Limited data exist on low-density lipoprotein-cholesterol (LDL-C) level variability or long-term persistence with the monoclonal antibody evolocumab in routine clinical practice. HEYMANS (NCT02770131) is the first multi-country, multicenter, observational study of European patients initiating evolocumab as part of their routine clinical management, based on local reimbursement criteria (overall data recently published). The aim of this analysis is to describe clinical characteristics, baseline and changes in LDL-C levels, treatment patterns and persistence to evolocumab over 30 months in the Spanish cohort using data from the HEYMANS Registry.
MethodsHEYMANS was a prospective study of adult patients (≥18 years) who received at least one dose of evolocumab. A total of 1951 patients were enrolled from 12 countries and were followed up for 30 months after evolocumab initiation. Data were collected for 6 months before evolocumab initiation and up to 30 months thereafter. The Spanish cohort included patients who started evolocumab in routine clinical practice from March 2016 to September 2019. Demographic and clinical characteristics, lipid-lowering therapies (LLT), and lipid levels were collected.
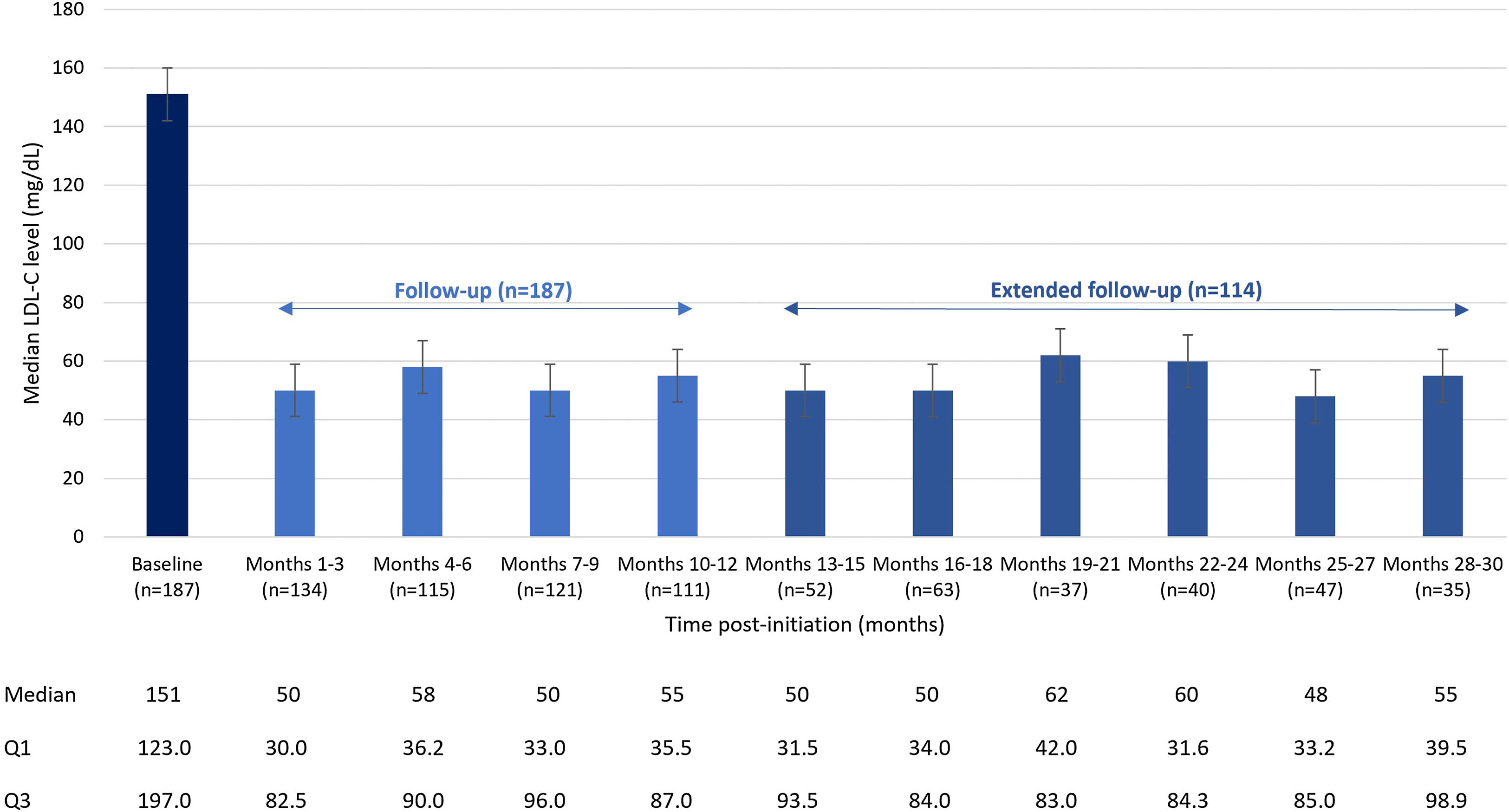
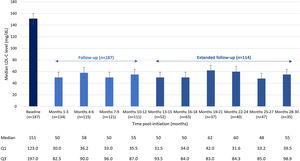
ResultsIn total, 201 patients were included in the Spanish cohort. Median follow-up (Q1–Q3) was 30.0 (12–30) months. A total of 61.7% of patients were men and the mean (standard deviation) age was 59.5 (10.8) years. Most patients (68.7%) had experienced a prior cardiovascular event, 45.3% had coronary artery disease or stable angina, and 60.2% had a diagnosis of familial hypercholesterolemia. Overall, 57.7% of patients were receiving treatment with statins, most of them with high-intensity statins (85.3%); 45.8% of patients were intolerant to statins, and 26.4% of patients did not receive any LLT. At baseline, median (Q1–Q3) LDL-C levels were 151 (123–197) mg/dL. After 3 months of treatment, baseline LDL-C decreased by 66% to a median of 50 (30–83) mg/dL and these levels were maintained over time, with a median LDL-C of 55 (40–99) mg/dL at 30 months. At months 10–12 of treatment, LDL-C levels<55mg/dL were achieved by 56.3% of patients. LDL-C levels<70mg/dL were achieved by 70.1% of patients, and a lowering of LDL-C levels ≥50% was achieved by 76.8% of patients. The percentage of patients on evolocumab treatment was 95% at 12 months and 93% at 30 months.
