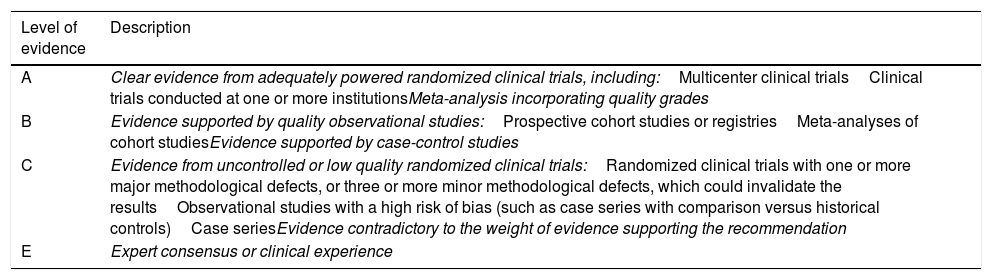
Levels of evidence of the recommendations.
| Level of evidence | Description |
|---|---|
| A | Clear evidence from adequately powered randomized clinical trials, including:Multicenter clinical trialsClinical trials conducted at one or more institutionsMeta-analysis incorporating quality grades |
| B | Evidence supported by quality observational studies:Prospective cohort studies or registriesMeta-analyses of cohort studiesEvidence supported by case-control studies |
| C | Evidence from uncontrolled or low quality randomized clinical trials:Randomized clinical trials with one or more major methodological defects, or three or more minor methodological defects, which could invalidate the resultsObservational studies with a high risk of bias (such as case series with comparison versus historical controls)Case seriesEvidence contradictory to the weight of evidence supporting the recommendation |
| E | Expert consensus or clinical experience |
Type 2 diabetes mellitus is associated with high early death and disability rates. Early glycemic control has been shown to reduce the complications, mortality and costs associated with the disease, as well as to increase patient quality of life.2 The parameter most commonly used to assess glycemic control in DM2 is glycosylated hemoglobin (HbA1c). The HbA1c target in DM2 has evolved in recent years. The ACCORD,3 ADVANCE4 and VADT trials5 examined whether intensive glycemic treatment, as compared to conventional treatment, affords CV benefits for patients with long-standing DM2 and a high percentage of cardiovascular complications. These studies found that intensive glycemic control afforded no benefits in terms of the development of cardiovascular events, and could increase mortality.2 By contrast, and although no significant effect of glycemic control upon cardiovascular complications was observed in the original study, the post-intervention analysis of the UKPDS trial6 showed that intensive blood glucose control reduced cardiovascular events and microvascular complications in patients with newly diagnosed DM2 and no associated complications. Overall, these conclusions underline the importance of strict glycemic control from the earliest stages of DM2, with a view to decreasing microvascular complications and morbidity and mortality of CV origin. However, they also suggest the need to be less aggressive with glycemic control in patients with more advanced stages of DM2.2,7
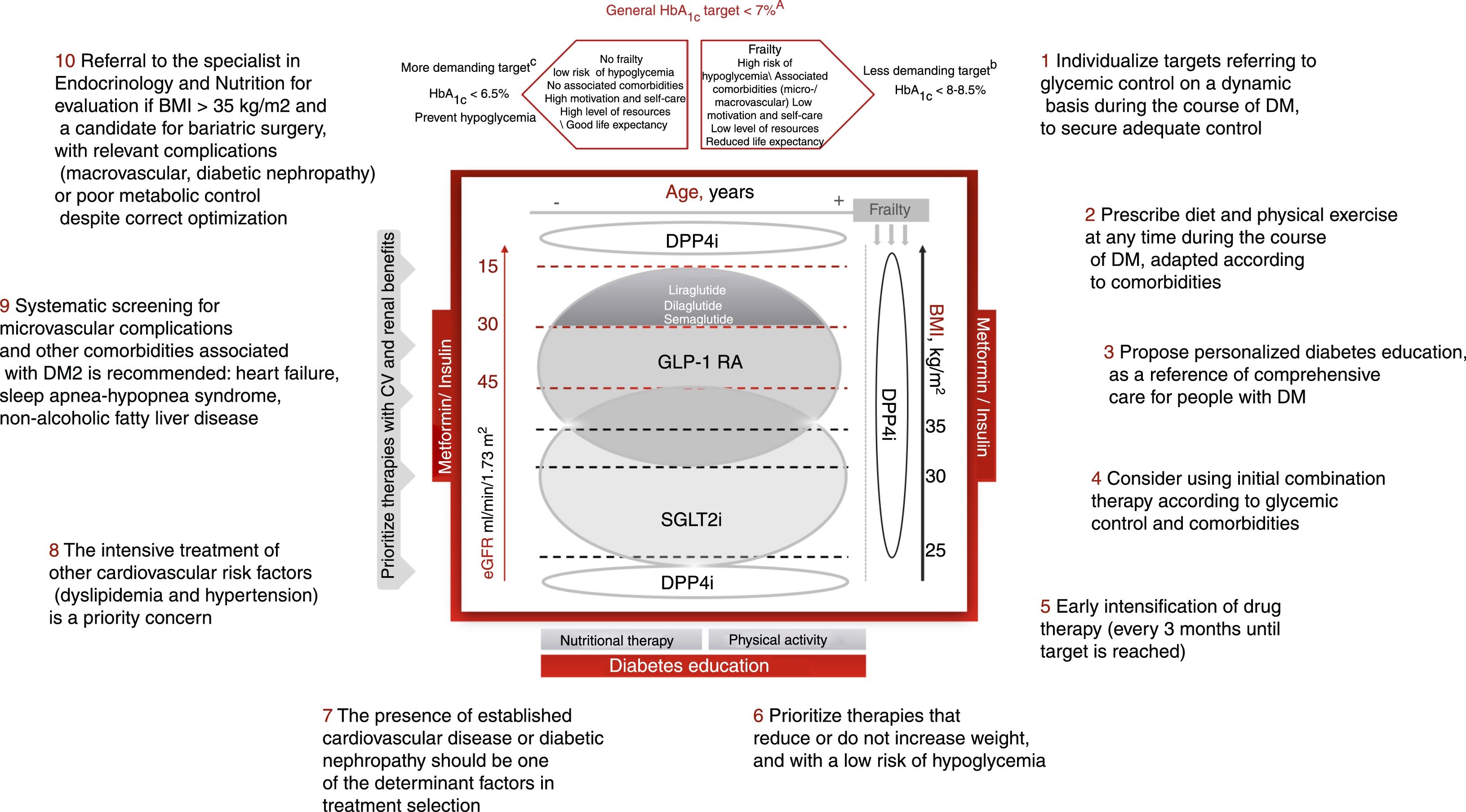
Individualization of the control targets in DM2, with their adaptation to the personal situation and characteristics and the preferences of each patient is essential (Fig. 1). The HbA1c target should be dynamic and be adapted to the changing clinical context of the patient. The assessment of HbA1c levels should be made together with the basal and postprandial blood glucose levels, and the presence of hypoglycemia. Likewise, we must be aware that the HbA1c target may change over time, and should be adapted to the situation of each patient. Although it is generally considered that the control target or goal in DM2 is to achieve HbA1c<7% (level of evidence A), a stricter target (HbA1c<6.5%) may prove adequate in younger patients with less advanced DM2, no associated complications, greater capacity for self-care and family support, provided the achieving of such HbA1c concentrations does not lead to hypoglycemia episodes and weight gain is avoided (level of evidence C). By contrast, less strict HbA1c levels (<8–8.5%) are advisable in frail elderly patients and/or those with a limited life expectancy, poor capacity for self-care, and poor family support (level of evidence B).2,7 In patients with diabetes and established CV disease, the HbA1c target should be 7–7.5%.2,7 Lastly, other control targets, in addition to HbA1c, are basal capillary blood glucose values in the range of 80–130mg/dl and blood glucose <180mg/dl 2h after food intake.
