Although it is known that resveratrol has anti-inflammatory and anti-atherogenic actions, its effect on vascular endothelial growth factor (VEGF) in atherosclerosis is unknown.
ObjectiveTo evaluate the effect of resveratrol on serum concentrations of VEGF during the progression and evolution of atherosclerosis, as well as and its evolution over time in rabbits fed with a cholesterol diet.
Materials and methodsA total of 48 New Zealand white male rabbits were randomly divided into four groups of 12 rabbits: group 1 (control): standard diet (commercial rabbit food); group 2: cholesterol diet (0.5% cholesterol); group 3 (control resveratrol): standard diet (commercial rabbit food) and resveratrol (2mg/kg); group 4: cholesterol diet (0.5% cholesterol) and resveratrol (2mg/kg), for 12weeks. Blood samples of overnight-fasted rabbits were collected at baseline and the sixth and twelfth weeks, and the lipid profile, VEGF, and C-reactive protein (CRP) levels were determined. Half of the animals were sacrificed on the sixth or twelfth week, and the aorta was dissected for histological studies.
ResultsVEGF and CRP levels were significantly higher in groups 2 and 4 than in groups 1 and 3, respectively, from the 6th week (p<0.001). VEGF and CRP were significantly lower in group 4 than in group 2 on 12th week (p<0.004). Supplementation of resveratrol reduced the formation of atherosclerotic lesions.
ConclusionsSerum VEGF and CRP levels are early markers of atherosclerosis. Oral supplementation of resveratrol exerts anti-inflammatory and anti-atherosclerotic effects, decreasing serum concentrations of VEGF and CRP and the formation and evolution of atherosclerotic lesions.
El resveratrol tiene propiedades antiinflamatorias y antiaterogénicas; sin embargo, se desconoce su efecto sobre el factor de crecimiento endotelial vascular (VEGF) en la aterosclerosis.
ObjetivoEvaluar el efecto del resveratrol sobre las concentraciones séricas del VEGF durante la progresión y evolución de la aterosclerosis y su evolución en el tiempo en conejos alimentados con dieta enriquecida con colesterol.
Materiales y métodosCuarenta y ocho conejos machos divididos en cuatro grupos de 12 conejos recibieron: grupo 1 (control): conejarina; grupo 2: conejarina suplementada con 0,5% colesterol; grupo 3 (control resveratrol): conejarina y resveratrol (2mg/kg); grupo 4: conejarina suplementada con 0,5% colesterol y resveratrol, durante 12 semanas. Se realizaron determinaciones séricas de triglicéridos, colesterol y sus fracciones, VEGF y proteína C reactiva (PCR) al inicio, a la 6.a y a la 12.a semana de experimentación. La mitad de los conejos fueron sacrificados a la 6.a y a la 12.a semana y se realizó estudio histológico de su aorta.
ResultadosEl VEGF y la PCR aumentaron en los grupos 2 y 4 desde la 6.a semana de experimentación con respecto a los grupos 1 y 3, respectivamente (p<0,001). En la duodécima semana se observó una disminución de los niveles de VEGF y PCR en el grupo4 con respecto al grupo 2 (p<0,004). El tratamiento con resveratrol disminuyó la formación de ateromas.
ConclusionesEl VEGF y la PCR séricos constituyen marcadores tempranos no invasivos de inflamación y aterosclerosis. La suplementación oral de resveratrol ejerce efectos antiinflamatorios y antiateroscleróticos, disminuyendo las concentraciones séricas de VEGF y PCR, y la formación y evolución de las lesiones ateroscleróticas.