ConclusionsIn the Spanish cohort in routine clinical practice, evolocumab therapy provided a reduction in LDL-C levels consistent with that reported in previous clinical trials, which was sustained during 30 months of follow-up. Treatment with evolocumab was started at LDL-C levels 50% higher than those recommended by The Spanish Society of Arteriosclerosis and the Therapeutic Positioning Report. The probability of achieving the 2019 ESC/EAS LDL-C goals would improve with combination therapy and also with a lower LDL-C threshold when starting evolocumab. Persistence to evolocumab remained high during follow-up, with a very low percentage of discontinuation (5% at 12 months; 7% at 30 months).
Existen datos limitados sobre la variabilidad del nivel de colesterol de lipoproteínas de baja densidad (cLDL) o la persistencia a largo plazo con el anticuerpo monoclonal evolocumab en la práctica clínica habitual. HEYMANS (NCT02770131) es el primer estudio observacional multicéntrico y multinacional de pacientes europeos que iniciaron tratamiento con evolocumab en la práctica clínica habitual, basado en criterios de reembolso locales. El objetivo fue evaluar las características clínicas, los cambios en los niveles de cLDL, los patrones de tratamiento y la persistencia a este con evolocumab en la cohorte española con un seguimiento de 30 meses, utilizando datos del registro HEYMANS.
MétodosHEYMANS fue un estudio prospectivo de pacientes adultos (≥18 años) que recibieron al menos una dosis de evolocumab prescrita. Se incluyeron 1.951 sujetos de 12 países. Los datos fueron recopilados desde los seis meses previos al inicio del tratamiento hasta los 30 meses posteriores. La cohorte española incluyó pacientes que comenzaron evolocumab en la práctica clínica habitual desde marzo del 2016 hasta septiembre del 2019. Se recogieron las características demográficas y clínicas, los tratamientos hipolipemiantes (LLT) y el perfil lipídico.
ResultadosEn la cohorte española se incluyeron 201 participantes. La mediana de seguimiento (Q1-Q3) fue de 30,0 (12-30) meses. De los pacientes, 61,7% eran hombres, con edad media (desviación estándar) de 59,5 (10,8) años. La mayoría (68,7%) había presentado un evento cardiovascular previo, 45,3% padecía enfermedad arterial coronaria o angina estable y 60,2% tenía diagnóstico de hipercolesterolemia familiar. En general, 57,7% de los pacientes recibían tratamiento con estatinas, la mayoría con estatinas de alta intensidad (85,3%), 45,8% eran intolerantes a las estatinas y 26,4% no recibían LLT. Al inicio del estudio, la mediana (Q1-Q3) de los niveles de cLDL fue de 151 (123-197) mg/dL. A los tres meses de tratamiento, el cLDL basal disminuyó 66% hasta una mediana de 50 (30-83) mg/dL y se mantuvo en el tiempo, con una mediana de 55 (40-99) mg/dL a los 30 meses. A los 10-12 meses de terapia, 56,3% de los pacientes alcanzaron niveles de cLDL < 55 mg/dL y 70,1% < 70 mg/dL. Además, 76,8% de los pacientes lograron una reducción de ≥ 50%. El porcentaje de pacientes en tratamiento fue de 95% a los 12 meses y 93% a los 30 meses.
ConclusionesEn España, en práctica clínica habitual, el tratamiento con evolocumab redujo los niveles de cLDL, y estos se mantuvieron constantes durante el seguimiento, similar a lo previamente reportado. El régimen con evolocumab se inició con niveles de cLDL 50% superiores a los recomendados. La probabilidad de alcanzar los objetivos de cLDL de la ESC/EAS 2019 mejoraría con la terapia combinada y también con un umbral de cLDL más bajo al iniciar evolocumab. La persistencia al tratamiento con evolocumab se mantuvo alta durante el seguimiento, con un porcentaje muy bajo de discontinuidad (5% a los 12 meses; 7% a los 30 meses).
