Bariatric surgery (BS) has shown to reduce cardiovascular morbidity and mortality in obesity. The BS has improved the dyslipidemia of the insulin resistant patient, our objective was to evaluate if there was a difference in the lipid profile between the laparoscopic roux-en-Y gastric bypass (RYGB) technique vs. the sleeve gastrectomy (SG) technique at 18 months of follow-up.
MethodsAn observational, open, prospective study of morbidly obese patients who underwent bariatric surgery at 18-month follow-up. Anthropometric analysis, body composition, energy expenditure at rest, glucose, insulin, Hba1c, LDL, HDL, TG and CT were performed.
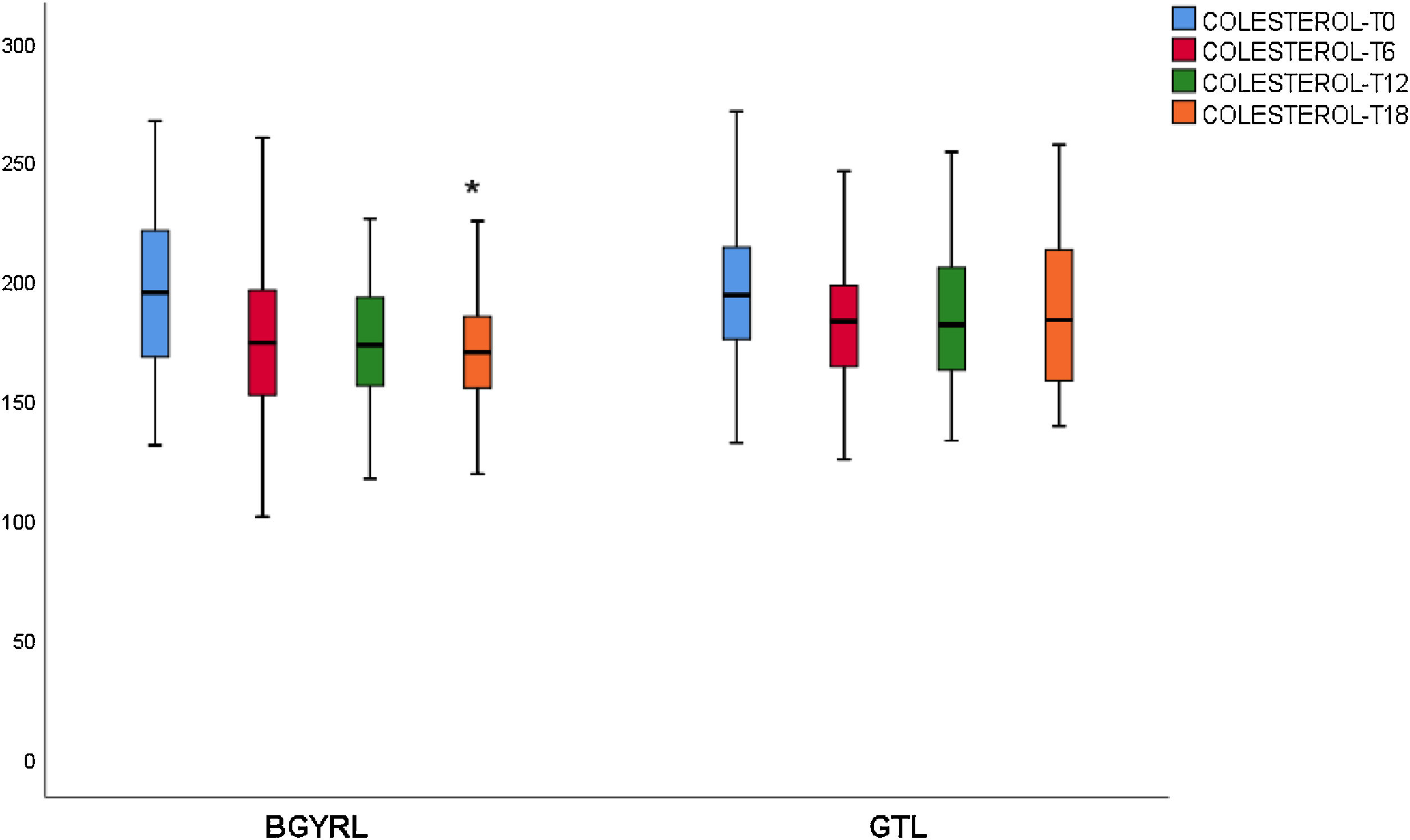
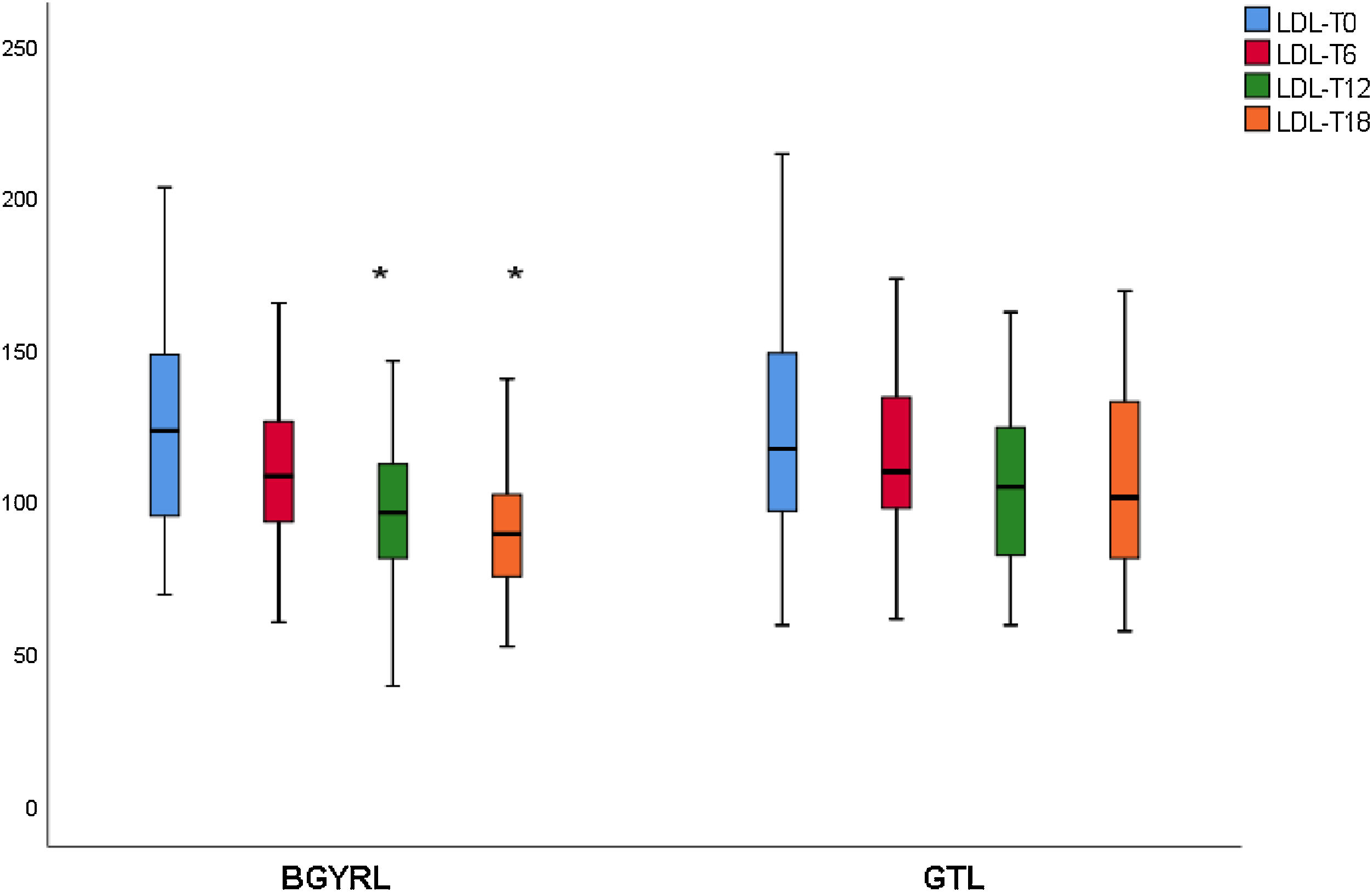
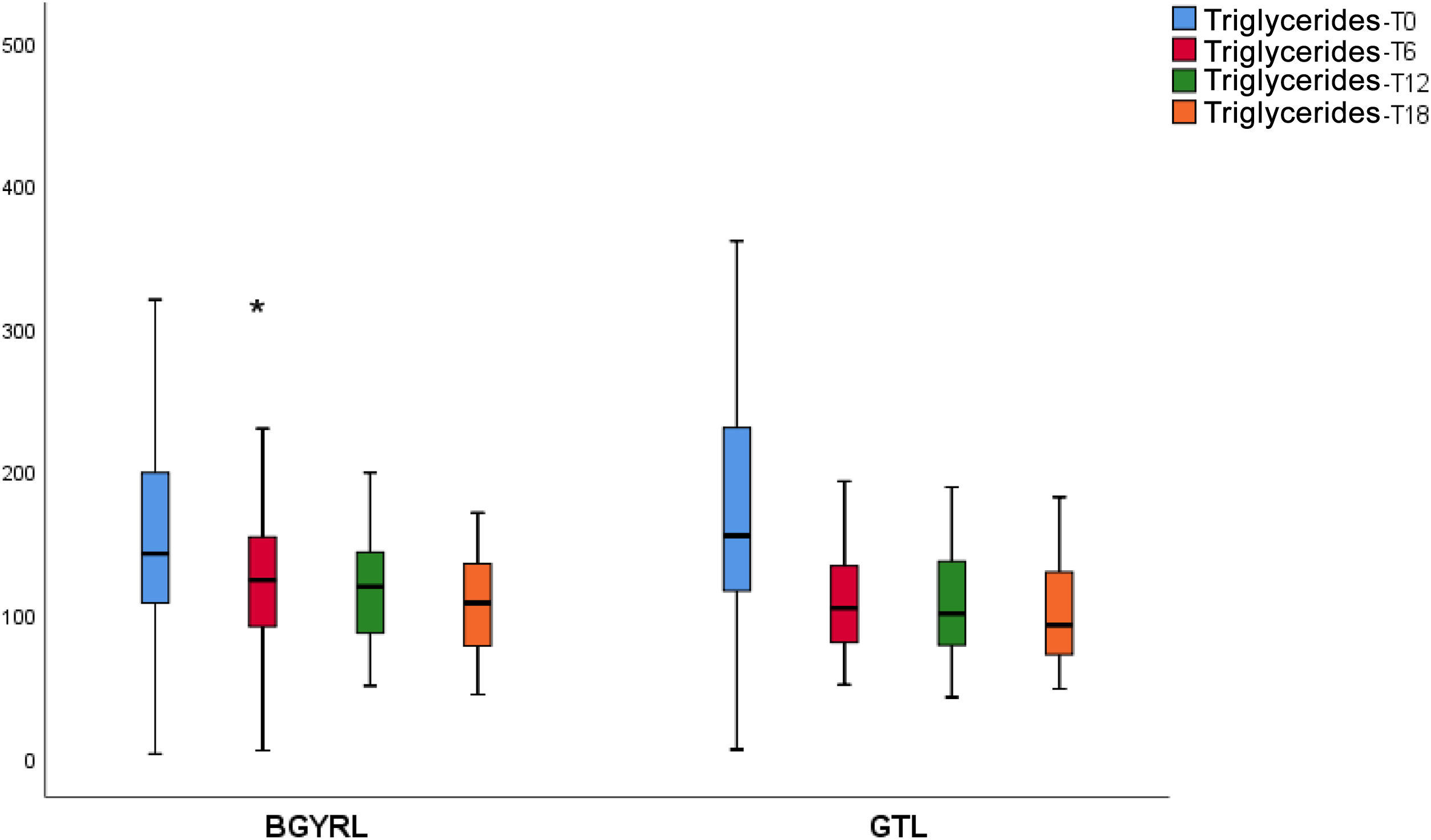