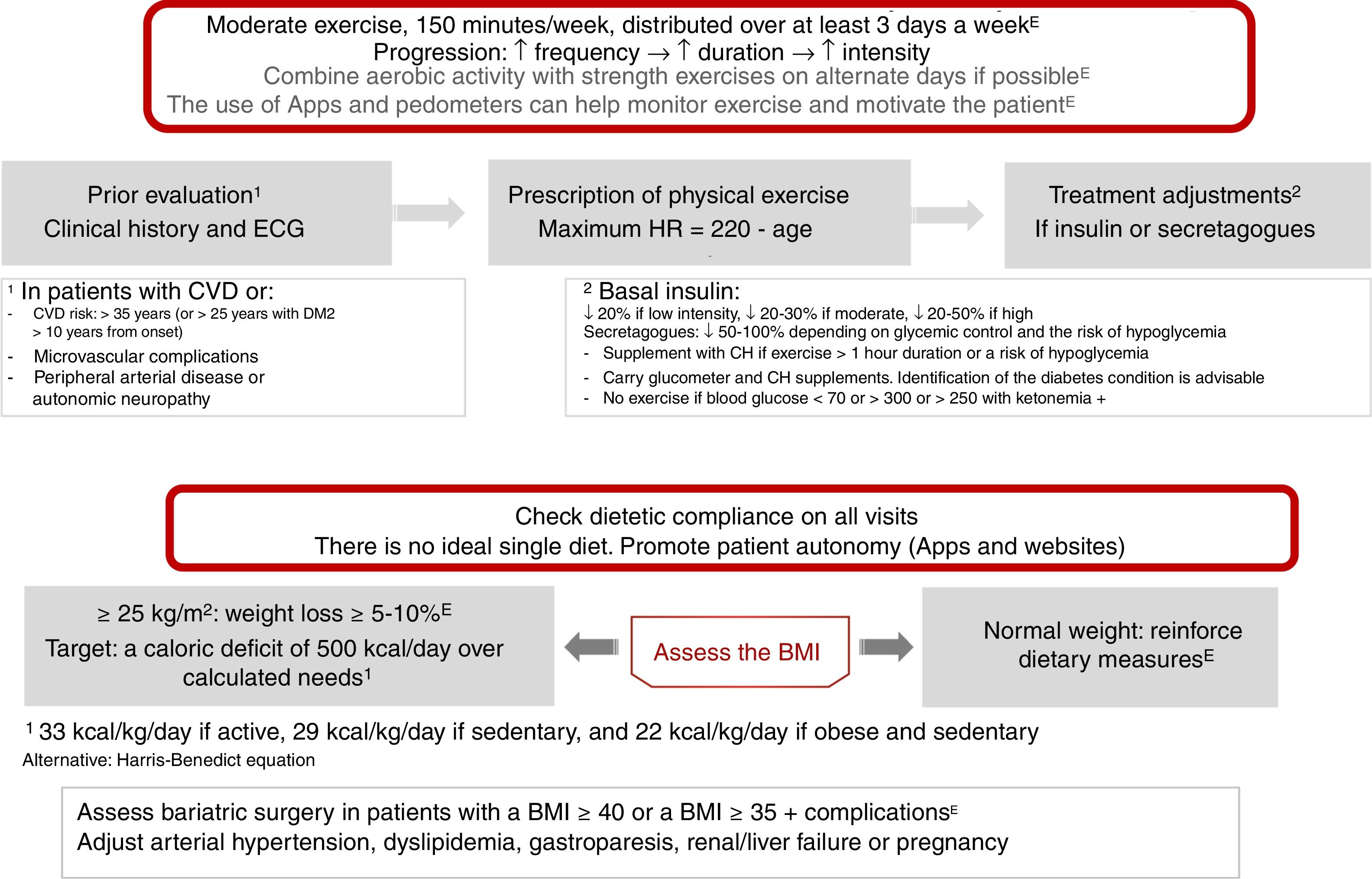
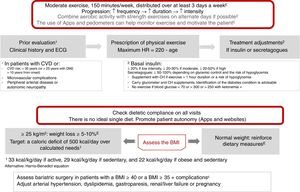
Physical exercise prescription and recommendationsPhysical exercise improves glycemic control, reduces insulin resistance, decreases CV risk, contributes to weight loss, and improves patient well-being and quality of life. The effects differ according to the type of exercise involved. Aerobic exercise fundamentally improves insulin sensitivity, in addition to other parameters of the CV system and lung capacity.8 Resistance exercises are beneficial for increasing muscle mass, strength, bone mineral density, insulin sensitivity, blood pressure and the lipid profile. Flexibility and balance exercises in turn help reduce falls and improve mobility, and can be performed even if neuropathy is present. In addition to recommending physical exercise, i.e., planned physical activity, increased physical activity in the context of daily activities is also advisable9 (level of evidence E for all recommendations in this section) (Fig. 2).
Before prescribing exercise, a complete clinical history focusing on symptoms suggesting cardiovascular complications, and an electrocardiographic (ECG) tracing, should be performed. In addition, when moderate-high intensity physical exercise is going to be performed, an exercise test is recommended if the patient has (or is suspected to have) CV disease or presents microvascular complications. Patients at risk of CV disease are defined as those over 35 years of age, or over 25 years with diabetes starting more than 10 years before, with other CV risk factors, microvascular complications, peripheral vascular disease, or autonomic neuropathy (level of evidence E).9,10
As a general recommendation, people with DM2 should perform at least 150min a week of moderate-vigorous aerobic activity, distributed over at least three days, with no more than two consecutive days without physical activity. For younger or fitter people, high-intensity training for at least 75min a week may be sufficient. It is advisable to include 2–3 sessions a week of resistance exercise on non-consecutive days.7,9,11 In addition, patients should be encouraged to reduce sedentary periods because the interruption of sedentary activity every 30min has been shown to improve glycemic control. In the case of elderly patients, flexibility and balance exercises are recommended 2–3 days a week. Physical exercise should be optimized in this order, its frequency, duration and intensity being gradually increased, and it is advisable to reach 60–70% of maximum heart rate (calculated as 220-age) (Fig. 2). Wearable physical activity monitoring devices may help with exercise monitoring, as well as contributing to self-motivation (level of evidence E).7,12
Regarding the treatment of patients with insulin or secretagogues (sulfonylureas and repaglinide), if exercise lasts for more than 1h, carbohydrate supplementation is advised. Exercise is not recommended in the following situations: blood glucose<70mg/dl, >300mg/dl, or >250mg/dl with positive ketone bodies. In these situations, physical activity may be performed following the adequate resolution of hypoglycemia. If the patient is on basal insulin, dose adjustment is advised based on exercise intensity (provided such exercise is performed regularly), with reductions of 20%, 20–30% and 20–50% for low, moderate and high intensity exercise, respectively. In the case of patients receiving secretagogues, it is also advisable to adjust the dose if regular exercise is performed: a 50–100% reduction in the presence of a high risk of hypoglycemia or with low HbA1c, a 25–50% reduction if control is adequate, and no change in dosage if control is off target (level of evidence E).8
In patients with chronic complications associated with diabetes, the individualization of physical activity is advised, as indicated by the algorithm table available on the SEEN website.8,12
Nutritional therapyNutritional intervention in DM2 may decrease HbA1c by 0.5–2%. In patients with normal body weight, the objective is to maintain an adequate weight.7,12 In patients with a body mass index (BMI)≥25kg/m2, a loss of at least 5–10% of body weight is indicated (Fig. 2). In order to achieve this, a calorie deficit of 500kcal a day with respect to the calculated daily requirements is needed: 33kcal/kg/day in active subjects, 29kcal/kg/day in sedentary subjects, and 22kcal/kg/day in obese and sedentary subjects. Alternatively, predictive equations such as the Harris-Benedict formula may be used.
There is no single ideal recommendation regarding the percentage distribution of macronutrients. Fresh and seasonal foods should be prioritized. Specialist support from a dietician-nutritionist or a nurse trained in nutrition has been shown to be cost-effective. In addition, it is necessary to promote patient autonomy, and digital resources such as Apps or websites may be useful for counseling and dietary monitoring. Some specific recommendations (level of evidence E)7,12 are provided below:
Carbohydrates: Low glycemic index foods, such as vegetables, fruits, whole grains and legumes, are recommended while high glycemic index foods and processed foods high in sugars should be avoided.
Fats: Recommended fats include extra virgin olive oil, avocado and oily fish. Saturated fats should be limited to <7%, trans fats to <1%, and cholesterol to <300mg/day. Oily fish (rich in eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]), nuts and seeds are recommended for the prevention and treatment of CV disease. There is no evidence regarding the CV benefit of omega-3 drug supplements.13
Proteins: The consumption of plant protein should be encouraged (legumes, soy, quinoa, etc.). Animal proteins should preferably come from fish, shellfish, eggs and skimmed dairy products. Consuming one egg a day in diabetes has been shown to be safe, and up to 12 eggs a week do not modify the lipid profile or glycemic control.14 Lean meats should be prioritized. Only occasional consumption of red meat and processed meat is indicated. Dietary protein intake should only be lowered in cases of moderate or severe renal failure, and it should not be reduced in situations of renal replacement therapy.
