Reduction of cardiovascular events in patients with hypertriglyceridaemia
More infoIcosapent ethyl, a highly purified ester of eicosapentoic acid, is the only omega-3 fatty acid authorized by the European Medicines Agency to reduce the risk of cardiovascular events in people at risk, treated with statins and with triglyceridemia ≥150 mg/dL. This authorization comes as a consequence of the clinical benefit observed in the "Reduction of Cardiovascular Events with Icosapent Ethyl Intervention Trial", in which icosapent ethyl demonstrated - compared to placebo - a 25% reduction in the relative risk of cardiovascular morbidity and mortality, a result consistent and independent of other variables in prespecified analyses and hypothesis generating in post-hoc analyses of several patient profiles. Although the mechanism of action for such benefit is not definitively established, it is known that it acts at different levels in the continuum of atherosclerotic cardiovascular disease (lipid-lowering, vascular endothelium and membrane protection, anti-inflammatory, atherosclerotic plaque stabilizing and antithrombotic effects) and that final anti-atherosclerotic action in the coronary territory has been demonstrated in the study “Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy”.
Icosapento de etilo, un éster del ácido eicosapentoico altamente purificado, es el único omega-3 autorizado por la Agencia Europea de Medicamentos para reducir el riesgo de episodios cardiovasculares en personas en riesgo, tratadas con estatinas y trigliceridemia ≥ 150 mg/dL. Tal autorización viene como consecuencia del beneficio clínico observado en el ensayo “Reduction of Cardiovascular Events with Icosapent Ethyl Intervention Trial”, en donde icosapento de etilo demostró -frente a placebo- un descenso del 25% en el riesgo relativo de morbimortalidad cardiovascular, resultado consistente e independiente de otras variables en análisis preespecificados y generador de hipótesis en los análisis post-hoc de varios perfiles de pacientes. Aunque el mecanismo de acción para tal beneficio no está definitivamente, se sabe que actúa a distintos niveles en el continuum de la enfermedad cardiovascular aterosclerótica (efecto hipolipemiante, protector de membranas y endotelio vascular, antiinflamatorio, estabilizador de placa aterosclerótica y antitrombótico) y esa acción final antiaterosclerótica en territorio coronario ha sido demostrada en el estudio “Effect of Vascepa on Improving Coronary Atherosclerosis in PeopleWith High Triglycerides Taking Statin Therapy”.
Icosapent ethyl (IPE) is a prodrug obtained from fish oil. Specifically, it is a highly purified (99.99%) and stable1 ethyl ester of eicosapentaenoic acid or EPA (an omega-3 with 20 carbon atoms) that does not contain docosahexaenoic acid (DHA), a 22-carbon omega-3. Currently, and based on the favourable results of the REDUCE-IT study (Reduction of Cardiovascular Events with Icosapent Ethyl Intervention Trial),2 IPE is the only omega-3 authorised by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to reduce the risk of cardiovascular events in people at risk, treated with statins, and with triglyceridaemia ≥150 mg/dl. In this chapter we will review the efficacy and safety of IPE in the REDUCE-IT trial and its anti-atherosclerotic effect demonstrated in the EVAPORATE3 study (Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy), previously analysing the various mechanisms of action studied and postulated as mediators of such clinical benefit.
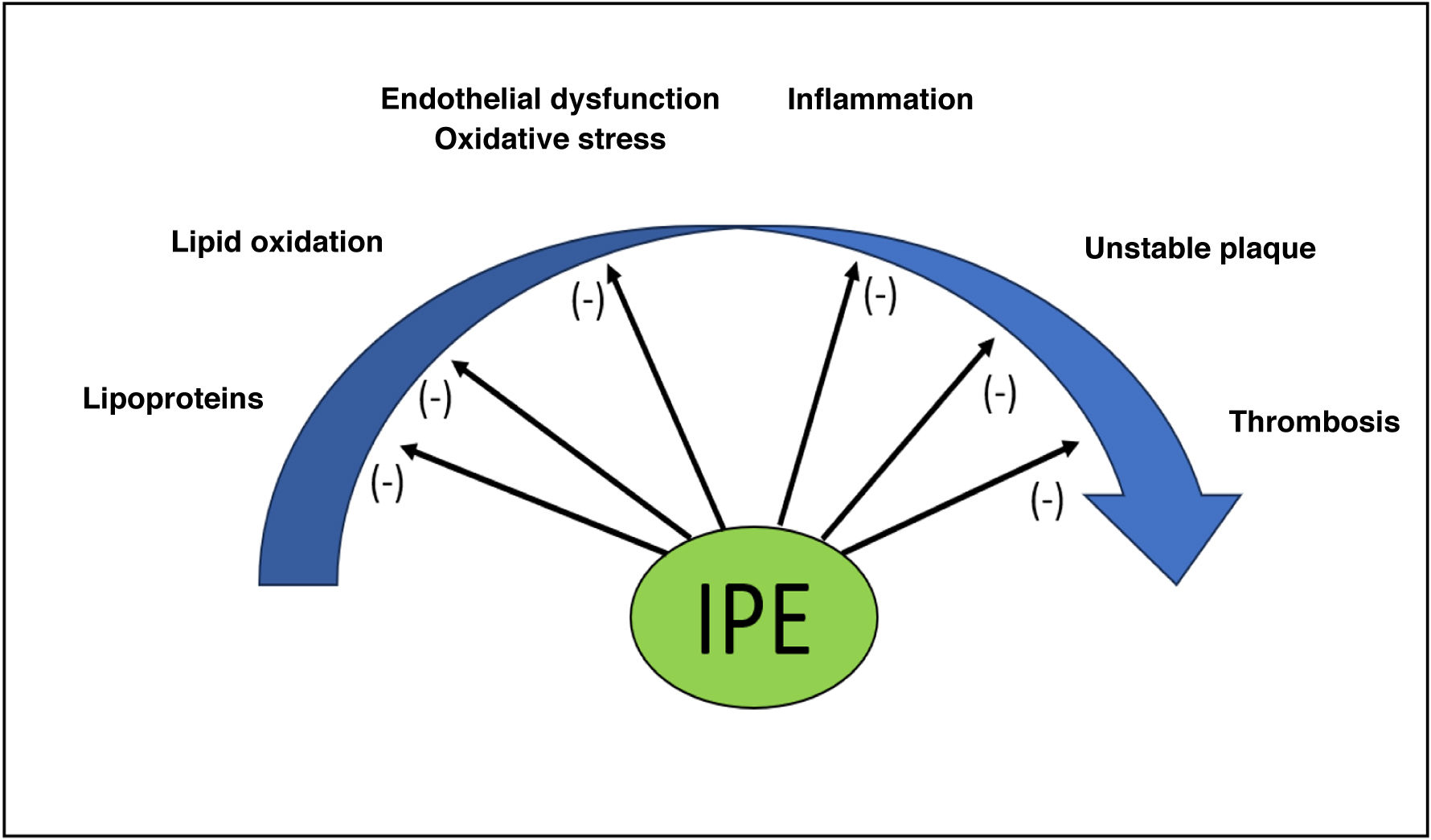