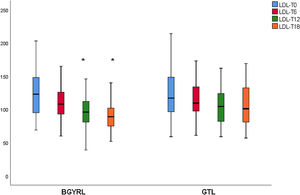
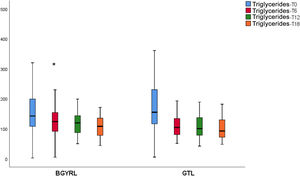
ResultsAbsence baseline differences were found in the proportion of patients with hypertension, diabetes, steatosis, and sex between the RYGB vs SG groups. A reduction of TG was observed at 6 months in favor of RYGB vs SG: 108.60 ± 34.86 vs. 124.59 ± 44.58, P = 0.044), however, a decrease in both LDL levels was found at 12 and 18 months in favor of the RYGB vs. SG group: 96.23 ± 24.33 vs. 107.83 ± 28.88, P = 0.025; 90.98 ± 20.62 vs 106.22 ± 31.48, P = 0.003; the decrease in CT was observed only at 18 months in favor of the RYGB vs. SG group: 171.39 ± 25.058 vs. 186.89 ± 31.81, P = 0.005.
ConclusionRYBG has shown to be more effective in reducing LDL and CT levels compared to SG, which provides an additional benefit of RYGB in relation to the lipid profile of the patient.
La cirugía bariátrica (CB) ha mostrado reducir la morbilidad y mortalidad cardiovascular en obesidad mórbida. La CB ha mejorado la dislipemia del paciente insulino-resistente (IR). El objetivo de nuestro trabajo fue evaluar si existe diferencia en el perfil lipídico entre la técnica de bypass gástrico laparoscópico en Y de Roux (BGYRL) vs la técnica de la gastrectomía tubular laparoscópica (GTL) a 18 meses de seguimiento.
MétodosEstudio observacional, abierto, prospectivo, de pacientes con obesidad mórbida sometidos que realizaron a cirugía bariátrica a 18 meses seguimiento. Se realizo análisis antropométricos, composición corporal, gasto energético de reposo, de glucosa, insulina, Hba1c, LDL, HDL, TG y CT.