High fiber intake improves glycemic control and the lipid profile. Such intake can be provided by 5 daily servings of whole fruit and vegetables. Juices, especially commercially produced fruit juices, should be avoided.
People with diabetes do not have increased micronutrient requirements and should not undergo routine supplementing unless there is a documented deficiency. Alcohol consumption is not advisable, due to the increased risk of hypoglycemia and the increased calorie intake. If alcohol is consumed, it should be limited to less than 15g in women (one standard drink unit) and to less than 30g in men (two standard drink units).
The consumption of salt should be reduced (<2300mg sodium daily, equivalent to 3g of salt a day), and should be further lowered in the presence of high blood pressure. Using non-caloric sweeteners instead of sugar may help reduce the total calorie content of the diet. In general, such sweeteners have been shown to be safe, and no concrete sweetener has been shown to be superior in diabetes.
Metabolic surgery should be considered in patients with DM2 and a BMI≥40kg/m2, or a BMI≥35kg/m2 with associated complications. Metabolic surgery involves the use of surgical procedures aimed at treating DM2 and the improvable cardiometabolic risk factors. It is mainly indicated for patients with obesity (BMI≥35kg/m2), particularly if DM2 or its comorbidities prove difficult to control with changes in lifestyle and drug treatment.15
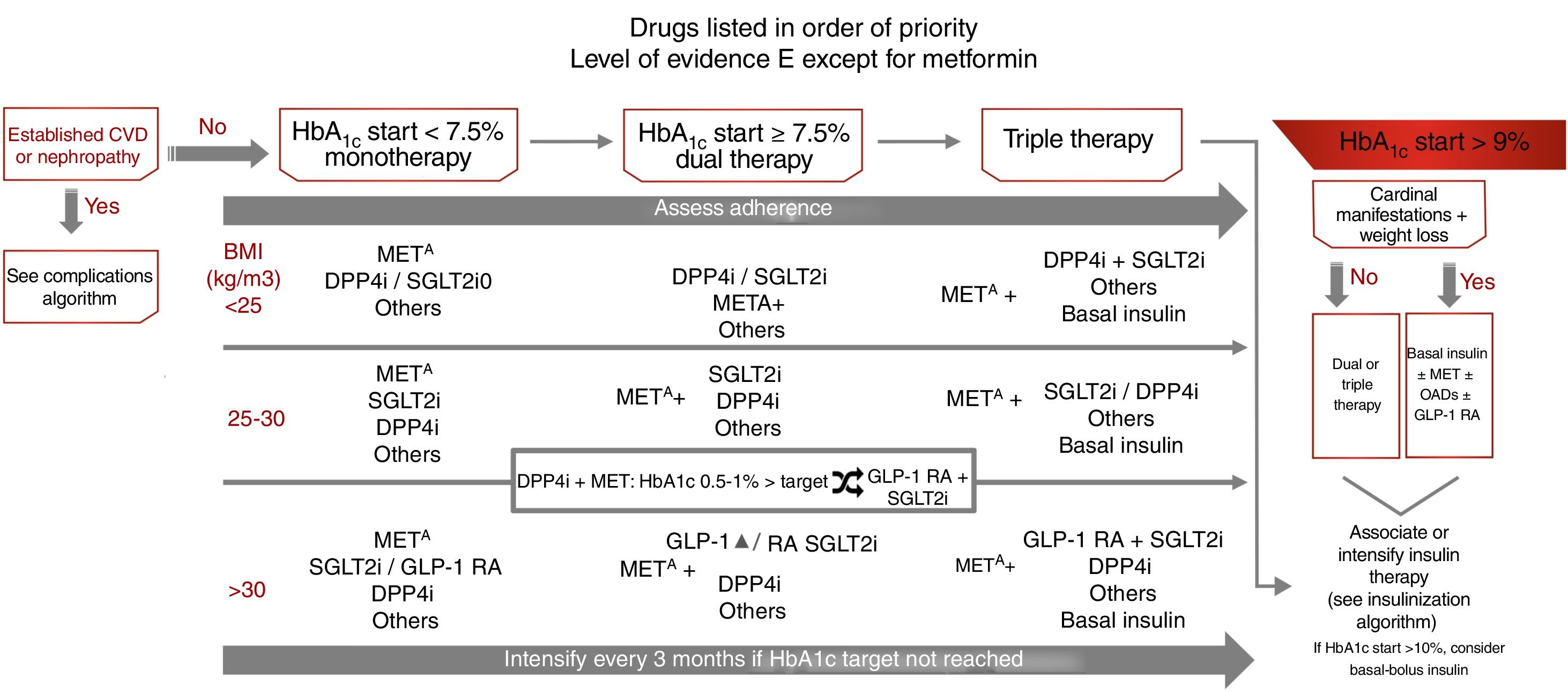
Treatment of type 2 diabetes focusing on glycemic controlThe treatment recommendations in this case are based on the degree of initial glycemic control: monotherapy if initial HbA1c<7.5%, dual therapy if initial HbA1c 7.5–9%, and triple therapy if initial HbA1c>9%.7 In this latter situation, in the presence of cardinal clinical signs, the recommendation is to use basal insulin associated with other antidiabetic therapies, and with initial HbA1c>10%, we may consider administering basal-bolus insulin as initial therapy. However, this regimen should subsequently be re-evaluated, with the possibility of switching to simpler treatment being considered (level of evidence E) (Fig. 3). As with other guides, the target for glycemic control which defines the limit for selecting dual or triple therapies has been arbitrarily established, and should be individualized.
Treatment algorithm focusing on glycemic control. Other drugs: SU, GLIN, PIO. Avoid their use if there is a risk of hypoglycemia (SU, repaglinide) or heart failure or fractures (PIO). CVD: cardiovascular disease; GLIN: repaglinide; MET: metformin; PIO: pioglitazone; SU: sulfonylureas. ΔIn favor of GLP-1 RA: difficult control of intake, BMI>35kg/m2, distance to HbA1c target>1%, ↑ risk fractures, recurrent genital infections. o If arterial hypertension or need to avoid weight gain. SGLT2i: dapagliflozin, empagliflozin, canagliflozin, ertugliflozin (approved by the European Medicines Agency).
Body weight is an important determinant in treatment selection. Weight control improves microangiopathic (kidney disease, polyneuropathy) and macroangiopathic alterations (abdominal adiposity is an independent CV risk factor [CVRF]), and helps control other CVRFs and comorbidities.7 If the BMI is≥25kg/m2, the preferred second-line therapies after metformin are those that reduce body weight: sodium-glucose cotransporter 2 inhibitors (SGLT2i) and GLP1 receptor agonists (GLP-1 RAs), though it should be taken into account that GLP-1 RAs only receive public funding in Spain in the case of a BMI≥30kg/m2. In patients with a BMI<25kg/m2, the advised second-line therapies after metformin are DPP4 inhibitors (DPP4i) and SGLT2i, with the same level of recommendation, but with SGLT2i being prioritized in the presence of high blood pressure or a need to avoid weight gain (level of evidence E).
After initiation or modification of the treatment regimen, glycemic control should be assessed at three months, and therapy should be further intensified if the target for glycemic control has not been reached. Therapeutic adherence should be assessed regularly throughout treatment and before any further intensification is introduced (level of evidence E) (Fig. 3). In patients treated with metformin+DPP4i in which HbA1c is 0.5–1% above the target concentration, it is advisable to add SGLT2i or replace DPP4i with GLP-1 RAs.
The different antidiabetic therapies are indicated in order of priority, emphasis being placed on those with a low risk of hypoglycemia (metformin [level of evidence A], DPP4i, SGLT2i and GLP-1 RAs [level of evidence E]) and with a positive effect on body weight and blood pressure (SGLT2i and GLP-1 RAs). Other therapies that increase the risk of hypoglycemia (sulfonylureas or repaglinide), or which have a less favorable adverse effects profile, such as pioglitazone (fluid retention, heart failure or fractures) are recommended as second-line options, but may be used in the case of intolerance or contraindication to first-line therapies7,16 (Fig. 3).