Icosapent ethyl in the atherosclerotic cardiovascular disease (ASCVD) continuumAlthough its beneficial effect on cardiovascular health is not definitively clarified, IPE may act at different levels in the continuum of atherosclerotic plaque development and its clinical complications (Fig. 1):
- •
Effect on lipoprotein metabolism: Both EPA and DHA reduce triglyceride (TGL) synthesis and promote their oxidation in the liver, although EPA is more potent than DHA in inhibiting the rate-limiting enzyme diacylglycerol acyltransferase.4 Furthermore, both molecules limit the incorporation of TGL into developing very low-density lipoproteins (VLDL), resulting in a smaller number and size of these particles, which in turn will produce larger low-density lipoproteins (LDL). Furthermore, at the circulatory level, IPE and DHA promote VLDL clearance by activating lipoprotein lipase (LPL).5 The final result is a reduction in plasma TGLs, non-HDL cholesterol, and lipoproteins containing apolipoprotein B (ApoB), which is more marked with EPA than with DHA. In turn, this reduction does not increase LDL-bound cholesterol (LDL-C)6,7 and limits the formation of oxidised LDL8 and other lipoprotein particles, as some experimental studies have shown.9 EPA also more significantly promotes the antioxidant and anti-inflammatory function of high-density lipoproteins (HDL) and the efflux of cholesterol into these particles from macrophage.10
- •
Action on vascular membranes and endothelium: given its chemical structure, it can displace arachidonic acid (AA) from cellular membrane phospholipids and modify their physical properties, acting, unlike DHA, as a potent antioxidant at this level and promoting membrane stabilisation under conditions of oxidative stress.11 Thus, IPE protects against damage caused by oxidative stress, improves endothelial function (increasing endothelial nitric oxide synthase or eNO) and vascular function (via prostacyclin), and inhibits monocyte migration and the conversion of macrophages to foam cells in early atherosclerotic lesions.12,13
- •
Anti-inflammatory effect: EPA and DHA, by competing with omega-6 for the cyclooxygenase (COX) enzyme, promote the formation of metabolites such as thromboxane A3 and prostacyclin I3 and limit the formation of leukotriene B4 (LTB4), thromboxane A2 (TXA2), and prostaglandin E2 (PGE2), molecules that promote inflammation and thrombosis.14 Furthermore, their metabolism produces resolvins, maresmins, and protectins. These metabolites—especially resolvins— can limit chronic inflammation and the activation of cells in the adaptive immune system.15,16
- •
Atherosclerotic plaque stabilisation: EPA and its metabolites tend to localise in plaques at risk of rupture, where they act on factors that promote atherosclerotic plaque stabilisation and minimise the risk of rupture by leading to fewer foam cells, T cells, and inflammatory markers. This effect is amplified when combined with statins.17–19
- •
Antithrombotic effect: IPE reduces thrombus formation by inhibiting platelet aggregation, probably through the action of thromboxane A3 and prostacyclin I3 and reduced TXA production.20
The REDUCE-IT2 trial was a phase 3, multicentre, double-blind, randomised, placebo-controlled study designed to determine whether IPE administration (2 g/12 h with food) was better than placebo (mineral oil) in reducing the so-called MACE-5 (composite of cardiovascular death, non-fatal acute myocardial infarction (AMI), non-fatal stroke, coronary revascularisation, and unstable angina). For this purpose, 8,179 participants (median age 64 years, 28.8% women) aged ≥ 45 years with established ASCVD (70.7%) or ≥ 50 years with diabetes mellitus (DM) and at least one additional cardiovascular risk factor (29.3%) were included, who were being treated with statins and who