Resveratrol (3,5,4′-trihydroxystilbene) is a polyphenolic compound found in grapes and red wine, described as having antioxidant, anti-inflammatory, antiproliferative and antiangiogenic properties.1,2 It has been proven beneficial in preventing the onset and progression of atherosclerosis, as it inhibits the oxidation of low-density lipoproteins (LDL), suppresses platelet aggregation, inhibits the proliferation of vascular smooth muscle cells in vitro, reduces endothelial cell damage and attenuates thickening of the intima media. It also reduces the size of atherosclerotic lesions, modulates the production of nitric oxide (NO), inhibits the production of chemokines and reactive oxygen species (ROS) and controls plasma cholesterol levels; all of the above being important factors in atherogenesis.2
Inflammation and angiogenesis are involved in the pathophysiology of atherosclerosis.3 Vascular endothelial growth factor (VEGF) is a powerful growth factor produced by different cells such as endothelial and smooth muscle cells, platelets and macrophages. VEGF plays a determining role in the rate and extent of vascular remodelling.4 Its role in atherosclerosis, however, is still not fully understood. VEGF exerts dual actions and induces both beneficial and harmful effects.5,6 It can protect endothelial cells by inducing the expression of antiapoptotic proteins and the production of NO,7 but it also has pro-atherogenic effects, as it stimulates the proliferation and growth of endothelial cells, induces angiogenesis, and increases the permeability of the endothelium and the expression of cell adhesion proteins.5
Evidence has been found of an increase in serum VEGF levels in patients with coronary heart disease, myocardial infarction and atherosclerosis.4,8 An increase has also been detected in the expression of VEGF in atherosclerotic plaques in the coronary and carotid arteries,9 primarily in the deep portions of the intima of affected vessels, associated with intraplaque haemorrhage,6 suggesting that VEGF is involved in the pathogenesis of atherosclerosis. However, the effect of resveratrol on serum VEGF levels during the development and progression of atherosclerosis remains unclear, and even less is known about the relationship between VEGF and inflammation and how this evolves over the course of the atherogenic process. We therefore decided to conduct a study to analyse the effect of resveratrol on serum levels of VEGF and how they evolve over time in rabbits fed a diet enriched with cholesterol.
Materials and methodsWe used 48 male 12-week-old New Zealand strain rabbits (Laboratory Animal Facility of the Instituto de Higiene Rafael Rangel, Caracas, Venezuela) weighing from 1200 to 1300g. The animals were kept in cages under controlled light and dark (L:D, 12:12) and temperature (25±1°C) conditions. After a week of acclimatisation at the Laboratory Animal Facility, Universidad de Carabobo (Valencia, Venezuela), the rabbits were randomly divided into four groups of 12: group 1 (control), fed daily with standard diet (commercial rabbit food from Protinal, Venezuela); group 2, fed daily with a standard diet enriched with 0.5% (w/w) cholesterol; group 3 (control resveratrol), fed daily with standard diet and supplemented with resveratrol in the food (2mg/kg body weight, oral); group 4: fed daily with standard diet, enriched with 0.5% (w/w) of cholesterol and resveratrol mixed in the food (2mg/kg body weight, oral).
The cholesterol was administered by enriching the standard diet with cholesterol dissolved in ethyl ether and absolute ethanol. The grains of rabbit feed were covered with the cholesterol solution at a ratio of 0.5g of cholesterol per 100g of feed and the solvent was removed by evaporation for 24h.10 The resveratrol concentration was selected based on in vivo studies in rabbits11 and tests performed in our laboratory, in which resveratrol was found to have biological effects at a dose of 2mg/kg orally. The resveratrol (3,5,4′-trihydroxystilbene) administered to the rabbits was of natural origin.
All the rabbits had free access to drinking water. The experimental period lasted 12 weeks. The rabbits were weighed before, during and after the experimentation. The experiments were approved by the Bioethics Committee and conducted in line with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Experimental procedureBiochemical measurementsThe blood samples were extracted from all the rabbits by intracardiac puncture, after fasting for 14h, at the start and weeks 6 and 12, using tubes without anticoagulant. After being cold-stored, the samples were centrifuged at 3000rpm for 15min and the serum obtained was then stored frozen at −70°C until processing. Enzymatic methods (Linear Chemicals, Spain) were used to measure serum total cholesterol (TC) and triglycerides (TG). High-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured by precipitation and subsequent enzymatic determination (Linear Chemicals, Spain). C-reactive protein (CRP) was measured quantitatively using the immunoturbidimetric method (Alpco, USA). Serum concentrations of VEGF were determined by immunoenzyme assay (NeoBiolab, Cambridge, Massachusetts, USA).
Killing of the animalsTissue preparation and histological typing of atherosclerotic lesionsAt week 6 and at the end of the study, half the animals in each group were killed by dislocation of the neck. Once the post-mortem examination had been performed, the aortic artery was dissected for histological study. The extracted tissue samples were fixed in 10% formaldehyde in PBS for 24h and processed according to the routine technique and then stained with haematoxylin-eosin, to be studied by optical microscopy. The lesions were classified according to the American Heart Association classification system.12
Data analysisMean and standard deviation were calculated for the variables studied. We performed the Shapiro–Wilk, Kolmogorov–Smirnov and Jarque–Bera normality tests. The Kruskall–Wallis analysis was used with post hoc analysis, applying the Mann–Whitney U test to each pair of groups. We used the Spearman correlation to relate VEGF to the study variables. A p value <0.05 was considered significant. The programme GraphPad Prism version 5 was used.