Hypoglycemia is associated with considerable morbidity and mortality. It is a possible risk factor for neurocognitive impairment, falls and related injuries, and impairs patient quality of life. Hypoglycemia is a major limiting factor for optimizing glycemic control and treatment adherence. The avoidance of hypoglycemic episodes is cost-effective, and the use of drugs with a low risk of hypoglycemia is very important.17
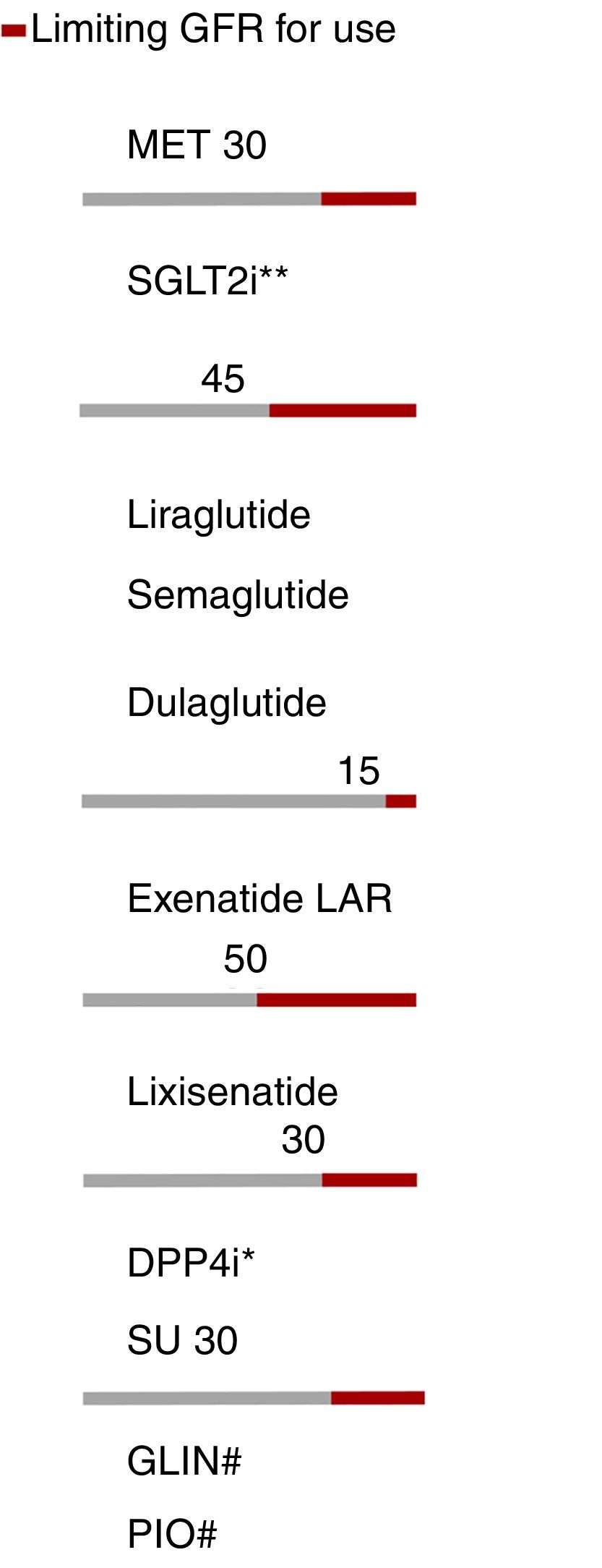
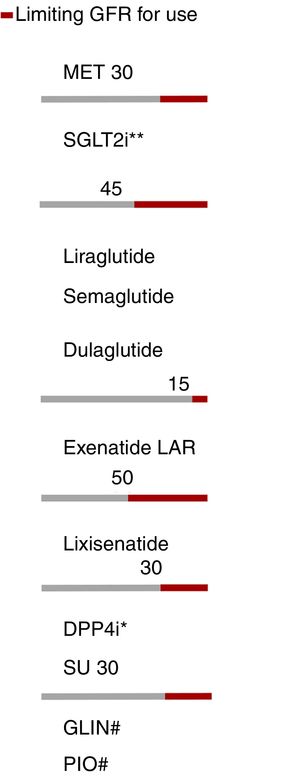
The presence of renal failure determines or limits the use of most antidiabetic therapies, except insulin. The treatment algorithm contemplates the limitations of each therapeutic group according to the glomerular filtration rate (GFR) (Fig. 4).
Adjustment of antidiabetic therapies according to renal function. The red line marks the limiting glomerular filtration rate (GFR) for the use of a given drug. eGFR: Estimated glomerular filtration rate. *Dose adjustment in chronic kidney disease (CKD), except linagliptin. **Dapagliflozin eGFR>60. #No dose adjustment required in CKD. In advanced kidney disease, monitor the repaglinide dose due to the risk of hypoglycemia.
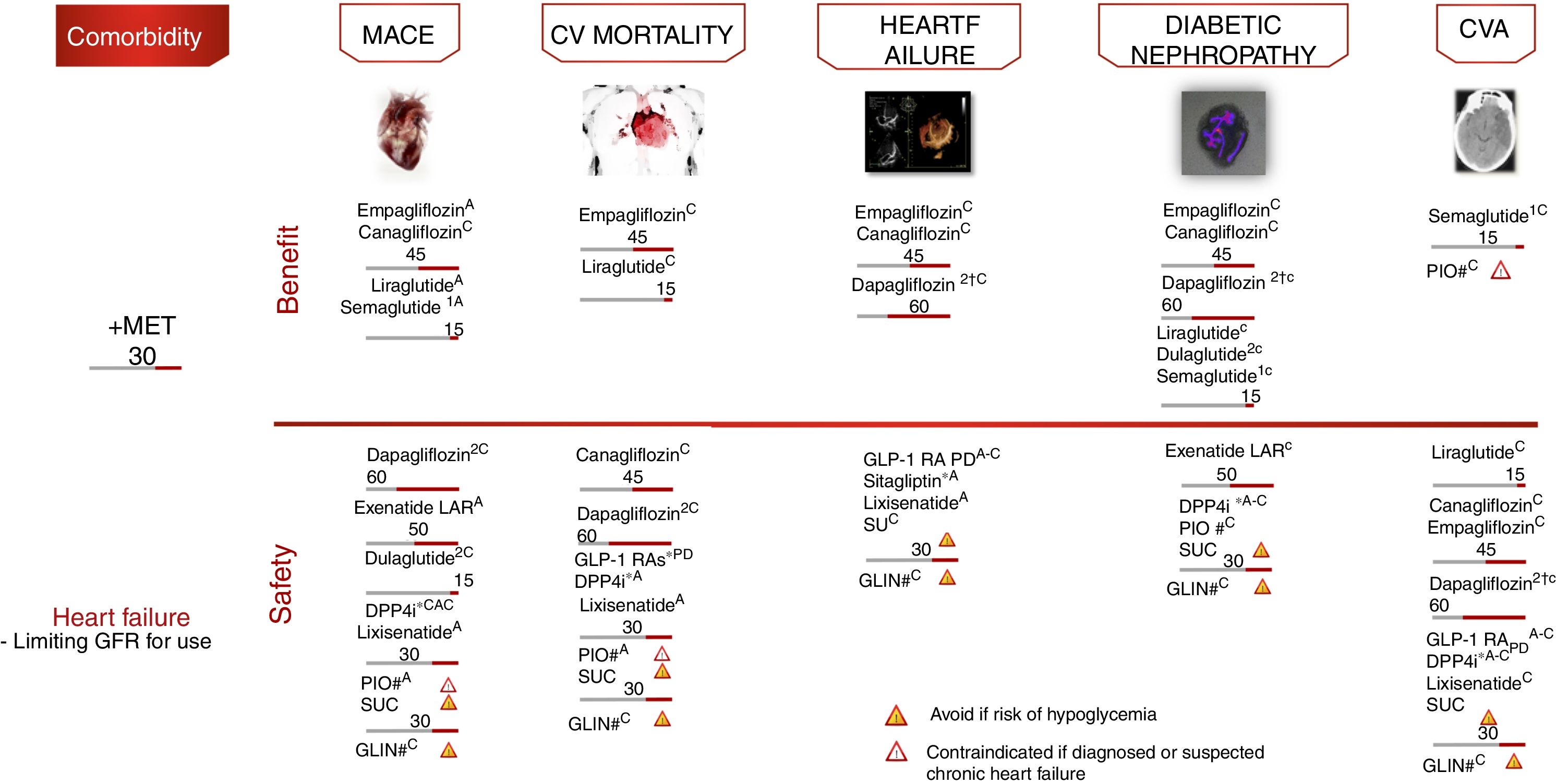
Atherosclerotic CV disease (CVD), defined as coronary artery disease, cerebrovascular disease or peripheral arterial disease, is the leading cause of morbidity and mortality in diabetic individuals, and is the main contributor to the direct and indirect costs of diabetes.16 For patients with DM2 and CVD or diabetic kidney disease, it is advisable that an agent known to reduce CV events or the progression of diabetic kidney disease be included, the characteristics of each individual patient being taken into account (level of evidence A).