ResultadosNo se encontraron diferencias basales de la proporción de pacientes con hipertensión arterial, diabetes de tipo 2, esteatosis y de sexo entre los grupos de BGYRL (91) vs GTL (77). Se observo reducción de TG a los 6 meses a favor de BGYRL vs GTL: 108,60 ± 34,86 vs 124,59 ± 44,58; P = 0,044), en cambio se encontró disminución tanto de niveles de LDL a los 12 y 18 meses a favor del grupo BGYRL vs GTL: 96,23 ± 24,33 vs 107,83 ± 28,88, P = 0,025; 90,98 ± 20,62 vs 106,22 ± 31,48, P = 0,003; la disminución de CT se observó solo a los 18 meses a favor del grupo BGYRL vs GTL: 171,39 ± 25,058 vs 186,89 ± 31,81, P = 0,005.
ConclusiónEl BGYRL ha mostrado ser más eficaz para reducir LDL y CT en comparación con GTL, lo cual otorga un beneficio adicional del BGYRL en relación al perfil lipídico del paciente.
Obesity is a public health problem in the whole world, and it is associated with comorbidities such as: type 2 diabetes mellitus (DM2) and cardiovascular diseases (CVD).1 The increasing prevalence of obesity is accompanied by a higher prevalence of DM2. Obesity does not only increase the risk of developing DM2, as it also aggravates the risks of the same for health and makes managing it more complex.2
CVD is the main cause of morbidity and mortality in patients with obesity and DM2.3 High levels of cholesterol in plasma with low density lipoproteins (C-LDL) are the main cardiovascular lipid risk factor.4 Nevertheless, atherogenic dyslipidaemia (AD) as seen in states of insulin resistance (IR), such as obesity and DM2, is a decisive factor in cardiovascular risk. AD is chiefly characterized by high levels of fasting and post-prandial triglyceride-rich lipoproteins (TRL) in plasma, small and dense low density lipoproteins (LDL) and low levels of cholesterol carried in high density lipoproteins (HDL-C).5
Bariatric surgery (BS) is currently the most effective treatment to induce weight loss in obese patients. It leads to the complete or partial remission of DM2 and improves cardiovascular risk factors such as arterial hypertension and lipid alterations in a significant proportion of patients.6
The most common BS procedures used worldwide are laparoscopic sleeve gastrectomy (LSG), a restrictive technique, and the laparoscopic Roux-en-Y gastric bypass (LRYGP), which is a combined restrictive and malabsorptive technique. As there is no strict recommendation regarding when to perform a LSG or a LRYGP, differences in the resulting glycosidic and lipid parameters may be highly useful when selecting the best surgical technique for a patient.7
Of the factors which are associated with a good prognosis, reducing TG and total cholesterol have been shown in multivariate analysis to be early markers of a reduction in cardiovascular events.8
Many of the random controlled clinical trials published do not evaluate lipid parameters in their initial end points. Many studies do not report on the percentage of patients who had been subjected to previous or subsequent lipid lowering treatments, or the class or dose of the lipid lowering drugs used.
This study evaluates lipid parameters after an 18 month follow-up of patients operated using LRYGP vs. LSG.
Material and methodsA total of 168 obese patients evaluated in the Department of Nutrition and Bariatric Surgery of the Nutrition, Endocrinology and Metabolism Centre (CIEN), Corrientes, Argentina, and the Instituto Modelo de Gastroenterología (IMG), Formosa, Argentina, were recruited for this study.
All of the patients were evaluated by a multidisciplinary team composed of an endocrinologist, a bariatric surgeon, a psychiatrist and a nutritionist during at least six months prior to surgery. All of the subjects fulfilled the criteria for bariatric surgery.9
The surgical techniques used included LRYGP and LSG. Follow-up after surgery was restricted to 18 months. Patients with kidney failure were excluded (glomerular filtration ≤60 mL/min/1.73 m2), as were minors and those older than 65 years, pregnant women and subjects with a severe disease that determined their survival. Patients whose follow-up was incomplete, lacked data or who had been subjected to surgery due to weight gain were excluded from this study. Clinical and analytical data corresponding to all of the patients were gathered after review of their computerized clinical histories. This study was undertaken according to the Helsinki Declaration and approved by the bioethics committee of the IMG, and all of the patients gave their informed consent in writing.
The diet of each subject was evaluated by a nutritionist, using a self-administered diary recording consumption over three days. Their weight, fasting biochemical parameters, body composition and energy intake were measured one month before surgery (T0) and at six months (T6), 12 months (T12) and 18 months (T18) after surgery. Our study was not designed to compare the efficacy of both surgical procedures, LRYGP vs LSG.