High levels of low-density lipoprotein cholesterol (LDL-C) represent a risk factor for cardiovascular disease (CVD).1–3 CVD is a leading cause of mortality and disability in Spain4 and the rest of the world.5 Therefore, reducing LDL-C levels can decrease the risk of major cardiovascular (CV) events6 and help to prevent CVD. The European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) recommend a risk-based approach to LDL-C goals and aim to attain the lowest LDL-C levels in those at very-high CV risk.7 In order to achieve the LDL-C goals of <55mg/dL or a >50% lowering in LDL-C level from baseline, a combined use of lipid-lowering therapies (LLT) may be required. Among the LLT, statins are recommended as a primary prevention in patients aged<75 years.7 Moreover, the use of a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i), as secondary prevention, is recommended for patients at very-high CV risk when LDL-C goals of <55mg/dL are not met despite receiving the maximum tolerated statin and ezetimibe therapy doses, or for patients who experience a second CV event within 2 years of treatment with the maximum tolerated statin dose and when LDL-C goals of <40mg/dL are not met.7
Use of evolocumab, either as monotherapy or as combination therapy with statins and ezetimibe, has been shown to reduce LDL-C levels by approximately 60%.1,8–10 The FOURIER-OLE study11 captured long-term safety, tolerability, lipids levels and risk of major CV events with prolonged treatment with evolocumab following completion of FOURIER trial.1 After long-term evolocumab treatment (median follow-up of 5 years), FOURIER-OLE patients originally randomized to evolocumab were less likely to have major CV events or die due to CV causes when compared to patients originally randomized to placebo in the parent FOURIER trial.11 However, limited data are available on how evolocumab is used in European clinical practice, including whether there is LDL-C level control variability or long-term persistence. The recently published HEYMANS study12 is, to date, the largest multi-country systematic registry of patients receiving evolocumab in European real-world practice. The study aimed to describe hyperlipidemic characteristics of patients at evolocumab initiation and treatment patterns in 12 European countries. Treatment with evolocumab was associated with sustained LDL-C level reductions for up to 30 months of follow-up. Moreover, persistence with evolocumab remained high, both at 12 months and 30 months of follow-up.
Despite the 2019 ESC/EAS guideline-recommendations,7 in routine clinical practice, the LDL-C thresholds at which evolocumab is reimbursed may be much higher12–14 than the recommended LDL-C thresholds for initiation of evolocumab. Additionally, the use of evolocumab in combination with other therapies may remain suboptimal in patients at very-high CV risk.15 Among European countries, LDL-C control is poor in Spain and, despite the availability of LLTs, they are underused, especially in secondary prevention.16,17 In the REPAR study, an observational prospective study, only 26% of Spanish patients with coronary heart disease (CHD) had an adequate lipid control.17 The DA VINCI study, which described the contemporary use of LLT and goal attainment in patients across 18 European countries, concluded that gaps between clinical guidelines and clinical practice for lipid management persist across Europe, and that these gaps were exacerbated by the 2019 ESC/EAS guidelines. Even with optimized statins, increased utilization of LLT is needed to reduce these gaps for patients most at risk.15
In Spain, evolocumab was initially approved in 2015 for the treatment of adult patients with primary hypercholesterolemia or primary mixed dyslipidemia, and as secondary prevention in combination with statins or a statin and another lipid-lowering agent or in monotherapy in patients with intolerance or contraindication to statins. In 2018, its indication was extended to adult patients with atherosclerotic cardiovascular disease (ASCVD).18 The Spanish Society of Arteriosclerosis (SEA in Spanish) recommends starting treatment with evolocumab when thresholds vary between 70mg/dL and 160mg/dL according to the subgroup of patients.19 In patients with coronary artery disease (CAD), treatment with evolocumab is recommended as secondary prevention in patients with LDL-C levels>70mg/dL, whereas in patients with familial hypercholesterolemia (FH) with less than four associated risk factors, evolocumab is recommended when LDL-C level is >160mg/dL.19 Therefore, in Spain, the conditions for evolocumab prescription are different to the 2019 ESC/EAS guidelines.18
Treatment persistence (taking the medication for the prescribed period) and treatment adherence (complying with drug schedules and dosage) are both important for an optimum LDL-C control. Currently, rates of adherence to lipid-lowering medications and treatment persistence are suboptimal in Spain.20 According to the REPAR study, treatment dropout rates in Spanish patients depend on the drug used. Statins (28.9%) and ezetimibe (33%) had lower rates of treatment discontinuation compared with bile acid sequestrants (68.3%), niacin (55.4%) or fibrates (39.9%).17 Additional data on persistence with evolocumab in Spanish clinical practice are required.
Gaining an understanding of patient characteristics is essential to achieving LDL-C goals and improving adherence and persistence to medication is critical to reducing the CV risk. For this purpose, here we describe the clinical characteristics, treatment patterns, control of LDL-C levels, and the persistence to treatment with evolocumab with 30-months of follow-up in the Spanish cohort of the HEYMANS study.