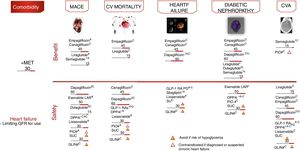
Cardiovascular safety clinical trialsFour large randomized controlled trials have demonstrated statistically significant and clinically relevant reductions in CV events among patients with established CVD or at high CV risk: EMPAREG-OUTCOME,18 with empagliflozin (level of evidence A); CANVAS,19 with canagliflozin (level of evidence C); LEADER,20 with liraglutide (level of evidence A); and SUSTAIN-6,21 with semaglutide (level of evidence A). In all the aforementioned studies, the evaluated therapy demonstrated non-inferiority in terms of major adverse CV events (MACE) reduction, and also superiority, except in the SUSTAIN-6 study, which was only designed to assess non-inferiority.
The empagliflozin and liraglutide trials demonstrated significant reductions in CV mortality (level of evidence A) (Fig. 5). By contrast, other GLP-1 RAs have shown no similar reductions in CV events.22,23 These studies examined the drugs in combination with metformin, which defines the latter as the drug of choice in combination.
Treatment algorithm focusing on comorbidities. Intensification. If no individualized target is reached in 3 months, add triple therapy with a drug exhibiting a complementary mechanism of action and CV benefit/safety. Assess the use of basal insulin therapy (see algorithm). CVA: cerebrovascular accident; CV: cardiovascular; PD: prolonged duration; GFR: glomerular filtration rate; GLIN: repaglinide; MACE: major CV events; IOP: pioglitazone; SU: sulfonylurea. *Dose adjustment in renal failure, except linagliptin. # No dose adjustment required in CKD. † Evidence based on observational studies. (1) Approved by the European Medicines Agency. (2) Dulaglutide, dapagliflozin, and linagliptin have not completed a CV safety trial. Vildagliptin has no CV safety trial.
Other contributions to the evidence of these trials have been derived from secondary objectives or post hoc analyses (level of evidence C).24–27 In these analyses, empagliflozin and canagliflozin were shown to reduce hospital admissions due to heart failure, and empagliflozin, canagliflozin, liraglutide and semaglutide improved the renal targets, defined as the progression of albuminuria, the progression to end-stage kidney disease and/or the doubling of serum creatinine levels. In other CV safety trials, different antidiabetic treatments such as sitagliptin,28 alogliptin,29 saxagliptin,30 lixisenatide23 and exenatide-LAR22 demonstrated a neutral effect upon CV events, thus supporting the use of these drugs when there is a need to improve glycemic control, with the aim of limiting microvascular complications without increasing CV risk. However, differences were observed among the different DPP4i in terms of heart failure risk, with an increased risk in the SAVOR trial with saxagliptin,30 and also with alogliptin in a post hoc analysis of the EXAMINE trial.29 By contrast, sitagliptin did not increase the risk of heart failure in the TECOS study.28
With regard to the insulins, glargine has also demonstrated its CV safety in patients with prediabetes and DM2.31 In subjects with established CVD and/or high CV risk, insulin degludec has confirmed its cardiovascular safety compared with insulin glargine.32
Vildagliptin has not been assessed in CV safety trials to date, while the CV clinical trials on dulaglutide (REWIND), dapagliflozin (DECLARE), ertugliflozin (VERTIS) and linagliptin (CARMELINA, CAROLINA) are still ongoing.
Real-life studiesIn addition to CV safety trials, “real-world” data analysis is an emerging area in diabetes, as a means of complementing the data obtained from CV safety trials. A number of observational studies have confirmed the positive effects of SGLT2i in everyday life (CVD-real, EASEL),33–35 this being in concordance with the findings regarding CV morbidity–mortality reduction in the CV safety trials, though most of the patients in these studies did not have established CVD.
Fig. 5 presents the additional recommendations referring to therapy in adults with DM2 and CV or renal comorbidity.
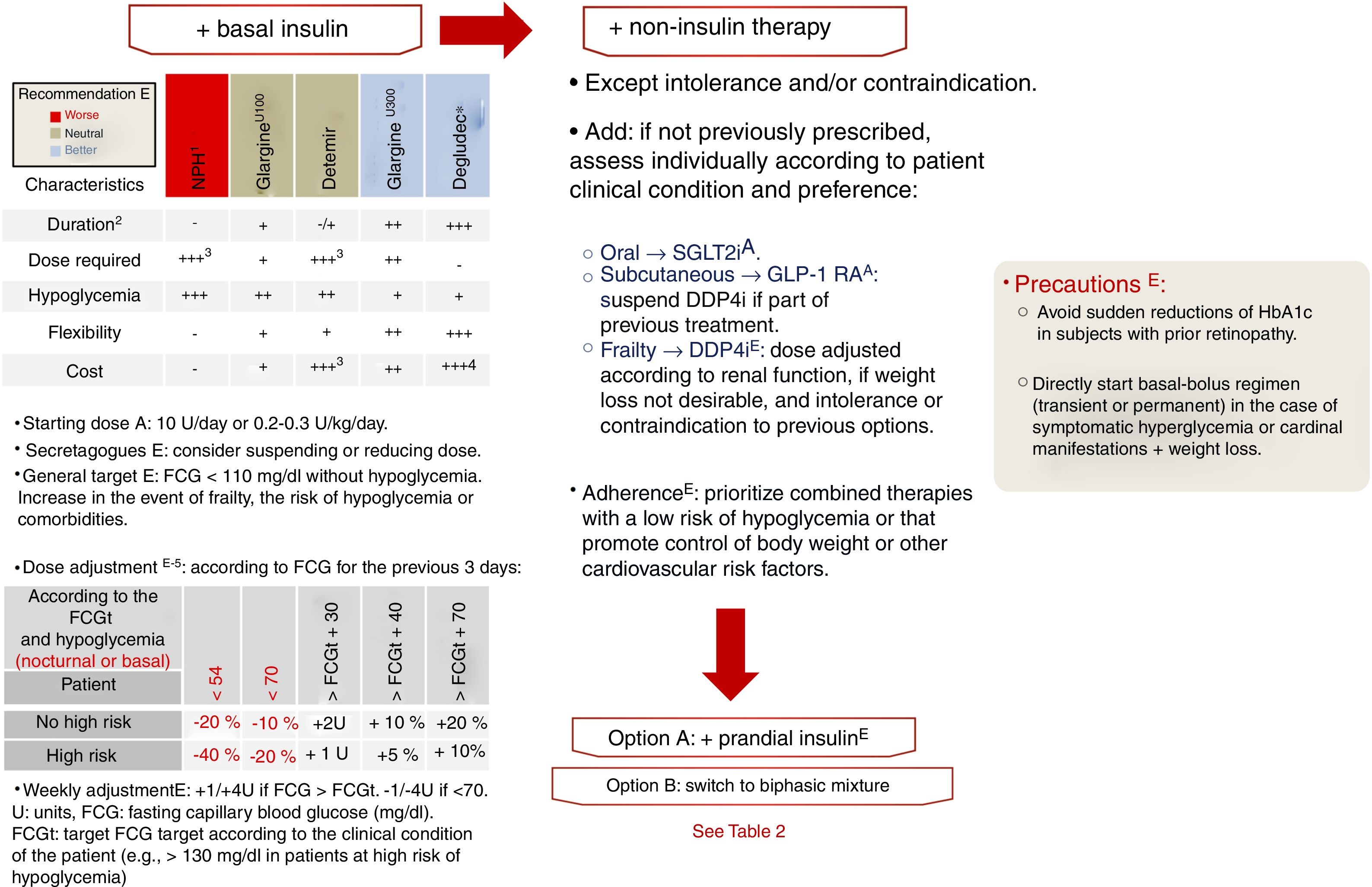
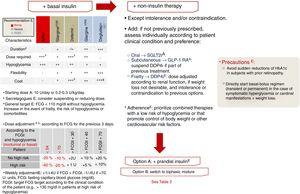
Insulinization algorithmIn DM2, insulin production gradually decreases. Consequently, if the course of the disease is long enough, insulin will eventually be required as replacement therapy. On the other hand, if the patient presents symptomatic hyperglycemia or a hypercatabolic state, even at the time of diagnosis, insulin treatment will be needed, either temporarily in the event of reversible decompensation episodes, or permanently.7 The global body of evidence points to the starting of replacement therapy with basal insulin as the most reasonable option, with assessment on a case-by-case basis as to whether the prior antidiabetic treatment should be maintained (in whole or in part),16 as explained in relation to non-insulin therapies in the previous sections. We currently have 5 types of insulin with which to perform this first step. The main differences among them are their duration of action, which conditions the number of daily administrations, and the variability of their effect. Both of these aspects condition the number of daily administrations required and the risk of hypoglycemia with each option (level of evidence E). Accordingly, NPH insulin has a half-life shorter than the time required to maintain adequate levels over the following 24h, plus a high variability of effect, leading to an increased risk of hypoglycemia.36 More than one daily administration of NPH insulin is usually required to achieve the required degree of metabolic control. In this document we classify the remaining options (the basal insulin analogs) into first-generation analogs (AB1, insulin glargine U100 and insulin detemir) and second-generation analogs (AB2, insulin glargine U300 and insulin degludec). As compared to NPH insulin, AB1 showed a longer duration of action, less variability, and a lower risk of hypoglycemia. Studies subsequently showed AB2 to be superior to AB1 in relation to all three of these parameters.37–43 Compared with glargine U100, insulin degludec showed a decreased risk of hypoglycemia in a clinical trial designed with hypoglycemic episodes as the primary objective,44 and of severe hypoglycemia in another clinical trial conducted in patients at high CV risk.32 These and the other differential characteristics of the basal insulins are reported in Fig. 6. Only one clinical trial comparing the AB2 drugs has been published to date.45 In the BRIGHT study,45 the primary objective was to assess non-inferiority in relation to changes in HbA1c after treatment with basal insulin (glargine U300 versus degludec) in patients with DM2 over a 24 week period. As in previous studies involving other basal insulins, no differences in HbA1c were observed at the end of the study period. Likewise, no differences were seen in the incidence of hypoglycemia, although the latter was not assessed as a primary or secondary objective or endpoint, but was recorded as an adverse effect.