ResultsLipid profile of the rabbitsThe rabbits’ serum lipid concentrations are summarised in Table 1. No statistically significant differences were found in serum concentrations of TC, HDL-C, LDL-C or TG among the groups of rabbits. There were no significant changes in TC, HDL-C, LDL-C or TG over the course of the study in groups 1 and 3. In weeks 6 and 12 of the experiment, however, there was a significant increase in the concentrations of TC, HDL-C, LDL-C and TG in groups 2 and 4 compared to their respective controls (groups 1 and 3, respectively) (p<0.0001). There were changes in serum lipid concentrations between the beginning and the end of the experiment in groups 2 and 4 (p<0.0001), but no statistically significant differences were found between the two groups in the serum concentrations of TC, HDL-C, LDL-C or TG over the course of the study (Table 1).
Lipid profile in the study rabbits.
| Groups | TC (mg/dl) | LDL-C (mg/dl) | HDL-C (mg/dl) | TG (mg/dl) |
|---|---|---|---|---|
| Baseline | ||||
| Group 1 | 69±7 | 36±8 | 29±3 | 73±7 |
| Group 2 | 69±8 | 36±6 | 28±4 | 70±8 |
| Group 3 | 70±7 | 35±7 | 26±4 | 74±7 |
| Group 4 | 71±7 | 35±6 | 26±5 | 70±7 |
| Week 6 | ||||
| Group 1 | 68±8 | 37±8 | 29±4 | 73±9 |
| Group 2 | 739±104a | 590±69a | 74±7a | 721±111a |
| Group 3 | 70±13 | 38±9 | 27±3 | 74±10 |
| Group 4 | 702±125b | 591±68b | 71±7b | 716±90b |
| Week 12 | ||||
| Group 1 | 70±7 | 37±7 | 29±4 | 72±8 |
| Group 2 | 1184±201a | 879±82a | 75±7a | 1103±315a |