had hypertriglyceridaemia (135−499 mg/dl) and a fasting LDL-C between 41−100 mg/dl. At 1 year after the start of the study, subjects randomised to IPE had a median change in plasma triglycerides of −19.7% (−44.5 mg/dl; p < .001) and in non-HDL cholesterol of −15.5% (−13.1 mg/dl; p < .001) compared to placebo, as well as a smaller increase in LDL-C (3.1% versus 10.2%; p < .001). Similarly, at 2 years the median change in ApoB levels ((–2 versus 7.8%; difference –9.7%) and ultrasensitive CRP (–13.9 versus 32.3%; difference –39.79%) were also significantly higher (p < .001 in both cases). Overall, in the intention-to-treat analysis and after a median follow-up of 4.9 years (maximum 6.2 years), there was a statistically significant 25% reduction in the relative risk (RR) of the primary endpoint compared to placebo (RR: 17.2% versus 22%; HR: .25; 95% CI: .68–.83; p < .001), which translated into an absolute risk (AR) reduction of 4.8% and a number needed to treat (NNT) of 21 to prevent a primary cardiovascular event. The results were consistent and independent of sex, age, presence or absence of ASCVD or DM, type of statin received, or baseline or study level of plasma triglycerides or LDL-C, among other pre-specified analyses.
Regarding safety, there were no significant differences between the randomisation groups in the overall rate of adverse events or serious adverse events leading to discontinuation of the study drug. Specifically, participants randomised to PEI had significantly higher rates of atrial fibrillation (AF) (5.3% versus 3.9%), AF-related hospitalisation or flutter (3.1% versus 2.1%; p = .004), and peripheral oedema (6.5% versus 5%) than those assigned to placebo. Similarly, there was a higher, although not significant, incidence of non-fatal severe bleeding (2.7 versus 2.1%; p = .06), haemorrhagic stroke (.3 versus .2%; p = .42), or gastrointestinal bleeding (1.5 versus 1.1%; p = .15) among those assigned to IPE.
The results of the REDUCE-IT study have been controversial when compared with those of the STRENGTH trial,21 in which a combination of omega-3 (EPA + DHA) at a dose of 4 g/day was not superior to placebo (corn oil) in reducing MACE-5. Some authors have suggested that part of the benefit observed in REDUCE-IT could have been due to the use of mineral oil as a placebo, stating its ability to inhibit statin absorption and increase lipoproteins and inflammatory markers.22 It is known, however, that such benefit was directly correlated with plasma levels of IPE, which were much higher in subjects assigned to active treatment in REDUCE-IT than in STRENGTH (144 and 90 μg/mL, respectively). Furthermore, a detailed analysis of the literature including different clinical trials in which mineral oil was used as a placebo concluded that at the dose used it is a biologically inert substance, with no systemic effects.23 This fact has been ratified by regulatory agencies (FDA, EMA and others) considering that any effect of mineral oil on cardiovascular events is clinically insignificant.24
Subgroup analysis in REDUCE-IT: generating hypothesesA clinical trial with the included population and favourable results such as those of REDUCE-IT has enable post-hoc analyses of numerous patient profiles. These analyses have led to the generation of highly attractive hypotheses regarding the beneficial effect of IPE in reducing cardiovascular morbidity and mortality in these groups. The results are summarised in Table 1.25–28 The efficacy results have been equally consistent regardless of LDL-C levels (<55 mg/dL or >55 mg/dL),29 lipoprotein (a) levels (<50 mg/dL or > 50 mg/dL),30 or renal function.31
Subgroup analysis of the REDUCE-IT trial.