Blood samples were taken after nocturnal fasting. The plasma was immediately separated by centrifuging at 2000 rpm during 15 min at 4 C. TG in plasma, total cholesterol, HDL-C and LDL-C were measured using an enzymatic colorimetric kit (Cobas 6000 c 501 analyser; Roche Diagnostics, Mannheim, Germany). Glucose in plasma was analysed using the hexokinase method (Cobas 6000c, 501 analyser). Concentrations of insulin in plasma were determined using the electrochemiluminescence method (Cobas Analyser 6000 and 601). Insulin resistance was estimated using the homeostasis model of assessment (HOMA-IR): fasting insulin (mUI/L) × fasting glucose (mmol /L) /22.5.10 HOMA-IR was not calculated for patients with diabetes to avoid the HOMA-IR calculation error due to the antidiabetic drugs taken by these patients.
Concentrations in plasma were determined for apoB and apoA-I by immunonephelometry (Roche Diagnostics) using a BN ProSpec analyser (Siemens Healthineers, Erlangen, Germany). Resting energy expenditure (REE) was measured by indirect calorimetry (Quark-RMR-Cosmed, Rome, Italy). Body composition was evaluated by dual-energy x-ray absorptiometry (DEXA) (Lunar iDXA and enCORE 2007 version software, GE Healthcare, Chalfont St Giles, United Kingdom).
Statistical analysisData were expressed as an average ± standard deviation for continuous variables. Categorical variables were shown as percentages. The normal distribution of variables was confirmed by the Kolmogorov- Smirnov test. Comparisons between groups were made by the X2 test for categorical variables, as well as the Student’s t-test and ANOVA for continuous variables. The relationship between continuous variables was examined by multiple regression analysis. All statistical analysis was performed using the IBM SPSS Statistics 25.0 programme (IBM Inc., NY). Tests were bilateral, and P < .05 was considered to be statistically significant.
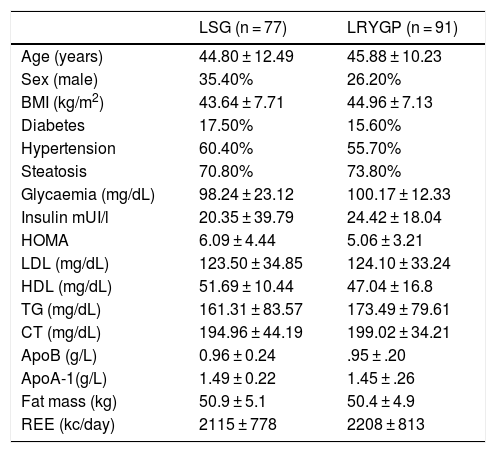
ResultsPatients’ basal characteristics are shown in Table 1, without significant differences. The average follow-up time was 1.5 years. Treatment prior to BS is shown in Table 2. No significant differences were found in terms of sex, energy expenditure, fat mass, glucose, lipids or apolipoproteins between the laparoscopic Roux-en-Y gastric bypass (LRYGP) group and the laparoscopic sleeve gastrectomy (LSG) group. The evolution of anthropometric and metabolic parameters after months’ follow-up after surgery are shown in Table 3. A fall in weight, body mass index (BMI), fasting glucose in plasma, HOMA-IR, energy intake and fat mass was detected after surgery in both groups during the months of follow-up.
Basal clinical and metabolic characteristics of the population.
| LSG (n = 77) | LRYGP (n = 91) | |
|---|---|---|
| Age (years) | 44.80 ± 12.49 | 45.88 ± 10.23 |
| Sex (male) | 35.40% | 26.20% |
| BMI (kg/m2) | 43.64 ± 7.71 | 44.96 ± 7.13 |
| Diabetes | 17.50% | 15.60% |
| Hypertension | 60.40% | 55.70% |
| Steatosis | 70.80% | 73.80% |
| Glycaemia (mg/dL) | 98.24 ± 23.12 | 100.17 ± 12.33 |
| Insulin mUI/l | 20.35 ± 39.79 | 24.42 ± 18.04 |
| HOMA | 6.09 ± 4.44 | 5.06 ± 3.21 |
| LDL (mg/dL) | 123.50 ± 34.85 | 124.10 ± 33.24 |
| HDL (mg/dL) | 51.69 ± 10.44 | 47.04 ± 16.8 |
| TG (mg/dL) | 161.31 ± 83.57 | 173.49 ± 79.61 |
| CT (mg/dL) | 194.96 ± 44.19 | 199.02 ± 34.21 |
| ApoB (g/L) | 0.96 ± 0.24 | .95 ± .20 |
| ApoA-1(g/L) | 1.49 ± 0.22 | 1.45 ± .26 |
| Fat mass (kg) | 50.9 ± 5.1 | 50.4 ± 4.9 |
| REE (kc/day) | 2115 ± 778 | 2208 ± 813 |
Data expressed as an average ± standard deviation or a percentage.