Materials and methodsStudy population and designHEYMANS (cHaractEristics of hYperlipidaeMic pAtieNts) is a multi-country, multicenter, prospective observational study of European patients initiating evolocumab as part of their routine clinical management, based on local reimbursement criteria.12,13 A total of 1951 patients were enrolled from 12 countries: Austria, Belgium, Bulgaria, Czech Republic, Germany, Greece, Italy, Portugal, Slovakia, Spain, Sweden, and Switzerland. Here we report on data from the Spanish cohort of this study. The baseline period included data collected 6 months before evolocumab initiation. Data were then collected up to 30 months thereafter. The index date was defined as the date of the first dose of evolocumab received as part of routine clinical practice. The study was originally designed with a follow-up period of up to 12 months. However, the follow-up period was extended to 30 months following the protocol amendment of February 2018. Patients yet to complete 12 months of follow-up at the date of the protocol amendment were followed-up for up to 30 months.12 Data were collected on baseline characteristics, CV risk factors and LLT use at the time of initiation of evolocumab and at the end of each 3-month period. LDL-C level measurements were collected as per clinical practice.
Adults aged 18 years or older who had initiated evolocumab as part of clinical management after 1 August 2015 were included in the HEYMANS study. Patients who were enrolled in a PCSK9i interventional study or who had received a commercially available PCSK9i within 12 weeks before evolocumab initiation were excluded from the study.
The study was performed in accordance with ethical principles that had their origin in the Declaration of Helsinki and were consistent with the International Council for Harmonization. The study was reviewed by an independent ethics committee. All patients signed the informed consent form.
OutcomesClinical characteristics of patients recorded at initiation of evolocumab included demographics, laboratory values, data on other LLTs and comorbidities. Comorbidities were defined by national criteria among sites following the 2019 ESC/EAS guidelines.21 Statin intolerance was determined through history of muscle-related or non-muscle-related intolerance to any statin and through patient-reported symptoms, at the discretion of the investigator. LDL-C measurements were collected as per clinical practice. LDL-C levels during each 3-month window from baseline and during the 30-month follow-up were calculated. The proportion of patients achieving their risk-based LDL-C goals (LDL-C levels<55mg/dL; LDL-C levels<70mg/dL; and LDL-C levels≥50% lowering) at least once during the entire follow-up, was calculated. The proportion of patients continuing treatment with evolocumab and remaining in the study at specified time points was estimated to determine treatment persistence. After baseline, the percentages are based on patients with available evolocumab intake data during the previous 3 months. Although treatment adverse events and fatal adverse events were reported, no formal safety analysis was planned for this study.
Statistical analysisData were presented descriptively using summary statistics, including percentage for categorical data, and mean with standard deviation (SD) or median [interquartile range (Q1–Q3)] for continuous data. Summaries are based on observed data, with no imputation applied for patients who did not have data at specific time points. For assessment of attained LDL-C goals, patients were included in the analysis if they had at least one post-baseline LDL-C value and were classified as high or very high-risk according to the 2019 ESC/EAS guideline.7 All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
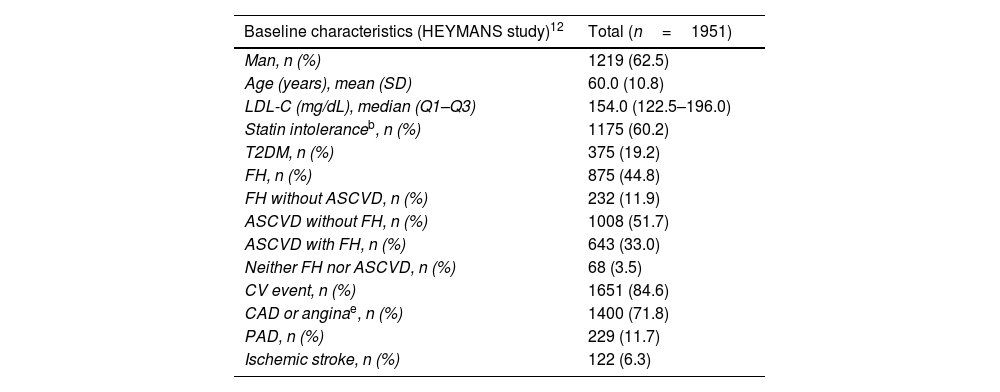
ResultsPatient characteristicsPatient characteristics are depicted in Table 1. A total of 201 adult patients were included in the Spanish cohort from March 2016 to September 2019. The median (Q1–Q3) follow-up was 30.0 (12–30) months. Of these patients, 61.7% were men and the mean (SD) age was 59.5 (10.8) years. Overall, 57.7% of patients were receiving treatment with statins at baseline, most of them with high-intensity statins (85.3%). Generally, the percentage of patients using background LLT did not change substantially over time. A total of 45.8% of patients were intolerant to statins, and 26.4% of patients did not receive any LLT. This high percentage of patients intolerant to statins is probably due to the fact that these patients had no other therapeutic alternative and, therefore, were prescribed evolocumab. Most patients (68.7%) had experienced a prior CV event, 45.3% had CAD or stable angina, and 60.2% had a diagnosis of FH (26.4% of them without ASCVD). More than half of patients had a diagnosis of ASCVD: 34.8% without FH and 33.8% with FH. Spanish patient characteristics are consistent with the data from the patients included in the overall HEYMANS study (Table 1).
