Chronic kidney disease (CKD) has to be considered as a high, or even very high risk cardiovascular risk condition, since it leads to an increase in cardiovascular mortality that continues to increase as the disease progresses.
An early diagnosis of CKD is required, together with an adequate identification of the risk factors, in order to slow down its progression to more severe states, prevent complications, and to delay, whenever possible, the need for renal replacement therapy.
Dyslipidaemia is a factor of the progression of CKD that increases the risk in developing atherosclerosis and its complications. Its proper control contributes to reducing the elevated cardiovascular morbidity and mortality presented by these patients.
In this review, an assessment is made of the lipid-lowering therapeutic measures required to achieve to recommended objectives, by adjusting the treatment to the progression of the disease and to the characteristics of the patient.
In CKD, it seems that an early and intensive intervention of the dyslipidaemia is a priority before there is a significant decrease in kidney function. Treatment with statins has been shown to be safe and effective in decreasing LDL-cholesterol, and in the reduction of cardiovascular events in individuals with CKD, or after renal transplant, although there is less evidence in the case of dialysed patients.
La enfermedad renal crónica (ERC) ha de ser considerada como una situación de alto e incluso muy alto riesgo cardiovascular, ya que provoca un aumento de la mortalidad cardiovascular que va incrementándose a medida que progresa la enfermedad.
Es preciso realizar un diagnóstico precoz de la ERC junto con la adecuada identificación de los factores de riesgo, al objeto de frenar su evolución a estadios más severos, evitar las complicaciones y retrasar, en lo posible, la necesidad de tratamiento sustitutivo renal.
La dislipidemia es un factor de progresión de la ERC que aumenta el riesgo de desarrollo de aterosclerosis y sus complicaciones. Su adecuado control contribuye a reducir la elevada morbimortalidad cardiovascular que presentan estos pacientes.
En esta revisión se evalúan las medidas terapéuticas hipolipemiantes necesarias para el logro de los objetivos recomendados, ajustando el tratamiento a la evolución de la enfermedad y a las características del paciente.
En la ERC parece prioritaria una intervención precoz e intensiva de la dislipidemia antes de que se produzca una disminución importante de la función renal. El tratamiento con estatinas ha demostrado ser seguro y eficaz en la disminución del cLDL y en la reducción de episodios cardiovasculares en individuos con ERC o después del trasplante renal; sin embargo, la evidencia en los pacientes dializados es menor.
Chronic kidney disease (CKD) is a clinical situation generated by a gradual, progressive loss of kidney function. The significance of the CKD is conditioned not only by the progressive decline in the patient's quality of life and life expectancy as it advances to later stages, but also by an increase in cardiovascular morbidity and mortality, which is the main cause of death in these patients.1 Mortality among final stage CKD patients is 30 times higher than in the general population, and it can be as much as 1000 times higher when it affects lower risk population groups, such as children and adolescents.2 The prevalence of CKD is clearly increasing due to the longer life expectancy in the general population, an increase in diabetes and obesity, and the higher survival rate of patients who have presented cardiovascular episodes or who have been diagnosed with chronic renal failure.
CKD is defined as the presence of alterations in kidney structure or kidney function lasting more than three months, secondary to a progressive decrease in the number of nephrons, with a subsequent deterioration in health derived from the inability of the kidneys to perform their excretion, filtration and metabolic functions.
In its everyday clinical treatment, diagnosis, classification and aetiology, CKD is determined by a decrease in estimated glomerular filtration rate (GFR), with levels of <60ml/min/1.73m2, and/or the presence of albuminuria3 (Table 1).