REE, Resting Energy Expenditure.
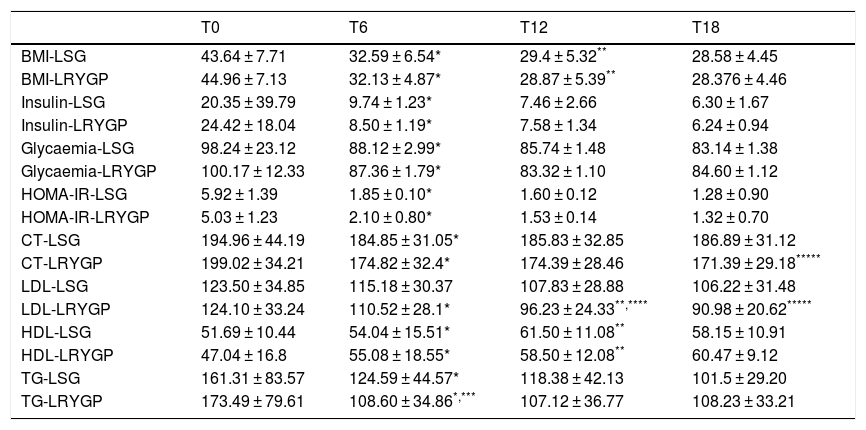
Clinical and metabolic characteristics at six, 12 and 18 months of follow-up.
| T0 | T6 | T12 | T18 | |
|---|---|---|---|---|
| BMI-LSG | 43.64 ± 7.71 | 32.59 ± 6.54* | 29.4 ± 5.32** | 28.58 ± 4.45 |
| BMI-LRYGP | 44.96 ± 7.13 | 32.13 ± 4.87* | 28.87 ± 5.39** | 28.376 ± 4.46 |
| Insulin-LSG | 20.35 ± 39.79 | 9.74 ± 1.23* | 7.46 ± 2.66 | 6.30 ± 1.67 |
| Insulin-LRYGP | 24.42 ± 18.04 | 8.50 ± 1.19* | 7.58 ± 1.34 | 6.24 ± 0.94 |
| Glycaemia-LSG | 98.24 ± 23.12 | 88.12 ± 2.99* | 85.74 ± 1.48 | 83.14 ± 1.38 |
| Glycaemia-LRYGP | 100.17 ± 12.33 | 87.36 ± 1.79* | 83.32 ± 1.10 | 84.60 ± 1.12 |
| HOMA-IR-LSG | 5.92 ± 1.39 | 1.85 ± 0.10* | 1.60 ± 0.12 | 1.28 ± 0.90 |
| HOMA-IR-LRYGP | 5.03 ± 1.23 | 2.10 ± 0.80* | 1.53 ± 0.14 | 1.32 ± 0.70 |
| CT-LSG | 194.96 ± 44.19 | 184.85 ± 31.05* | 185.83 ± 32.85 | 186.89 ± 31.12 |
| CT-LRYGP | 199.02 ± 34.21 | 174.82 ± 32.4* | 174.39 ± 28.46 | 171.39 ± 29.18***** |
| LDL-LSG | 123.50 ± 34.85 | 115.18 ± 30.37 | 107.83 ± 28.88 | 106.22 ± 31.48 |
| LDL-LRYGP | 124.10 ± 33.24 | 110.52 ± 28.1* | 96.23 ± 24.33**,**** | 90.98 ± 20.62***** |
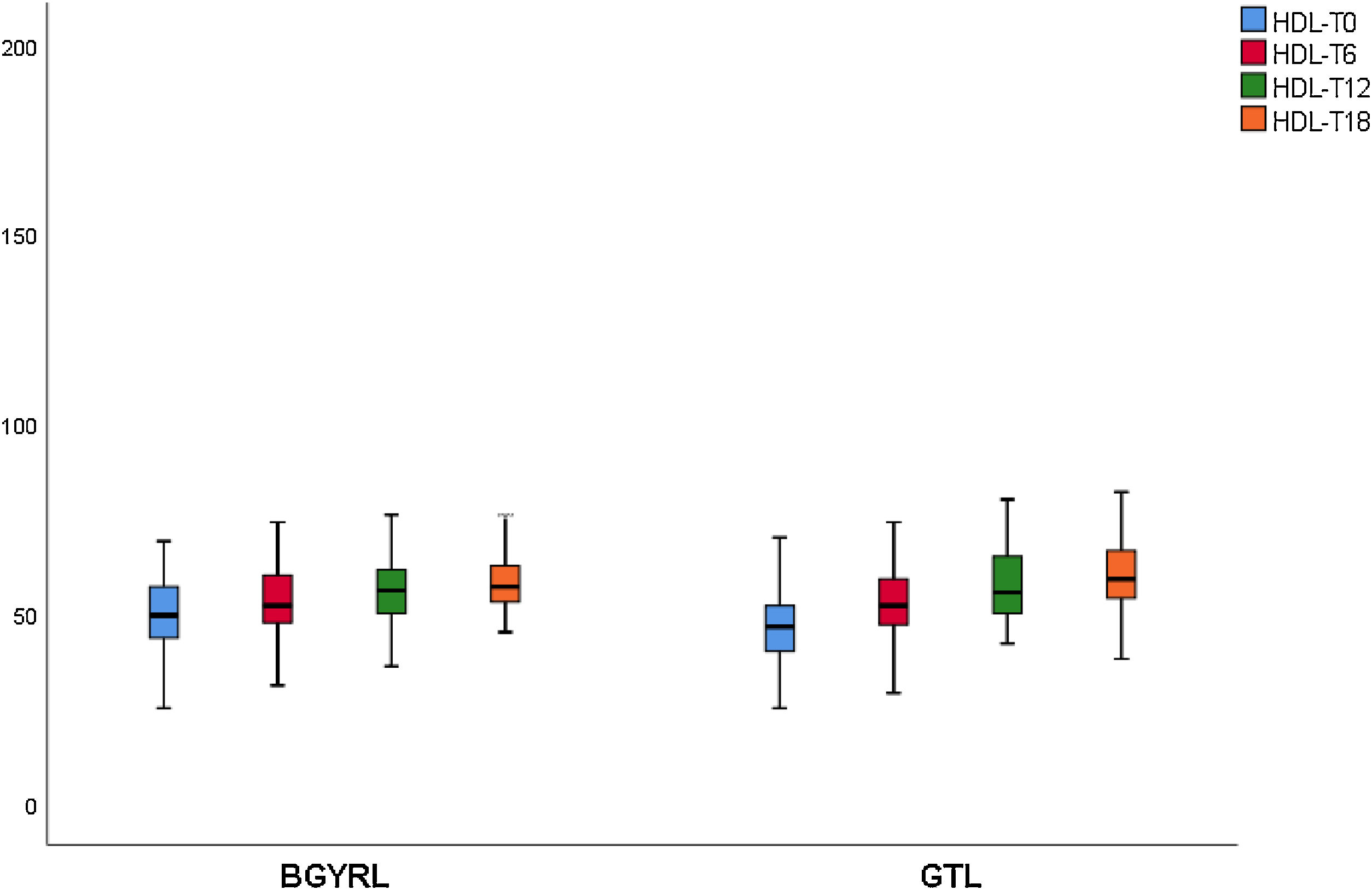
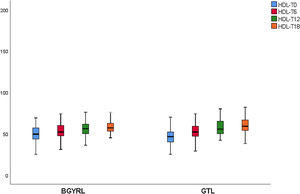
| HDL-LSG | 51.69 ± 10.44 | 54.04 ± 15.51* | 61.50 ± 11.08** | 58.15 ± 10.91 |