Baseline characteristics of the total population included in the HEYMANS study and of the Spanish cohort.
| Baseline characteristics (HEYMANS study)12 | Total (n=1951) |
|---|---|
| Man, n (%) | 1219 (62.5) |
| Age (years), mean (SD) | 60.0 (10.8) |
| LDL-C (mg/dL), median (Q1–Q3) | 154.0 (122.5–196.0) |
| Statin intoleranceb, n (%) | 1175 (60.2) |
| T2DM, n (%) | 375 (19.2) |
| FH, n (%) | 875 (44.8) |
| FH without ASCVD, n (%) | 232 (11.9) |
| ASCVD without FH, n (%) | 1008 (51.7) |
| ASCVD with FH, n (%) | 643 (33.0) |
| Neither FH nor ASCVD, n (%) | 68 (3.5) |
| CV event, n (%) | 1651 (84.6) |
| CAD or anginae, n (%) | 1400 (71.8) |
| PAD, n (%) | 229 (11.7) |
| Ischemic stroke, n (%) | 122 (6.3) |
| Baseline characteristics in the Spanish cohort | Total (n=201) |
|---|---|
| Man, n (%) | 124 (61.7) |
| Age (years), mean (SD) | 59.5 (10.8) |
| LDL-C (mg/dL), median (Q1–Q3) | 151.0 (123.0–196.8) |
| Patients without LLTa, n (%) | 53 (26.4) |
| Patients with LLTa, n (%) | 148 (73.6) |
| Patients receiving treatment with statinsa, n (%) | 116 (57.7) |
| High-intensity statins | 99 (85.3)c |
| Moderate-intensity statins | 9 (7.8)c |
| Low-intensity statins | 4 (3.4)c |
| Unknown intensity statins | 4 (3.4)c |
| Statin intoleranceb, n (%) | 92 (45.8) |
| T2DM, n (%) | 32 (15.9) |
| FH, n (%) | 121 (60.2) |
| FH without ASCVD, n (%) | 53 (26.4) |
| ASCVD without FH, n (%) | 70 (34.8) |
| ASCVD with FH, n (%) | 68 (33.8) |
| Neither FH nor ASCVDd, n (%) | 10 (5.0) |
| CV event, n (%) | 138 (68.7) |
| CAD or anginae, n (%) | 91 (45.3) |
| PAD, n (%) | 16 (8.0) |
| Cerebrovascular disease, n (%) | 44 (21.9) |
| Ischemic stroke, n (%) | 18 (9.0) |
ASCVD, atherosclerotic cardiovascular disease; AVD, atherosclerotic vascular disease; CAD, coronary artery disease; CV, cardiovascular; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; PAD, peripheral artery disease; SD, standard deviation; T2DM, type 2 diabetes mellitus.
At baseline, the median (Q1–Q3) LDL-C level was 151 (123–197) mg/dL. At 3 months of treatment, baseline LDL-C decreased by 66% to a median of 50 (30–83) mg/dL and was maintained over time, with a median LDL-C of 55 (40–99) mg/dL at 30 months (Fig. 1).
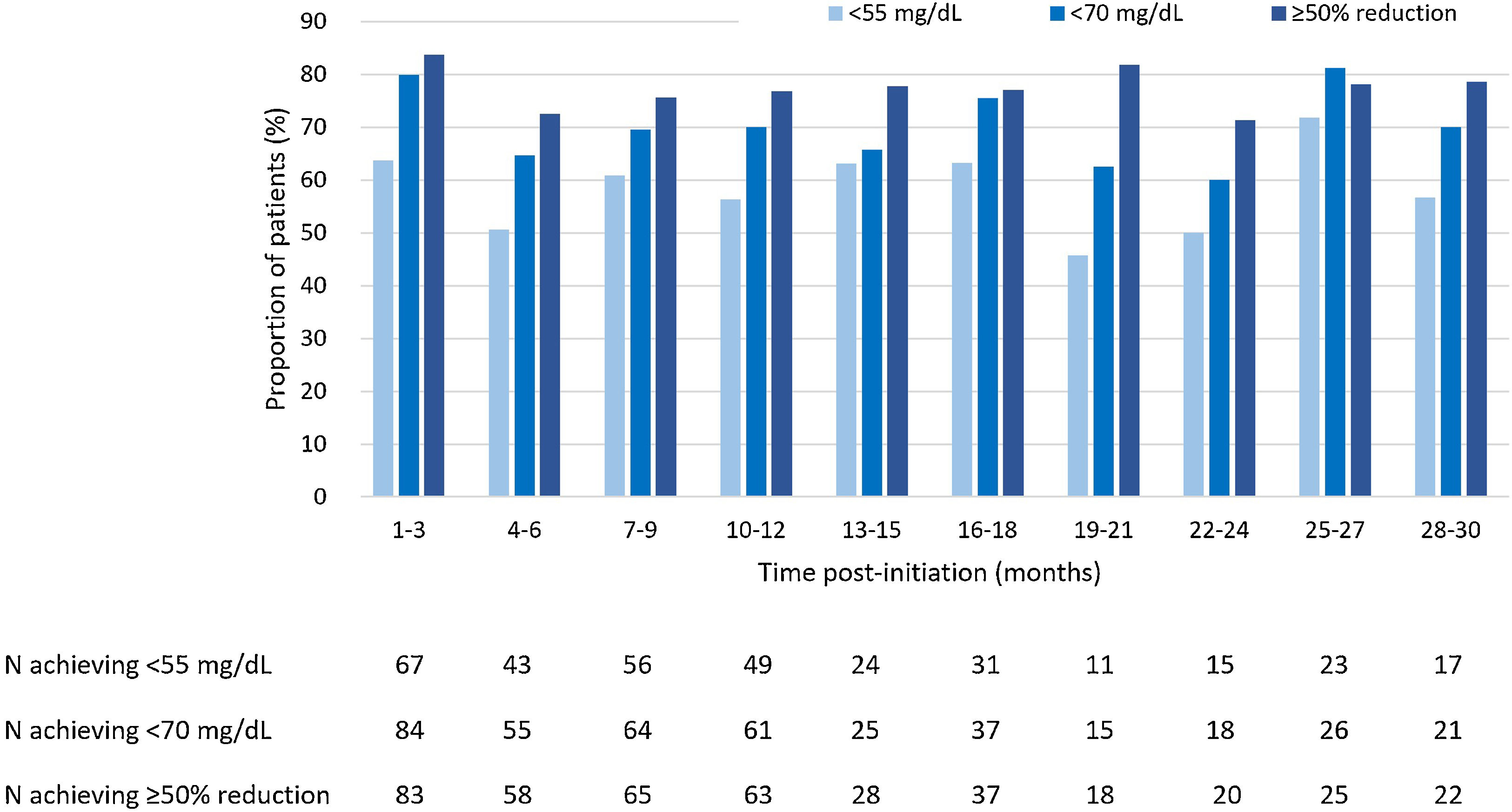
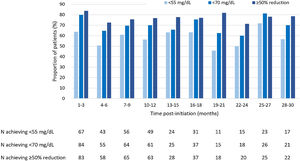
LDL-C goals achievementAt 3 months of treatment with evolocumab, 63.8% of patients that received LLT at baseline (n=148) achieved LDL-C levels<55mg/dL, and 56.3% of patients achieved it at months 10–12 of treatment. At 30 months of treatment, LDL-C levels<55mg/dL were achieved by 56.7% of patients receiving LLT (Fig. 2). Regarding patients receiving evolocumab without LLT at baseline (n=53), 27.3% of them achieved LDL-C levels<55mg/dL at 3 months of treatment, 33.3% of them at 10–12 months, and 20.0% at 30 months.
Among patients that received LLT at baseline, LDL-C levels<70mg/dL were achieved by 80.0% at 3 months of evolocumab treatment, by 70.1% of patients at months 10–12 and by 70.0% of patients at 30 months (Fig. 2). Whereas, among patients without LLT at baseline, LDL-C levels<70mg/dL were achieved by 30.3% at 3 months of treatment, by 45.8% of patients at 10–12 months and by 40.0% of patients at 30 months of treatment.