Cardiovascular risk in chronic kidney disease according to estimated glomerular filtration (GFR) category and albuminuria.
| Persistent albuminuria | ||
|---|---|---|
| A1 | A2 | A3 |
| Normal or slight increase | Moderate increase | Drastic increase |
| <30mg/g | 30–300mg/g | >300mg/g |
| Risk | ||
| GFR category (ml/min/1.73m2) | |||||
|---|---|---|---|---|---|
| G 1 | Normal or high | ≥90 | Low | Moderate | High |
| G 2 | Slight decrease | 60–89 | Low | Moderate | High |
| G 3a | Slight–moderate decrease | 45–59 | Moderate | High | Very high |
| G 3b | Moderate–drastic decrease | 30–44 | High | Very high | Very high |
| G 4 | Drastic decrease | 15–29 | Very high | Very high | Very high |
| G 5 | Renal failure | <15 | Very high | Very high | Very high |
Source: Adapted from “KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease”.3
According to data from the EPIRCE study,4 the prevalence of CKD in Spain is close to 10% when jointly screening for GFR and albuminuria, but this increases to 21.4% in subjects over 64 years old when screening for only GFR<60ml/min/1.73m2. Age is the risk factor most related to CKD, with the early stages of kidney function loss observed in the third decade of life, and with a significant loss in kidney function after age 60 (OR 1.12 [1.10–1.14; p<0.0001] for each additional year of age).
CKD is an independent risk factor of cardiovascular disease,5 even in children and adolescents with less exposure to cardiovascular risk factors than adults.2 Both pre-existing and newly presenting atherosclerosis in CKD patients clearly has an accelerated progression,6 with the early onset of cardiovascular episodes increasing (in men<age 55 or women<age 65).7 This effect seems to be related to a diffuse inflammatory pattern that persists in spite of the possibility of correcting possible trigger factors, such as bypass surgery in cases of renal artery stenosis.8
All of these factors have led CKD to be deemed a high- or even very-high cardiovascular risk situation, making early detection and treatment necessary to address the risk factors that can be modified as the disease progresses.
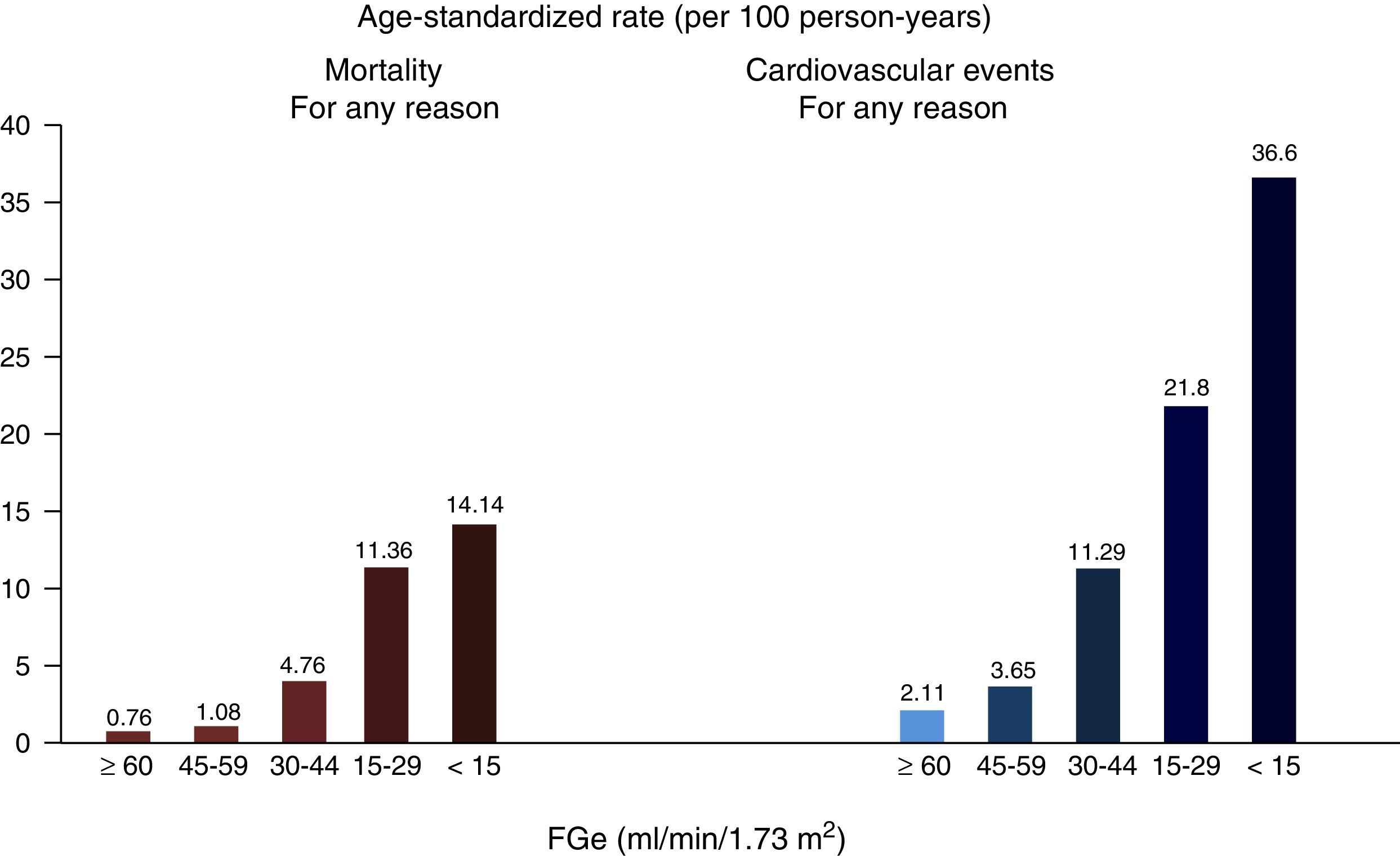
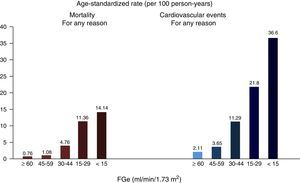