| Group 3 | 64±14 | 36±8 | 25±3 | 67±11 |
| Group 4 | 1345±179b | 867±79b | 72±10b | 1136±226b |
| p valuesc | ||||
| Group 1 | 0.9436 | 0.9399 | 0.9890 | 0.9108 |
| Group 2 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Group 3 | 0.3843 | 0.6896 | 0.4564 | 0.3449 |
| Group 4 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
HDL-C: cholesterol combined with high-density lipoproteins; LDL-C: cholesterol combined with low-density lipoproteins; TC: total cholesterol; TG: triglycerides.
The results are expressed as mean±standard deviation. Significance: p<0.05.
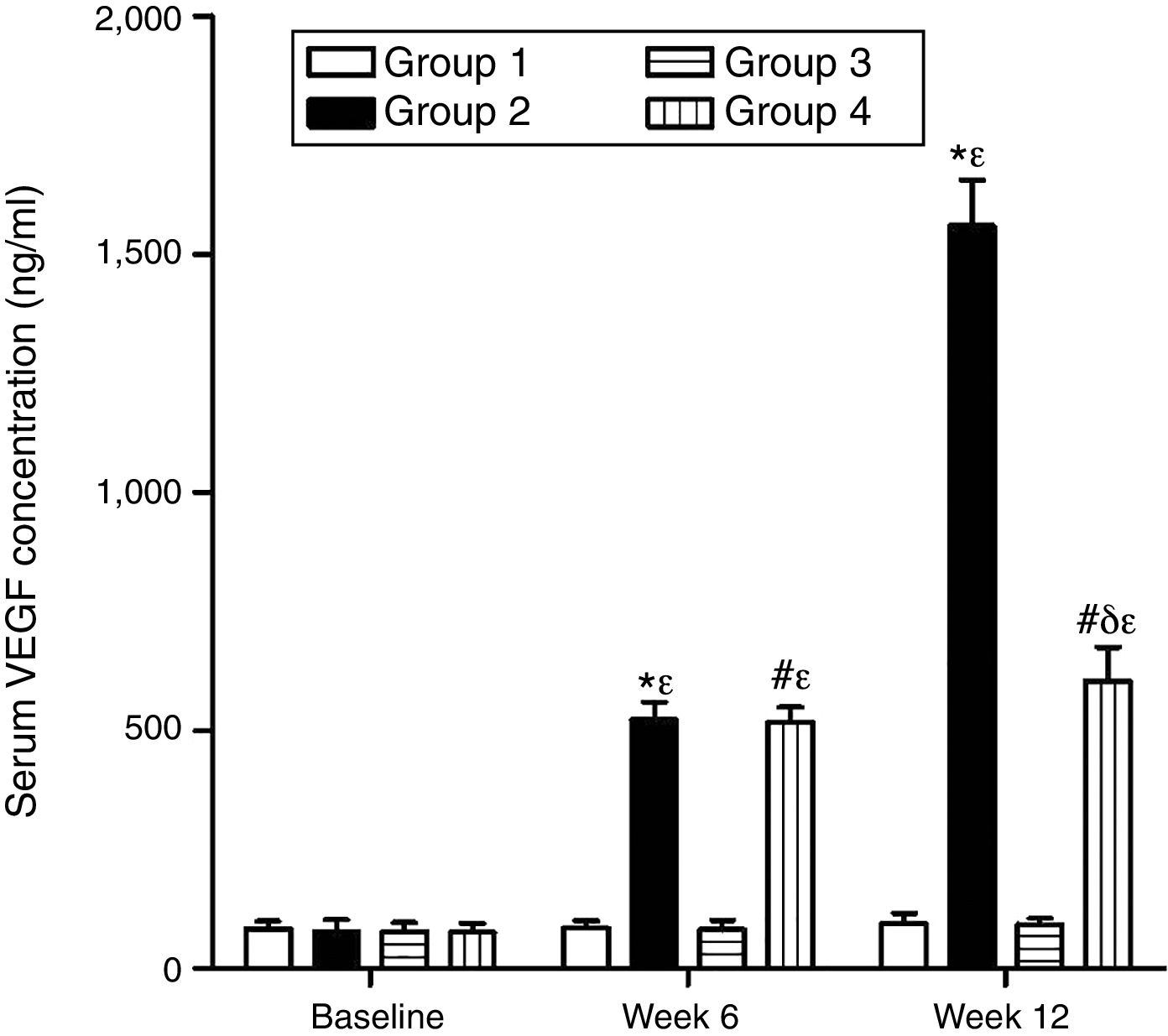
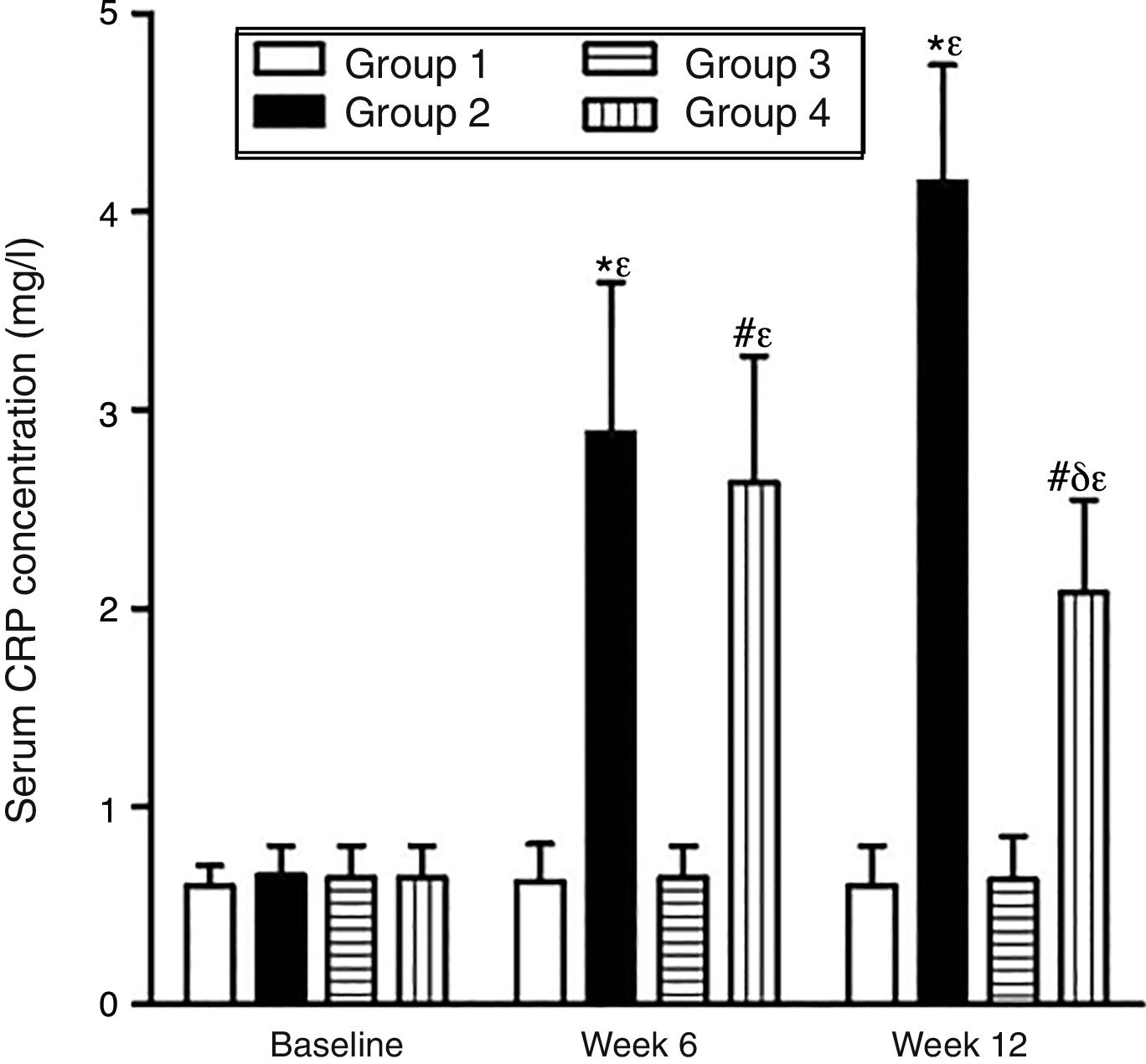
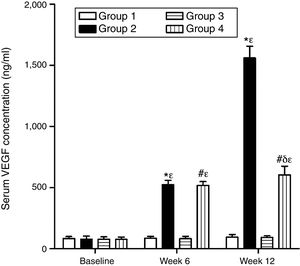
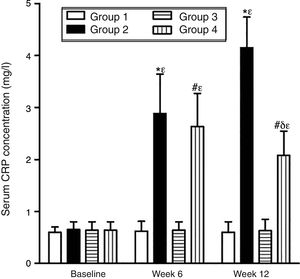
As can be seen in Figs. 1 and 2, no statistically significant differences were found among the groups of rabbits in the baseline serum concentrations of VEGF or CRP. The VEGF and CRP in groups 1 and 3 remained unchanged throughout the experiment. There were changes, however, in the levels of VEGF and CRP in groups 2 and 4 over the course of the study (p<0.0001) In week 6 and at the end of the experiment, serum concentrations of VEGF and CRP increased in group 2 compared to group 1 (p<0.0010), and in group 4 compared to group 3 (p<0.0010). However, in week 12 a significant decrease in VEGF and CRP levels was found in group 4 compared to group 2 (p<0.0049).