Moreover, of patients receiving baseline LLT, a ≥50% reduction in LDL-C levels was achieved by 83.8% at 3 months, by 76.8% at months 10–12 and by 78.6% of patients at 30 months (Fig. 2). As for patients without LLT at baseline, 46.7% of them achieved a ≥50% reduction in LDL-C levels at 3 months of treatment, 68.2% of them at 10–12 months of treatment, and 40.0% of them at 30 months of treatment.
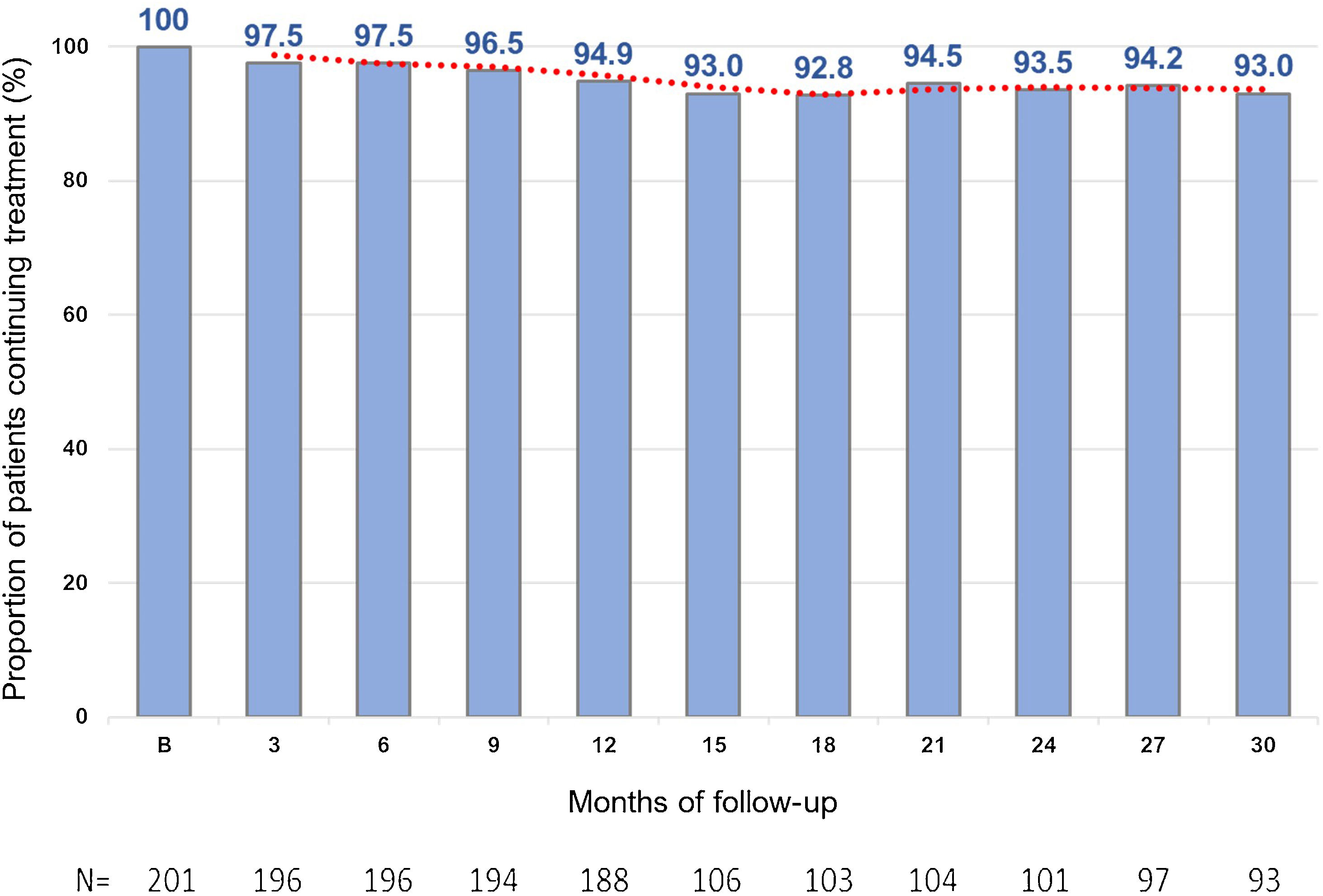
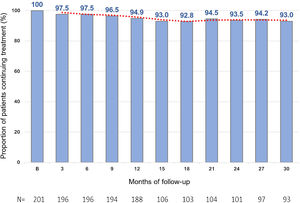
Treatment persistenceThe percentage of patients remaining on evolocumab treatment was 94.9% at month 12 and 93.0% at month 30 (Fig. 3).
DiscussionInformation from clinical practice provides invaluable real-world data on the characteristics and health conditions of patients with high levels of LDL-C and on the use of available therapeutic resources, as prescribed per current reimbursement criteria. The HEYMANS study, which is representative of routine clinical practice, provides a unique opportunity to assess clinical characteristics, treatment patterns, LDL-C levels and persistence to therapy in patients receiving evolocumab over a 30-month follow-up period in a Spanish cohort.
The 2019 ESC/EAS guidelines recommend the use of PCSK9i as a secondary prevention when the LDL-C goals of <55mg/dL are not met in patients at very-high risk despite receiving the maximum tolerated statin and ezetimibe therapy doses, or when the LDL-C goals of <40mg/dL are not met in patients who experienced a second CV event within 2 years of treatment.7 On the other hand, SEA recommends evolocumab as secondary prevention at thresholds starting at values between 70 and 160mg/dL according to the subgroup of patients, reflecting the different LDL-C thresholds mandated for reimbursement.18,19 Therefore, in Spanish clinical practice, the 2019 ESC/EAS guideline recommended thresholds at which evolocumab should be initiated are not usually met. In this study, patients had median (Q1–Q3) LDL-C levels of 151 (123–197) mg/dL at baseline, LDL-C levels more than 50% higher than that recommended by SEA19 and the Therapeutic Positioning Report (TPR), issued by the Spanish Agency of Medicines and Medical Devices (AEMPS)18 for evolocumab initiation. Furthermore, these values were almost three time higher than the 2019 ESC/EAS goals.7 This disparity between the guidelines and the reimbursement thresholds was also observed in the overall HEYMANS study when assessing the full dataset across 12 European countries and also reflecting the fact that reimbursement thresholds were much higher than those recommended in clinical guidelines.12,13 Even so, evolocumab was associated with a reduction in LDL-C levels of about 60% from baseline to 3 months of treatment. This reduction maintained over the study. Our results obtained from Spanish clinical practice support previous observations from clinical trials.1,22