Chronic kidney disease and cardiovascular riskCKD is associated with the presence of coronary heart disease, heart failure, cardiac arrhythmias, and ischaemic and haemorrhagic strokes,9 as well as being associated with a higher incidence of sudden death,10 and increased mortality from cardiovascular causes and all other causes,11 which is exponential with the greatest reduction in GFR12 (Fig. 1). In the most advanced stages of CKD (Stages 4–5 [GFR<30ml/min/1.73m2]), mortality is much higher than in the general population, and it is significantly higher in dialysis patients than in other CKD patients. Meanwhile, the risk decreases in kidney transplant patients compared to dialysis patients.13
Relationship between GFR and overall mortality and cardiovascular mortality.
Source: from Go et al.12
An attempt was made to compare the cardiovascular risk of CKD patients with that of subjects who have already suffered a myocardial infarction (MI) and with that of diabetic patients. In spite of these three diseases (MI, diabetes mellitus and CKD) progressing differently over time, it was nevertheless observed that, in patients over 65, the risk of new cardiovascular episodes at ten years is similar in both CKD patients and diabetes patients, compared with the subjects who had suffered an MI.14 These cardiovascular complications occur simultaneously with increased loss of glomerular function and longer exposure over time to both CKD and other cardiovascular risk factors. In another study, conversely, the patients with diabetes mellitus or CKD did not have the same risk of coronary episodes as patients who had suffered an MI, but in CKD patients with diabetes the risk was similar to that of patients who had suffered an MI. In this same study, the diabetes patients had a lower incidence of MI than the CKD patients with GFR<45ml/min/1.73m2 accompanied by a drastic increase in proteinuria.15
Microalbuminuria, per se, increases the relative risks of severe cardiovascular episodes (RR 1.83; 95% CI: 1.64–2.05), overall mortality (RR 2.09; 95% CI: 1.84–2.38) and hospitalisations for heart failure (RR 3.23; 95% CI: 2.54–4.10),16 with similar repercussions in both diabetic patients and non-diabetic patients. Albuminuria and proteinuria were also observed to be better predictors of a risk of stroke than glomerular filtration,17 with the risk increasing as albuminuria increased.18 It is important to remember that 16% of CKD patients with a negative albumin/creatinine ratio had a positive protein/creatinine ratio. The latter group presented a higher risk of their kidney disease advancing, with an increased need for substitute treatment and increased mortality, which was even higher in frank proteinuria patients.19