Insulinization algorithm. If the HbA1c target has not been reached in 3 months, after evaluating adherence and dose adjustments. FCG: fasting capillary blood glucose. 1: An intermediate acting insulin is considered due to the frequent need to administer more than one daily dose; 2: the longer the duration, the longer the period required to achieve the expected effect; 3: a higher dose will be required if administered more than once daily; 4: prescription reimbursement limited to visa conditions; 5: individualize the basal insulin titration method (e.g., mean or lowest of the 3 previous FCG values).
The recommended starting dose of basal insulin is 10units/day or 0.2–0.3units/kg/day (level of evidence A). Subsequently, this starting dose has to be titrated to reach the level required by each individual patient, based on fasting capillary blood glucose (FCBG), until the established target FCBG is reached for each subject (level of evidence E).7,16 Studies have validated dose adjustment based on the mean or on the lowest of the three previous FCBG values or, preferentially with AB2, on the weekly FCBG value.46,47 If, following a minimum period of three months of basal insulin therapy, the dose required to secure the target FCBG is reached, with HbA1c values remaining above the established target for the patient, we should consider the next step in treatment intensification. This is done in the same way as if the latter target had been achieved but the patient presented new hyperglycemic decompensation in subsequent laboratory tests.7,16
Before starting an advanced regimen including rapid insulin, the treatment regimen may be intensified with non-insulin options if these have not been previously prescribed and pose no problems of intolerance and/or contraindications. In this regard, priority should focus on therapies that facilitate adherence (e.g., combined therapies), with a low risk of hypoglycemia, and favoring the control of body weight or other CV risk factors (Table 2).7,16 If the patient continues to show inadequate glycemic control despite these therapeutic measures, we should consider the possibility of adding rapid insulin or switching the previous treatment regimen to biphasic mixtures, depending on the characteristics of the patient (level of evidence E).
Algorithm on insulinization, intensification with multiple insulin doses.
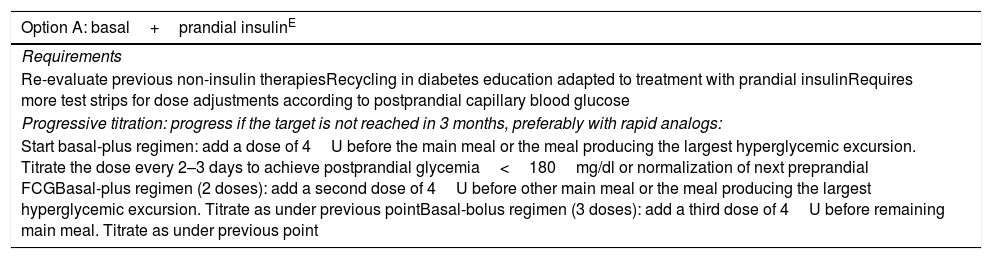
| Option A: basal+prandial insulinE |
|---|
| Requirements |
| Re-evaluate previous non-insulin therapiesRecycling in diabetes education adapted to treatment with prandial insulinRequires more test strips for dose adjustments according to postprandial capillary blood glucose |
| Progressive titration: progress if the target is not reached in 3 months, preferably with rapid analogs: |
| Start basal-plus regimen: add a dose of 4U before the main meal or the meal producing the largest hyperglycemic excursion. Titrate the dose every 2–3 days to achieve postprandial glycemia<180mg/dl or normalization of next preprandial FCGBasal-plus regimen (2 doses): add a second dose of 4U before other main meal or the meal producing the largest hyperglycemic excursion. Titrate as under previous pointBasal-bolus regimen (3 doses): add a third dose of 4U before remaining main meal. Titrate as under previous point |
| Option B: switch to biphasic mixtureE |
|---|
| Indication |
| In patients at low risk of hypoglycemia, lifestyle measures plus relatively stable schedules and a limited ability to apply the necessary adjustments to insulin regimens (basal and/or bolus) |
| Progressive adjustment: titrate dose every 2–3 days to achieve postprandial glycemia<180mg/dl or normalization of next preprandial FCG. |
| The risk of hypoglycemia is greater with these regimens. Level of evidence AStarting dose: 0.2–0.3U/kg/day divided into 2 (2/3 with breakfast plus 1/3 with dinner) or 3 doses (1/2 with breakfast, 1/4 with lunch, 1/4 with dinner) |
Superscript text indicates the level of evidence.
FCG: fasting capillary blood glucose (mg/dl); U: units.
Different control targets are distinguished according to the patient risk level7:
- 1.
High CV risk (most patients with DM2, excluding those <40 years of age with no other CVRF or target organ damage due to their lesser risk, and those included under points 2 and 3 due to their increased risk): keep LDLc<100mg/dl, non-HDLc<130mg/dl, triglycerides<150mg/dl and ApoB<90mg/dl (level of evidence A).
- 2.
Very high CV risk (DM2 with other major CVRF such as smoking or arterial hypertension, target organ damage or stage 4–5 chronic kidney disease): keep LDLc<70mg/dl, non-HDLc<100mg/dl, triglycerides<150mg/dl and ApoB<80mg/dl (level of evidence A).
- 3.
Extreme CV risk (DM2 with established atherosclerotic CV disease, cerebrovascular disease or peripheral vascular disease): keep LDLc<55mg/dl, non-HDLc<80mg/dl, triglycerides<150mg/dl and ApoB<70mg/dl (level of evidence A).
Hygiene-dietary measures are essential in the treatment strategy and comprise a decrease in the intake of saturated and trans fats and cholesterol, with an increased intake of omega-3 fatty acids and dietary fiber. The consumption of plant sterols should be assessed, weight loss should be sought in patients that are overweight or obese, and physical activity should be gradually increased (level of evidence A).
The addition of pharmacological treatment should be considered in patients who fail to reach the control targets after three months of hygiene-dietary measures. When the LDLc targets are not reached, the following should be added on a stepwise basis (level of evidence A)48:
- -
Low, moderate or high-potency statins (Table 3), according to whether the required lowering of LDLc is <30%, 30–50% or >50%, respectively.
Table 3.Statins classified according to potency (daily dose).
Low potency Moderate potency High potency Simvastatin 10mgPravastatin 10–20mgLovastatin 20mgFluvastatin 20–40mgPitavastatin 1mg Atorvastatin 10–20mgRosuvastatin 5–10mgSimvastatin 20–40mgPravastatin 40–80mgLovastatin 40mgFluvastatin extended release 80mgFluvastatin 40mgPitavastatin 2–4mg Atorvastatin 40–80mgRosuvastatin 20–40mg Use according to LDLc reduction requirements of <30%, 30–50% or >50%.
- -
Ezetimibe 10mg/day.
- -
PCSK9 inhibitors: alirocumab (75mg/15 days; patients requiring LDLc reduction>60% can start with 150mg once every 2 weeks or 300mg once every 4 weeks, via the subcutaneous route), evolocumab (140mg/15 days or 420mg/month).