It is worth highlighting the importance of maintaining background medication and not modifying it when introducing evolocumab, since we showed that achievement of LDL-C goals improves when used in combination with LLT treatment.12,13 In our Spanish cohort, 57.7% of patients received LLT with statins at the start of evolocumab treatment and a high percentage of these patients achieved LDL-C levels<55 and <70mg/dL and a reduction of LDL-C levels ≥50% during follow-up. LDL-C goals were already achieved in a high proportion of patients after 3 months of treatment. At months 10–12 of treatment, 56.3% of patients receiving LLT at baseline achieved LDL-C levels<55mg/dL, and 70.1% achieved LDL-C levels<70mg/dL. Moreover, 76.8% of patients achieved a reduction of LDL-C ≥50% at months 10–12. Our results are in line with other clinical studies demonstrating that achievement of LDL-C goals for those at very-high risk is increased when PCSK9i are used in combination with oral LLT.13,15 In DA VINCI study, which described the contemporary use of LLT and goal attainment in patients across 18 European countries,15 between 7% and 29% of patients receiving low to high-intensity statins achieved the 2019 ESC/EAS LDL-C goals.7 The percentage of patients achieving the 2019 ESC/EAS LDL-C goals reached 21% of patients when receiving combined therapy with ezetimibe and 33% of patients when receiving PCSK9i. As was concluded in the DA VINCI study, gaps between clinical guidelines and clinical practice for lipid management across Europe were exacerbated by the introduction of the stricter 2019 ESC/EAS guidelines. Therefore, greater utilization of non-statin LLT is needed to reduce these gaps for patients at highest risk.15
In our Spanish cohort, 68.7% had experienced a prior CV event and 45.3% had a medical history of CAD or stable angina. Hence, more than half of patients were undertreated. Moreover, 33.8% of patients had a diagnosis of ASCVD with FH and 34.8% of patients had a diagnosis of ASCVD without FH. These clinical characteristics are not accidental and are primarily based on the recommended criteria for the use of PCSK9i in Spain: very-high LDL-C levels in primary prevention or those who had already suffered an CV event and patients with diagnosis of ASVD with or without FH.7,18,19 The fact that patients with these clinical characteristics, who had exhausted all other therapeutic options to achieve LDL-C goals, have managed to further reduce their LDL-C levels with evolocumab shows that LLT therapy combined with PCSK9i is practically the only option to currently achieve the 2019 ESC/EAS goals.
It is probable that the sustained effects on LDL-C levels observed in this study are in part due to the high rates of persistence with evolocumab. In this Spanish cohort, not only was the effectiveness of treatment with evolocumab very good, but persistence to evolocumab treatment was excellent, with 94.9% of patients continuing treatment at months 10–12 (188 out of 201 patients) and 93% of patients at month 30 (93 out of 100 evaluable patients). Other real-world data have also shown persistence with PCSK9i to be high.23,24 In a recent study in a Spanish FH population, persistence to PCSK9i treatment after a median of 3.7 years was over 95%.25 In the overall analysis of the HEYMANS study, evolocumab therapy was associated with sustained LDL-C level reductions for up to 30 months, and persistence with evolocumab remained high, both at 12 months and 30 months of follow-up.12 Conversely, results of other previous studies have shown a low adherence to and persistence with some oral LLTs, such as statins, which could potentially lead to an increase in CV risk.26 Moreover, self-administration of evolocumab has been shown to be both feasible and acceptable in the at-home setting,27 with high treatment persistence.23