Therefore, the joint screening for GFR and albuminuria in CKD is a better predictor of cardiovascular risk than estimates based on traditional risk factors.20
Based on these data, it is no wonder that the risk of a new cardiac episode after an MI increases in patients who previously had CKD, with a rate of 30.8 per 1000 people compared to 18.8 per 1000 people in subjects who do not present CKD. This is a significant difference,21 with an increase both in the risk of progression to late stages of CKD and in overall mortality.22
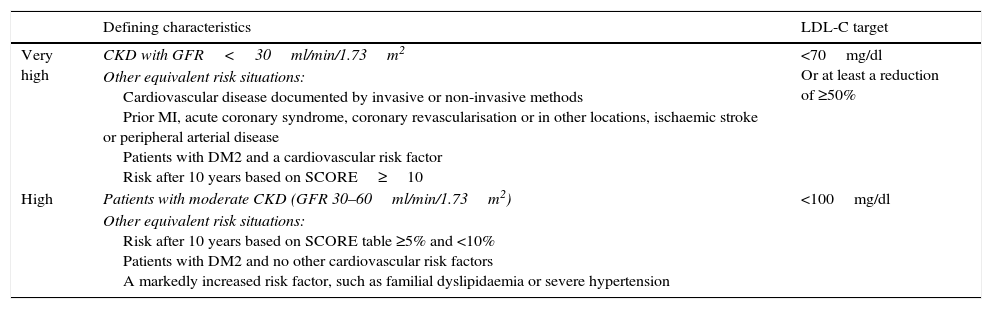
Based on all of this, the 2012 European Cardiovascular Prevention Guidelines23 classify CKD patients with GFR<30ml/min/1.73m2 as having a very high cardiovascular risk; the same control targets are set for them as for patients with arteriosclerotic vascular disease (secondary prevention), and setting their target LDL-C to below 70mg/dl or to, at least, 50% of their baseline LDL-C. In addition to this, the guidelines also classify CKD patients with a GFR between 30 and 60ml/min/1.73m2as being at high cardiovascular risk, assigning them an LDL-C target of under 100mg/dl (Table 2). In order to reach these therapeutic targets, statins are the lipid-lowering medication of choice.
Recommended LDL-C control targets in chronic kidney disease patients by various cardiovascular risk categories.
| Defining characteristics | LDL-C target | |
|---|---|---|
| Very high | CKD with GFR<30ml/min/1.73m2 | <70mg/dl Or at least a reduction of ≥50% |
| Other equivalent risk situations: Cardiovascular disease documented by invasive or non-invasive methods Prior MI, acute coronary syndrome, coronary revascularisation or in other locations, ischaemic stroke or peripheral arterial disease Patients with DM2 and a cardiovascular risk factor Risk after 10 years based on SCORE≥10 | ||
| High | Patients with moderate CKD (GFR 30–60ml/min/1.73m2) | <100mg/dl |
| Other equivalent risk situations: Risk after 10 years based on SCORE table ≥5% and <10% Patients with DM2 and no other cardiovascular risk factors A markedly increased risk factor, such as familial dyslipidaemia or severe hypertension |
LDL-C: cholesterol transported by low-density lipoproteins; DM2: Type-2 diabetes mellitus; CKD: chronic kidney disease; GFR: estimated glomerular filtration; MI: myocardial infarction.