Effect of resveratrol on the serum concentrations of VEGF in the experimentation rabbits in weeks 0, 6 and 12. The results are expressed as mean±standard deviation (n=12 baseline and week 6; n=6 at week 12). *p<0.0001 vs. control (group 1). #p<0.0010 vs. control resveratrol (group 3). δp=0.0051 vs. group 2. ¿p<0.0001 vs. baseline value.
Effect of resveratrol on the serum concentrations of CRP in the experimentation rabbits in weeks 0, 6 and 12. The results are expressed as mean±standard deviation (n=12 baseline and week 6; n=6 at week 12). *p<0.0010 vs. control (group 1). #p<0.0010 vs. control resveratrol (group 3). δp=0.0049 vs. group 2. ¿p<0.0001 vs. baseline value.
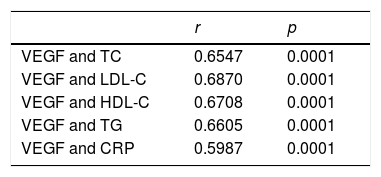
Table 2 shows Spearman's correlations between VEGF concentration, lipid profile and CRP, with a significant positive correlation being found between VEGF and the lipid profile and CRP (p<0.0001).
Analysis of the Spearman correlation between the concentrations of vascular endothelial growth factor (VEGF), C-reactive protein (CRP) and the lipid profile.
| r | p | |
|---|---|---|
| VEGF and TC | 0.6547 | 0.0001 |
| VEGF and LDL-C | 0.6870 | 0.0001 |
| VEGF and HDL-C | 0.6708 | 0.0001 |
| VEGF and TG | 0.6605 | 0.0001 |
| VEGF and CRP | 0.5987 | 0.0001 |
CRP: C-reactive protein; HDL-C: cholesterol combined with high-density lipoproteins; LDL-C: cholesterol combined with low-density lipoproteins; TC: total cholesterol; TG: triglycerides; VEGF: vascular endothelial growth factor.