These results suggest that, to achieve the 2019 ESC/EAS LDL-C goals, a greater use of combination therapies is required. For this purpose, the TPR-recommended thresholds for PCSK9i initiation would need to be lowered so that more patients would be eligible to receive combination therapy and, thus, would be more likely to achieve the 2019 ESC/EAS LDL-C goals. These results emphasize the importance of real-world evidence to support guideline development and potential changes to reimbursement criteria.
Due to the observational nature, limitations of this study include potential misclassifications of data. Moreover, patients initiating evolocumab at least 6 months before enrolment could have been more likely to continue with their treatment than those who initiated treatment closer to the enrolment date. Thus, the high persistence observed could be slightly overestimated. An additional limitation is that participating centers could be motivated healthcare professionals who could be likely to encourage persistence with treatment. Therefore, it is possible that the high persistence with evolocumab observed in this study may reflect a slightly better than average scenario. Nevertheless, our results are similar to those of previous studies on evolocumab persistence.23,24 Despite these limitations, this study provides valuable information for the clinical practice on efficacy and persistence of long term (30 months) evolocumab treatment in patients with high and very-high LDL-C levels.
ConclusionsTo sum up, in Spanish routine clinical practice, treatment with evolocumab was started in patients with LDL-C levels 50% higher than those recommended by SEA19 and the TPR.18 The reductions in LDL-C levels observed in this study were consistent with those reported in previous studies1,10,15,22,28 and were sustained during follow-up. The probability of achieving the 2019 ESC/EAS LDL-C goals7 would improve with combination therapy and also with a lower LDL-C threshold when starting evolocumab. Persistence to treatment with evolocumab remained high during follow-up, with a very low percentage of discontinuation.
Funding sourceThis publication was funded by Amgen (Europe) GmbH, Rotkreuz, Switzerland.
Conflict of interestKKR reports grants/personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cargene, Daiichi Sankyo, Esperion, Kowa, Lilly, New Amsterdam Pharma, Novartis, Pfizer, Sanofi-Regeneron, Silence Therapeutics and Scribe Therapeutics. NPG reports grants/personal fees from Sanofi, Amgen, and Servier. IB is employee and stockholder of Amgen Ltd, ND is employee of Amgen Inc. and SG is employee of Amgen S.A. All other authors have no conflicts of interest to declare except being researchers of the HEYMANS study.
The authors would like to thank Alina Gavrus Ion, PhD, from TFS HealthScience for editorial assistance and writing support.