In patients with triglycerides>150mg/dl and HDLc<40mg/dl (males) or<45mg/dl (females), glycemic control should be improved, with emphasis on the avoidance of alcohol and unhealthy habits. In patients with triglycerides>500mg/dl, drug treatment in monotherapy or combination treatment should be added. In patients with triglyceride levels between 200 and 500mg/dl and low HDLc, once the LDLc target has been reached, the following additional drug treatment can be considered (level of evidence A)49:
- 1.
Fibrates (of choice): fenofibrate (145–250mg/day), gemfibrozil (900–1200mg/day), or bezafibrate (200–400mg/day). In combination with statins, fenofibrate should preferably be used.
- 2.
Omega-3 fatty acids (2–4g/day).
The general control target is systolic blood pressure (SBP)<140mmHg and diastolic blood pressure (DBP)<90mmHg (level of evidence A). The blood pressure control target for high CV risk patients, young individuals or patients with albuminuria is<130/80mmHg, provided this pressure is reached without side effects (level of evidence C).50
Hygiene-dietary measures include the reduction of sodium (<2.3g/day) and alcohol intake, an increase in fruit and vegetable consumption, weight loss in patients that are overweight or obese, and increased physical activity (level of evidence B).50
- –
In patients with SBP 140–159mmHgand/or DBP 90–99mmHg, it is advisable to start pharmacological treatment with a single drug, which may be an angiotensin converting enzyme inhibitor (ACEI), an angiotensin II receptor antagonist (ARA2), a dihydropyridine calcium antagonist, or a thiazide diuretic. If the patient presents albuminuria (an albumin/creatinine ratio ≥30mg/g), either ACEI or ARA2 should be the first choice (level of evidence A).48,50
- –
In patients with blood pressure≥160/100mmHg, it is advisable to start combination therapy with two drugs, which should include an ACEI or ARA2 if the patient presents albuminuria (level of evidence A).50
- –
If the target is not reached with such treatment, a second or third drug may be added, avoiding the combination of ACEI and ARA2 drugs (level of evidence A).
- –
Beta-blockers may be used in patients with ischemic heart disease or heart failure (level of evidence A).
- –
It is essential to emphasize the importance of smoking cessation. In this regard, the patient may be enrolled in structured programs, with drug support where necessary (level of evidence A). With regard to antiplatelet medication, aspirin (75–150mg/day) is advised, or clopidogrel (75mg/day) in patients with intolerance or allergy to the former drug48:
- •
In secondary prevention (previous ischemic event), whenever possible (level of evidence A).
- •
In primary prevention if the patient is at high CV risk (males and females >50years of age with other CVRFs: a family history of early CV disease, arterial hypertension, smoking, dyslipidemia or albuminuria), provided the risk of bleeding is low (level of evidence C).
- •
The individualization of treatment in patients with DM2 is essential for the success of the chosen therapy. The adverse effects profile and cost-effectiveness analysis help in therapeutic decision-making. In this regard, non-insulin antidiabetic treatments that do not increase the risk of hypoglycemia (DPP4i, GLP-1 RAs, SGLT2i), in combination with metformin and/or pioglitazone, should be preferred to secretagogues and insulin. In turn, the beneficial effect of GLP-1 RAs and SGLT2i upon body weight makes them the drugs of choice in overweight or obese patients versus treatments that exert a neutral (DPP4i) or harmful effect upon weight (secretagogues, insulin, pioglitazone). Both metformin and GLP-1 RAs are associated with gastrointestinal adverse effects (dyspepsia, nausea, vomiting); gradual dose introduction is therefore important, with discontinuation in the event of intolerance. Although the data are inconclusive and the risk appears to be low, a previous presence of pancreatitis (GLP-1 RAs, DPP-4i) and biliary disease (GLP-1 RAs) limits the use of incretin therapies. In the case of SGLT2i, the most important adverse effect is the occurrence of genital infections (with an incidence of 5–10%). These are typically mild, usually resolve with a single course of antifungal treatment, and do not require the discontinuation of SGLT2i treatment. Canagliflozin has been associated with an increased risk of lower limb amputations as well as an increased risk of fractures, and its use should be limited in patients at high risk of suffering such conditions.19 Furthermore, in individuals with high fracture risk (particularly women), the use of pioglitazone and – to a lesser extent – sulfonylureas and insulin should be limited due to the increased risk of fractures.51 Pioglitazone is also contraindicated in patients with a history of bladder cancer or macroscopic hematuria of indeterminate origin. Although infrequent, metformin has been associated with lactic acidosis (its use should be avoided in these situations during intercurrent conditions) and vitamin B12 deficiency. Monitoring of levels is recommended on at least an annual basis, especially in risk situations (elderly people, the use of proton pump inhibitors [PPIs]). Lastly, both liraglutide52 and pioglitazone53 have shown benefits in relation to the course of non-alcoholic fatty liver disease (NAFLD).
Given the progressive increase in healthcare expenditure, both the Ministry of Health and important international associations, such as the National Institute for Health and Care Excellence (NICE), recommend that cost-effectiveness analyses be conducted in order to prioritize healthcare resource allocation. In this regard, and in the context of the Spanish National Health System, several studies have shown that the novel non-insulin antidiabetic therapies (GLP-1 RAs, SGLT2i) are the dominant (more effective and less expensive) or cost-effective choice (≤30,000 euros per quality-adjusted life year gained [QALY]) versus other treatment options.54–57 In turn, the use of insulin degludec versus glargine U100 has also been shown to be a cost-effective option.58
Detection of comorbiditiesNon-alcoholic fatty liverNon-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disorder worldwide, with a prevalence of 25–30% in the general population and of 70% in patients with DM2. The condition encompasses simple steatosis, non-alcoholic steatohepatitis (NASH) and fibrosis, in the absence of other causes of liver disease and alcohol abuse (<20g/day in men and <10g/day in women). Non-alcoholic fatty liver disease can progress to cirrhosis and hepatocellular carcinoma. Patients with DM2 are particularly susceptible to more severe forms of NAFLD, and have an increased risk of hepatocellular carcinoma. Furthermore, the coexistence of DM2 and NAFLD usually implies a poorer metabolic profile and an increased CV risk.59
Ultrasound is the first choice technique for establishing the diagnosis, though its sensitivity is low when steatosis is less than 33% (e.g., in patients with fibrosis) or in the presence of abdominal obesity.60 Liver enzymes are not a sensitive marker of NAFLD, since the more severe forms of liver disease usually show normal values. The use of risk scales, such as the fatty liver index (https://www.medicalalgorithms.com/fatty-liver-index), has been shown to be useful for establishing the diagnosis.59,60
In the presence of steatosis or altered liver enzymes, fibrosis risk scales are applied. These make use of clinical variables and surrogate markers of liver inflammation or fibrosis (NAFLD fibrosis score [http://nafldscore.com]: age, the BMI, glucose intolerance/diabetes, GOT/GPT, platelets, albumin; FIB-4 [http://www.mdcalc.com/fibrosis-4-fib-4-index-liver-fibrosis]: age, GOT, GPT, platelets).60 Patients at moderate or high risk of fibrosis as revealed by these scales should be referred to the gastrointestinal specialist in order that other liver diseases can be discarded and the use of elastographic study and/or biopsy assessed. In the remaining cases, regular monitoring should be performed, with the assessment of liver enzymes and ultrasound, in patients without steatosis, based on liver enzymes and fibrosis risk scales in patients with steatosis and a low risk of fibrosis. In patients without steatosis and with normal liver enzyme levels, assessment should be repeated on a regular basis (every 3–5 years).
There is currently no approved drug treatment for NAFLD. Changes in lifestyle seeking to achieve 7–10% weight loss are the mainstay of treatment (metabolic surgery should be considered for a BMI≥35kg/m2).59,60 Some antidiabetic treatments have shown positive effects upon NASH (pioglitazone 45mg/day53 and liraglutide52) and fibrosis (pioglitazone 45mg/day) in the context of randomized clinical trials.