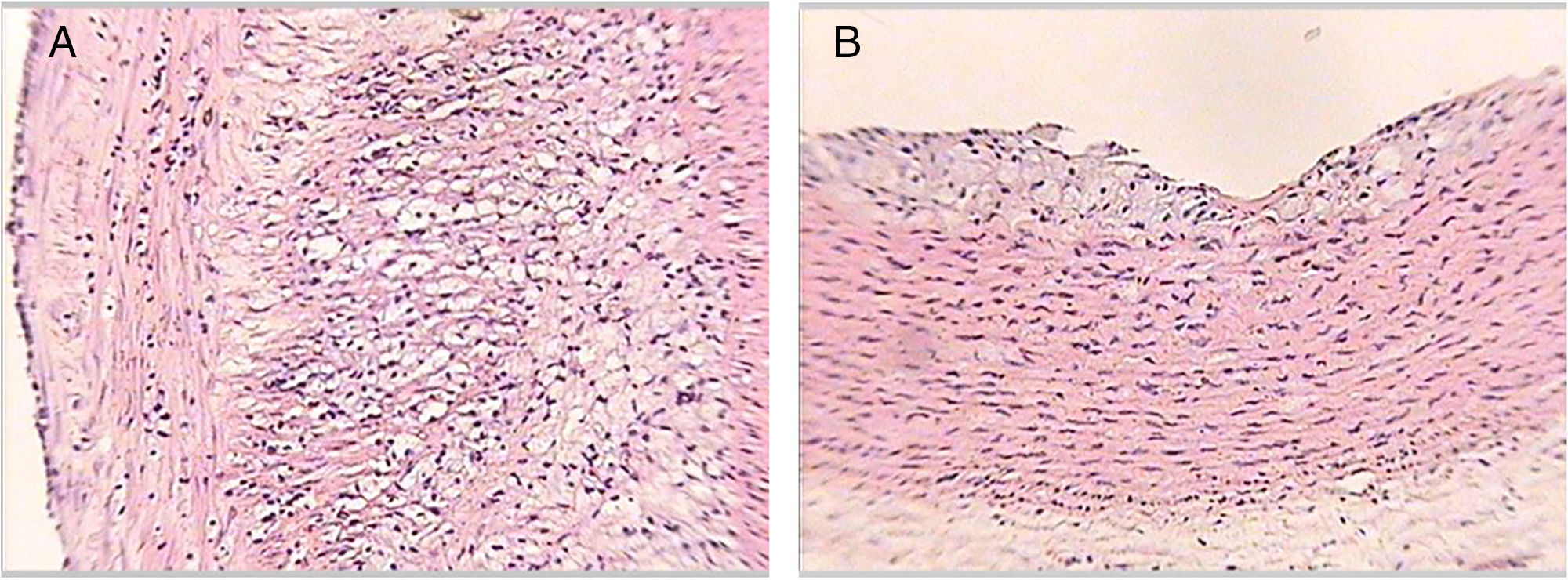
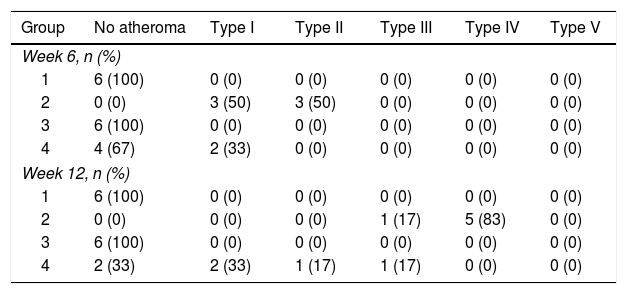
Table 3 shows the distribution of the rabbits according to the maximum degree of atheroma found in the aorta sections, with none of the rabbits from groups 1 or 3 having atherosclerotic lesions over the course of the study. The rabbits in group 2 did develop lesions of variable degrees over the course of the study. In group 4, some rabbits had no lesions at all and others had lesions of variable degrees (Fig. 3).
Distribution of the rabbits according to the maximum degree of atheroma found in the sections of aorta.
| Group | No atheroma | Type I | Type II | Type III | Type IV | Type V |
|---|---|---|---|---|---|---|
| Week 6, n (%) | ||||||
| 1 | 6 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2 | 0 (0) | 3 (50) | 3 (50) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 6 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 4 | 4 (67) | 2 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Week 12, n (%) | ||||||
| 1 | 6 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2 | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 5 (83) | 0 (0) |
| 3 | 6 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 4 | 2 (33) | 2 (33) | 1 (17) | 1 (17) | 0 (0) | 0 (0) |
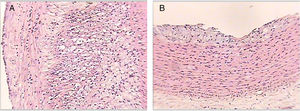
Histological sections of rabbit aortas at the end of the study. Type IV lesion (panel A). Intracellular and extracellular lipid clusters can be seen to a greater extent in the arterial intima in a rabbit belonging to group 2. Type II lesion (panel B). Intracellular lipid clusters can be seen in the arterial intima of a rabbit belonging to group 4. Haematoxylin-eosin staining, 50×.