| HDL-LRYGP | 47.04 ± 16.8 | 55.08 ± 18.55* | 58.50 ± 12.08** | 60.47 ± 9.12 |
| TG-LSG | 161.31 ± 83.57 | 124.59 ± 44.57* | 118.38 ± 42.13 | 101.5 ± 29.20 |
| TG-LRYGP | 173.49 ± 79.61 | 108.60 ± 34.86*,*** | 107.12 ± 36.77 | 108.23 ± 33.21 |
Data expressed as an average ± standard deviation or a percentage.
LRYGP, Laparoscopic Roux-en-Y gastric bypass; LSG, laparoscopic sleeve gastrectomy.
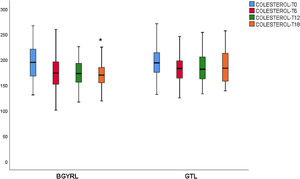
A fall in apoB and a rise in apoA-1 was observed at six months, as was a fall in TG and a rise in HDL in both groups (LRYGP and LSG) after six months, while on the other hand a fall in LDL and CT after 12 and 18 months only occurred in the LRYGP group (Figs. 1–4).
Finally, step-by-step multiple lineal regression analysis showed that the fall in HOMA-IR independently predicted the reduction in LDL at 18 months, explaining 72% of this fall (R2 = 0.720) (Table 4).
Multiple lineal regression models which predict the evolution of LDL T18-T0 (final model after step-by-step selection with an exclusion threshold at P < .05).
| Regression coefficient | Confidence interval | P | |
|---|---|---|---|
| HOMA-IR | .69 | .12 to .40 | <.01 |
| Constant | .16 | 5 |
The complete model was calculated for all of the obese subjects in the cohort, T18-T0 for BMI, apoB, apoA-I, fasting glucose and HOMA-IR (final adjusted model R2 = 0.72).