Heart failureHeart failure is currently the most common CV complication in DM2, with a greater incidence than myocardial infarction or stroke.61 If heart failure is suspected (previous coronary disease, arterial hypertension, orthopnea or paroxysmal nocturnal dyspnea), and in the presence of clinical signs and symptoms (dyspnea, edema and fatigue; elevated jugular venous pressure, pulmonary crepitants), associated or not with electrocardiographic changes, the patient may require referral to Cardiology for echocardiography. A useful option in the event of clinical suspicion is the determination (if available at the center) of brain natriuretic peptide (BNP>35pg/ml) and N-terminal pro-brain natriuretic peptide (NT-ProBNP>125pg/ml) in order to identify patients requiring echocardiographic evaluation.61
Sleep apnea–hypopnea syndromeSleep apnea–hypopnea syndrome (SAHS) is characterized by excessive drowsiness and cognitive-behavioral, respiratory, cardiac, metabolic or inflammatory disorders secondary to repeated episodes of upper airway obstruction during sleep. The prevalence of SAHS in patients with DM2 is higher than in the general population, and is estimated to be about 50%.62 The concomitant presence of SAHS may aggravate the course of DM2. In addition, there is an association between the macrovascular complications of diabetes and SAHS, the latter representing an independent predictor of proliferative retinopathy.62
Screening using validated questionnaires (the Stop-Bang tool is considered the most effective choice, with 93–100% sensitivity and 43% specificity) allows for early diagnosis and management by the pneumologist. Treatment focuses on body weight reduction. Continuous positive airway pressure (CPAP) is considered the best treatment for moderate to severe SAHS, and improves insulin sensitivity in patients with severe SAHS (apnea–hypopnea index>30) as compared to patients with less severe SAHS.62
Treatment of type 2 diabetes in frail patientsThe presence of frailty is an important determinant in selecting the treatment for diabetes.63 In diabetic patients over 70 years of age, the assessment of frailty using the Fried criteria is advised64:
- -
Unintentional weight loss (>4.5kg or 5% in one year).
- -
Low energy or resistance: an affirmative reply (5–7 days/week) to either of the following questions:
- •
Over the last week, on how many days did you feel that everything you do requires an effort? On how many days did you not feel like doing anything?
- •
- -
Walking speed: time required to walk 4.6m>7s (males<175cm or females<159cm) or >6s (males>175cm or females>159cm).
- -
Low level of physical activity (in males walking≤2h and 30min/week; in females≤2h/week).
- -
Muscle weakness measured by hand dynamometry.
If the patient meets frailty criteria (3 or more criteria), the following treatment targets should apply (level of evidence E): in the presence of established CVD, HbA1c 7.5–8%; in frail patients, HbA1c 8–8.5%. It is essential to avoid hypoglycemic episodes, which are more common in elderly subjects if blood glucose control is very strict and/or there are a greater number of comorbidities. In frail subjects, treatment discontinuation should be considered if HbA1c lies below target.63
Metformin is recommended as the first-line drug treatment (level of evidence A), with adjustment for renal function and monitoring for side effects, which may be of great relevance in fragile subjects (gastrointestinal intolerance, dysgeusia, hyporexia and vitamin B12 deficiency).16 Renal function should be monitored at least on an annual basis. The DPP4i are recommended as a second-line therapy, or as the first choice in the case of adverse effects with metformin (level of evidence E).63 If adequate glycemic control is not achieved with these therapies, basal insulin should be assessed (prioritizing basal analogs with a lower risk of hypoglycemia) (level of evidence E). In frail patients, it is advisable to avoid the use of other therapies: pioglitazone if there is a risk of heart failure or a risk of fractures and/or falls; secretagogues (sulfonylureas and repaglinide) due to the risk of hypoglycemia; and SGLT2i and GLP-1 RAs due to weight loss, which might not be adequate in frail patients.
Comprehensive approach to type 2 diabetes mellitusIn conclusion, the present document proposes a comprehensive management of DM2, individualized according to patient characteristics, and prioritizing treatments for diabetes with a low risk of hypoglycemia and with positive effects upon CV risk factors, including body weight. In addition, the presence of CVD or renal disease is given priority in treatment selection (Fig. 1). Lastly, the adequate prescription of diet and exercise is essential from the time of diagnosis and throughout the course of the disease. The key recommendations are presented in Fig. 1.
The document has been updated in 2019 based on new published evidence, and is available at http://www.seen.es/docs/apartados/791/abordaje%20integral%20dm2019.pdf.
AuthorshipRebeca Reyes-García and Óscar Moreno-Pérez contributed equally to the development and preparation of the manuscript.
Conflicts of interestR. Reyes: speaker/consultant for Almirall, Ascensia, Astra-Zeneca, Boehringer-Ingelheim, Esteve, Ferrer, Faes Farma, Glaxo Smith Kline, Janssen-Cilag, Lilly, MSD, Novartis, NovoNordisk, Sanofi-Aventis. Clinical investigator for Astra-Zeneca, Glaxo Smith Kline, Lilly, NovoNordisk, Roche.
O. Moreno: speaker/consultant for NovoNordisk, Lilly, MSD, Novartis, Boehringer-Ingelheim, Astra-Zeneca. C. Tejera: speaker/consultant for Sanofi-Aventis, NovoNordisk, Astra-Zeneca, R, Lilly, Boehringer-Ingelheim, MSD. Clinical investigator for NovoNordisk, Sanofi-Aventis, Astra-Zeneca.
V. Bellido: speaker/consultant for Astra-Zeneca, Boehringer-Ingelheim, Esteve, Lilly, MSD, NovoNordisk, Roche, Sanofi-Aventis. Clinical investigator for Sanofi-Aventis.
M. López de la Torre: speaker/consultant for NovoNordisk, Lilly, Novartis, Sanofi-Aventis, MSD, Boehringer-Ingelheim, Astra-Zeneca, Bristol-Myers Squibb, Esteve, Faes Farma, Almirall, Glaxo Smith Kline, Janssen-Cilag, Abbot and Ferrer. Clinical investigator for NovoNordisk, Lilly, Intarcia, Bristol-Myers Squibb, Boehringer-Ingelheim, Sanofi-Aventis, Glaxo Smith Kline, Astra-Zeneca, Amgen, Novartis.
P. Rozas: speaker/consultant for Astra-Zeneca, Boehringer-Ingelheim, Esteve, Ferrer, Faes Farma, Janssen-Cilag, Lilly, MSD, Novartis, NovoNordisk, Sanofi-Aventis.
J.C. Fernández: speaker/consultant for Astra-Zeneca, Boehringer-Ingelheim, Esteve, Lilly, NovoNordisk, Sanofi-Aventis. Research grants: M., NovoNordisk.
A. Marco: speaker/consultant for Almirall, Astra-Zeneca, Boehringer-Ingelheim, Esteve, Faes Farma, Lilly, MSD, NovoNordisk, Sanofi-Aventis. Clinical investigator: Sanofi-Aventis.
J. Escalada: speaker/consultant for Astra-Zeneca, Boehringer-Ingelheim, Lilly, MSD, NovoNordisk, Sanofi-Aventis.
M. Gargallo: speaker/consultant for Astra-Zeneca, Boehringer-Ingelheim, Janssen-Cilag, Lilly, Sanofi-Aventis/Lilly, Sanofi-Aventis. Clinical investigator: Sanofi-Aventis.
M. Botana: speaker/consultant for Astra-Zeneca, Boehringer-Ingelheim, Esteve, Ferrer, Faes Farma, Janssen-Cilag, Lilly, Novartis, NovoNordisk, Sanofi-Aventis.
J. López and J.M. Gómez: speaker/consultant for Astra-Zeneca, Boehringer-Ingelheim, Esteve, Janssen-Cilag, MSD, NovoNordisk, Sanofi-Aventis. Clinical investigator: Janssen-Cilag.
E. Jódar: speaker/consultant for Almirall, Astra-Zeneca, Boehringer-Ingelheim, Ferrer, Faes Farma, Janssen-Cilag, Lilly, MSD, Novartis, NovoNordisk. Clinical investigator: Astra-Zeneca, Boehringer-Ingelheim, Glaxo Smith Kline, Janssen-Cilag, Lilly, MSD, NovoNordisk, R, Sanofi-Aventis.
P. Mezquita: speaker/consultant for Lilly, Almirall, Novartis, NovoNordisk, Astra-Zeneca, Bristol-Myers Squibb, Esteve, MSD, Sanofi-Aventis, Faes Farma. Clinical investigator: Lilly, Roche, Sanofi-Aventis, NovoNordisk, MSD, Boehringer-Ingelheim, Theracos, Glaxo Smith Kline.
Please cite this article as: Reyes-García R, Moreno-Pérez Ó, Tejera-Pérez C, Fernández-García D, Bellido-Castañeda V, López de la Torre Casares M, et al. Documento de abordaje integral de la diabetes tipo 2. Endocrinol Diabetes Nutr. 2019;66:443–458.
The full document can be found at: http://www.seen.es/docs/apartados/355/2018%2005%2005%20Abordaje%20Integral%20DM2_SEEN_2018_GTDMSEEN%201.pdf.