We found evidence in our study to suggest that VEGF and CRP may be non-invasive early markers of atherosclerosis. The administration of a diet enriched with cholesterol caused intermediate and advanced atherosclerotic lesions and an increase in serum concentrations of VEGF and CRP from week 6 of the study, suggesting involvement of these molecules in atherosclerosis from the early stages. Other studies have shown an increase in plasma levels of VEGF in patients with coronary heart disease, myocardial infarction and atherosclerosis, which can reflect the severity of the lesion.8,13 In fact, a number of studies support the pro-atherogenic role of VEGF. It has been reported that treatment with recombinant human VEGF causes an increase in the density of macrophages in the plaque, and an increase in plaque growth and neovascularisation,14 all of which points to the importance of VEGF and its involvement in the growth and extent of inflammation in the atherosclerotic plaque.7
However, the role of VEGF in low-intensity inflammatory processes is still under debate because of its dual action. On the one hand, VEGF-A can increase endothelial permeability and expression of cell adhesion proteins and chemotactic factors, promoting the adhesion of monocytes, their transendothelial migration and activation,15 but, on the other, it protects endothelial cells by inducing anti-apoptotic proteins and increasing the expression of endothelial nitric oxide synthase (eNOS) and NO synthesis, and it can act as a mitogen that promotes re-endothelialisation, preventing or repairing the endothelial damage begun by the atherosclerosis.5
The increase in VEGF concentrations found in this study could be a result of the hypoxia and inflammation conditions that exist in atherosclerosis; the rabbits developed intermediate and advanced atherosclerotic lesions. Other authors have reported that during atherosclerosis the thickening of the intima causes a decrease in oxygen supplementation to the arterial wall, which in turn causes the release of angiogenesis-stimulating factors such as VEGF,16 promoting neovascularisation. This could allow nutrient supplementation, in addition to macrophage infiltration, wall thickening, lipid deposition, inflammation and progression of the atherosclerotic lesion.5,17
Therefore, the increase in VEGF and CRP concentrations found in our study suggests the existence of an inflammatory state induced by the diet enriched with cholesterol, which caused intermediate and advanced atherosclerotic lesions. It is thought that the increase in the expression of cell adhesion molecules and cytokines promoted by hyperlipidaemia, with the consequent accumulation of monocytes in the intima, may be involved in the development of these atherosclerotic lesions,18 given that inflammation plays an important role in the pathophysiology of atherosclerosis.10
In fact, CRP is the most studied marker of inflammation related to cardiovascular prognosis.19–21 Different authors have detected CRP in atherosclerotic lesions in humans and animals, and suggested that it may have a direct effect on atherogenesis because it is synthesised in the macrophages of the lipid nucleus and is associated with the thickness of the fibrous cap.19 The increase in serum CRP levels we found after week 6 of our experiment in the groups given a cholesterol-enriched diet therefore suggests that it is a systemic marker of inflammation which increases early on, as found in other studies,1,22–27 as it is involved from the initial phase of atherosclerosis, promoting the increase in the expression of cell adhesion molecules, increasing the adhesion and migration of monocytes, determining the synthesis of chemotactic factors and inducing the endothelial secretion of other pro-inflammatory factors such as nuclear factor-kappa B (NF-κB), interleukin 6 (IL-6) and IL-8.28 Additionally, epidemiological and clinical studies have shown that elevated serum levels of CRP are a risk factor as well as a marker of cardiovascular disease, as CRP is a predictor of risk of myocardial infarction and peripheral arterial disease, even in apparently healthy individuals.21
Our findings showed a positive association between VEGF and the lipid profile and CRP, in line with other studies,29,30 suggesting that the increase in the serum lipid concentration may help trigger the systemic inflammatory process, which is then accompanied by the increase in VEGF and CRP and contributes to the atherosclerotic process. These data also suggest that serum VEGF may be a marker of risk and of atherosclerosis, as the increase in serum lipids is accompanied by an increase in the inflammatory state.