This study showed that LRYGP is superior in terms of reducing LDL and CT at 12 and 18 months of follow-up.
BS reduces cardiovascular mortality.11 This fall has been attributed in part to a reduction in plasmatic lipids, through unknown mechanisms. This study shows that the clinically important improvements in plasmatic lipids after LRYGP surgery arise in the final postoperative period, with substantial weight loss. The possible underlying mechanism for the improvement or resolution of the dyslipidaemia after bariatric surgery may be associated with weight loss. Weight loss is expected to improve insulin sensitivity, which in turn may reduce TG levels and increase the levels of HDL-C. This curve is similar to the weight loss curve after LRYGP and LSG.12,13
Levels of total cholesterol and LDL-c fall at 12 and 18 months, which supports the hypothesis that these changes are a direct consequence of surgery rather than absolute weight loss. The intestine plays a crucial role in cholesterol metabolism and participates in the absorption of cholesterol (from foods and bile) as well as in trans-intestinal cholesterol excretion. It has been known for a long time that an ileac bypass (which creates poor absorption, without closing the stomach as occurs in bariatric surgery) reduces cholesterol levels and cardiovascular diseases.6 Curiously, all of the studies which compare the different effects of LSG and LRYGP on concentrations of cholesterol in plasma show a constant 20%–30% reduction in cholesterol after LRYGP, which is maintained at five years.14
Contrary to the previous finding, cholesterol levels do not change after LSG.15 The 20%–30% reduction in cholesterol after LRYGP is equivalent to the fall that is obtained with an average dose of statins. The malabsorption technique of LRYGP significantly alters the flow of nutrients in the intestine, and the metabolic changes observed after this technique are in part independent of weight loss. These changes are multifactorial and complex, and they constitute a broad field for exploration.
Characterizing the factors which cause the constant 20%–30% fall in cholesterol after LRYGP may make it possible to identify new components of intestinal cholesterol metabolism and new therapeutic targets to reduce cholesterol. The potential factors which may be involved in reducing LDL after LRYGP are: (i) changes in cholesterol metabolism (a reduction in intestinal absorption, an increase in intestinal excretion, an increase in hepatic catabolism or a fall in hepatic synthesis), (ii) an increase in the intestinal conversion of cholesterol into coprostanol (an inactive type of sterol that is neither absorbed nor eliminated in the faeces), (iii) changes in the metabolism of the biliary acids (which are critical factors in intestinal cholesterol metabolism), (iii) changes in the intestinal hormones, and (iv) changes in the intestinal microbial ecosystem (microbiota).16,17
Statins may affect this mechanism, as they inhibit the synthesis of cholesterol in the liver, which leads to the activation of the LDL receptors and increasing hepatic capture of HDL from the circulation. The 20%–30% fall in cholesterol after LRYGP is equivalent to the reduction caused by an average dose of low-intensity statins.18 The administration of low therapeutic intensity statins is associated with average falls in total cholesterol, LDL-c and TG of 20%, 28% and 13%, respectively, with a 5% increase in HDL-c.18,19 In long-term cohort studies of patients treated with statins, for every 1% fall in LDL-C, there is a ∼1% reduction in the relative risk of major cardiovascular events, in a lineal relationship.19
A 69% negative association was found in the multivariate analysis between the HOMA-IR index and LDL levels. This may be explained by the fact that insulin regulates the expression of the LDL receptor, so that within the context of increased insulin sensitivity the catabolism of LDL would improve.20
The fall in TG levels and the increase in HDL with both BS techniques may be associated with weight loss and reduced IR. In a recent work on the kinetics of triglyceride-rich lipoproteins (TRL) with a hepatic and intestinal origin, BS led to a lower production of B-100 apoproteins (apo) by the liver and lower production of apoB-48 by the intestine.21 This characteristic fall in TG and rise in HDL are not only due a reduction in the plasma content of apoC-III, as there is also redistribution of apo-CIII at six and 12 months after surgery, from the TRL fraction to the HDL fraction.22
Cholesterol synthesis increases when there is obesity, and it is associated with a fall in HDL-c, while weight loss reduces cholesterol synthesis.17 The increase in HDL-c after LRYGP is compatible with the secondary benefit of weight loss, perhaps through the altered absorption and reduced synthesis of cholesterol.
ConclusionBoth BS techniques have been shown to reduce cardiovascular mortality and to improve dyslipidaemia and atherogenisis. Only LRYGP improves LDL levels, and this may mean that this technique has an additional benefit that should be taken into account when selecting the surgical technique to use for patients with previous hypercholesterolaemia.
FinancingThe authors declare that they received no financing.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Ackerman M, Serra E, Flecha P, Nogueira JP, Efecto selectivo de bypass gástrico laparoscópico en Y de Roux sobre el metabolismo lipídico, Clin Investig Arterioscler. 2022;34:68–74.