| Subgroup analysis | Population in REDUCE-IT, n (%) | Baseline LDL-C (mg/dl) | Baseline TGL (mg/dl) | RRR MACE-5 | ARR MACE-5 | NNT | RRR MACE-3 | RRR CVM |
|---|---|---|---|---|---|---|---|---|
| Previous AMI | 3,693 (45.2%) | 75 | 220 | 26% | 5.9% | 17 | 29% | 30% |
| ACS < 12 m | 840 (10.3%) | 219 | 37% | 9.3% | 11 | 36% | ||
| CABG | 1,837 (22.5%) | 75 | 221.5 | 24% | 6.2% | 16 | 31% | 29% (NS) |
| PCI | 3,408 (41.7%) | 74 | 218 | 34% | 8.5% | 12 | 34% | 36% |
ACS: acute coronary syndrome; AMI: acute myocardial infarction; ARR: absolute risk reduction; CABG: coronary artery bypass graft (CABG); LDL-C: low-density lipoprotein cholesterol; MACE-3: includes cardiovascular death, acute myocardial infarction, and stroke; MACE-5: includes cardiovascular death, acute myocardial infarction, stroke, revascularisation, and angina; MCV: cardiovascular mortality; NNT: number needed to treat; PCI: percutaneous coronary intervention; REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial; RRR: relative risk reduction; TGL: triglycerides.
These analyses have also enabled the safety of IPE to be explored in the different subgroups. Subjects who had suffered an AMI and were assigned to IPE had a higher rate of hospitalisation for AF than those assigned to placebo (3.6% versus 2.2%; log-rank P = .01) and also a higher rate of bleeding (10.6% versus 8.7%; Fisher's exact test p = .05), although there was no difference in the rate of major bleeding (2.7% versus 2.2%; Fisher's exact test p = .46) despite the frequent use of antithrombotic therapy, including dual antiplatelet therapy and anticoagulation. It is also noteworthy that in subjects with coronary syndrome of under 12 months onset assigned to IPE, there was no higher rate of bleeding (6.9% versus 8.1%; Fisher's exact test, p = .60) or major bleeding (1.6% versus 3.2%; Fisher's exact test, p = .17), despite the fact that combined antiplatelet and anticoagulant therapy was more common in the IPE group (6.2% versus 2.9%; p = .02).26 Finally, it should be noted that in patients who had undergone prior surgical coronary revascularisation, assignment to IPE was associated with a higher frequency of hospitalisation for AF or flutter (5.0% versus 3.1%; p = .03).27
Effect of icoaspent ethyl on coronary atherosclerosis: EVAPORATE trialRegardless of the unknown relative importance of the various mechanisms involved in the clinical benefit observed with IPE in REDUCE-IT, the EVAPORATE3 trial has clarified that there is a clear underlying anti-atherosclerotic component. Indeed, in this multicentre, double-blind study, 80 individuals aged 30–85 years with coronary atherosclerosis (at least 20% stenosis in a coronary artery) who were being treated with statins and who had hypertriglyceridaemia (135–499 mg/dL) and fasting LDL cholesterol (LDL-C) between 40–115 mg/dL were randomised to receive IPE (2 mg/12 h with meals) or placebo (mineral oil at the same dosage). The coronary tree was explored by multidetector computed tomography (MDCT) coronary angiography with semi-automated software for plaque analysis (QangioCT). The primary endpoint of the study was the change in low-attenuation plaque (LAP) volume at 18 months. This plaque type represented 5.1% and 6.5% of total plaque volume in the IPE and placebo groups, respectively, at baseline. Thus, although no significant changes in the lipid profile (LDL-C, HDL-C and triglycerides) were observed during follow-up between groups, a reduction in LAP volume was observed in the angiography performed at 18 months in individuals randomised to IPE compared to placebo (–.3 ± 1.5 versus .9 ± 1.7 mm3; p = .006). Similarly, in a pre-specified analysis, there were also significant changes in total plaque volume (-9 with IPE versus -11 with placebo; p = .002), total non-calcified plaque volume (-19 versus -9%; p = .0005), fibrofatty plaque (-34 versus -32%; p = .0002), and fibrous plaque volume (-20 versus 1%; p = .003), but changes in calcified plaque volume were not significant (-1 versus -15%; p = .053).
FundingThis study was sponsored by the Spanish Society of Arteriosclerosis with funding from Amarin, which did not participate in the design or preparation of this manuscript.
Supplement informationThis article is part of the supplement entitled “Reduction of cardiovascular events in patients with hypertriglyceridaemia,” which was sponsored by the Spanish Society of Atherosclerosis, with funding from Amarin.
The author has no potential conflict of interests to declare.