In vivo and in vitro studies have shown resveratrol to have an effect on atherosclerosis.1,2,11 Resveratrol is a phytoalexin with cardioprotective effects. These effects are due to its antioxidant properties, as it is capable of inhibiting oxidative damage by acting on the superoxide anion and hydroxyl radicals, inhibiting ROS production and lipid peroxidation.31 Resveratrol also has anti-inflammatory effects, inhibiting activation of transcription factors associated with inflammation such as NF-κB and reducing serum levels of pro-inflammatory cytokines such as chemokine ligand 3, interleukin 1 beta (IL-1β) and tumour necrosis factor alpha (TNF-α).1,2,11,31
Our study showed that resveratrol has an anti-inflammatory and anti-atherogenic effect; in the rabbits supplemented with resveratrol and given a cholesterol-enriched diet, serum VEGF and CRP levels had decreased in week 12 and we found a decrease in the formation of atherosclerotic lesions. We demonstrated for the first time in vivo the effect of resveratrol on serum concentrations of VEGF in atherosclerosis. Resveratrol can regulate the expression of VEGF in various cells; it reduces the levels of VEGF in gingival fibroblasts,32 in squamous cell carcinoma of the tongue and in human hepatoma cells.33
It has also been shown to reduce the increase in VEGF expression and secretion and cellular hyperpermeability induced by high glucose in culture of monolayers of aorta endothelial cells.34 Additionally, resveratrol prevents up-regulation of VEGF in macrophages incubated with 1-oxo cholesterol, reinforcing its potential therapeutic role in counteracting the pro-atherogenic signalling of the oxysterols within the atherosclerotic plaque.35 Considering that resveratrol was able to reduce serum concentrations of VEGF, which has been shown to stimulate migration, permeability and endothelial proliferation, we can conclude that one of resveratrol's anti-atherogenic effects comes from reducing VEGF secretion in vivo during the atherosclerosis process, which may contribute to decreasing endothelial permeability. It is important to point out that the effect of resveratrol on VEGF and CRP levels was observed in rabbits with atherosclerosis induced by a hypercholesterolaemic diet, suggesting the need for a “stimulated” or inflamed environment, just as previously seen in vitro.35
Also of great relevance is the fact that resveratrol was able to reduce serum concentrations of CRP, an important acute-phase protein and cardiovascular risk marker.19 This confirms resveratrol's anti-inflammatory properties, as reported by other studies.11,31,36
Although the evidence suggests that resveratrol has lipid-lowering properties,2 we did not detect this effect in our study; as also reported in other studies,11,37 with the suggestion that it may have been due to the short experimental period.11 However, our data and the evidence suggest that the absence of the lipid-lowering effect in our experimental model may be a result of the accumulation of exogenous lipids from their diet, as they were not able to increase the excretion of sterols.26
Nevertheless, despite the fact that resveratrol supplementation had no effect on serum lipids, it was able to reduce the formation and progression of atherosclerosis induced by the hypercholesterolaemic diet from week 6 of the study, as has previously been demonstrated.2,11 A decrease was also reported in the expression of pro-inflammatory proteins in the aorta, such as vascular cell adhesion molecule-1 (VCAM-1), monocyte chemoattractant protein-1 (MCP-1) and IL-6,11 and of factor NF-κB.2 Resveratrol has also been described as having a preventive effect on the development of atherosclerotic lesions, possibly through inhibition of LDL oxidation, platelet aggregation and proliferation of vascular smooth muscle cells, and by inhibiting the expression of pro-inflammatory and pro-atherogenic genes, such as factor NF-κB and activating protein-1 (AP-1), in endothelial cells.31 However, in vitro studies have also found that the antioxidant effects mediate the anti-atherogenic action of resveratrol; pre-treatment with resveratrol in a macrophage cell line was able to inhibit the formation of foam cells and lipopolysaccharide-induced ROS production by inhibiting the expression of nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase).38 We would therefore suggest that the anti-atherogenic effect of resveratrol observed in this study could be partly due to its anti-inflammatory effect, indicating that it may act as a preventive agent in the development of atherosclerotic lesions.
In conclusion, our results suggest that serum VEGF and CRP are non-invasive early markers of inflammation and atherosclerosis. Concentrations of both VEGF and CRP increased from week 6 of the study onwards, confirming their role in the pathophysiology of atherosclerosis. We therefore believe that quantification of VEGF and CRP in blood could be useful for providing diagnostic information about atherosclerosis. We found oral resveratrol supplementation to have anti-inflammatory and anti-atherosclerotic effects, as it decreased serum concentrations of VEGF and CRP and reduced the formation and progression of atherosclerotic lesions, suggesting that administration of this polyphenol could be beneficial in preventing atherosclerosis.
FundingThis study was made possible thanks to the funding received from Distribuidora Gonzalab.
Conflicts of interestNone.
We would like to thank María José de Freitas and Manuel Avelino Dinis for their technical assistance.
Please cite this article as: Figueira L, González JC. Efecto del resveratrol sobre las concentraciones séricas del factor de crecimiento endotelial vascular durante la aterosclerosis. Clin Invest Arterioscler. 2018;30:209–216.