Source: Adapted from Perk et al.23
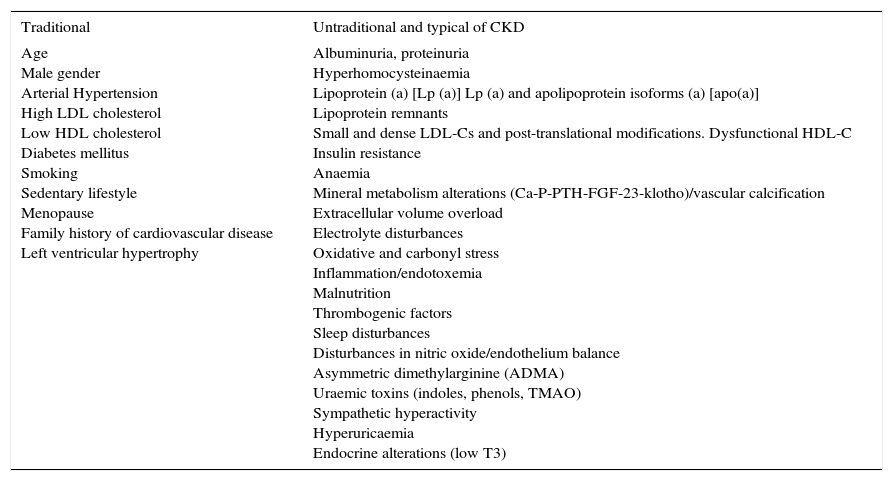
In addition to GFR and albuminuria, we can identify other cardiovascular risk factors in CKD patients, some of which are considered traditional and some non-traditional, some of which consolidate their specific impact, compared to others, as the disease progresses24–27 (Table 3).
Cardiovascular risk factors in chronic kidney disease.
| Traditional | Untraditional and typical of CKD |
|---|---|
| Age Male gender Arterial Hypertension High LDL cholesterol Low HDL cholesterol Diabetes mellitus Smoking Sedentary lifestyle Menopause Family history of cardiovascular disease Left ventricular hypertrophy | Albuminuria, proteinuria Hyperhomocysteinaemia Lipoprotein (a) [Lp (a)] Lp (a) and apolipoprotein isoforms (a) [apo(a)] Lipoprotein remnants Small and dense LDL-Cs and post-translational modifications. Dysfunctional HDL-C Insulin resistance Anaemia Mineral metabolism alterations (Ca-P-PTH-FGF-23-klotho)/vascular calcification Extracellular volume overload Electrolyte disturbances Oxidative and carbonyl stress Inflammation/endotoxemia Malnutrition Thrombogenic factors Sleep disturbances Disturbances in nitric oxide/endothelium balance Asymmetric dimethylarginine (ADMA) Uraemic toxins (indoles, phenols, TMAO) Sympathetic hyperactivity Hyperuricaemia Endocrine alterations (low T3) |
CKD is a progressive disease that in many cases is irreversible, and it is caused by various factors, as is its primary complication, cardiovascular disease. An early diagnosis of the disease is required, together with an adequate identification of the risk factors, in order to slow down its progression to more severe stages, to prevent complications, and to delay, whenever possible, the need for renal replacement therapy.
There are some well-established indications for treating CKD, such as inhibiting the renin-angiotensin system in patients with high blood pressure, or micro/macroalbuminuria, glycaemic control, lifestyle changes to improve metabolic syndrome components, decreasing salt consumption and the ingestion of proteins, and correcting anaemia if present. However, insufficient data exists for recommending the benefits of reducing uricaemia,3 despite some studies demonstrating the usefulness of allopurinol or febuxostat in impeding the disease's progress27,28 and in delaying cardiovascular morbidity/mortality in CKD.29
Dyslipidaemia is a factor in the progression of both CKD30 and cardiovascular disease. Nevertheless, there is still a debate around the importance of treating dyslipidaemia in CKD patients, especially in late-stage CKD.31
The difference in the LDL-C targets for CKD patients with a GFR between 30 and 60ml/min/1.73m2 indicated in the various European guidelines keeps this debate alive, although it may be aggravated by the different behaviour of statins according to the patient's stage of CKD. Statins yield better results when they are used in the initial stages of CKD, presenting a relative risk of cardiovascular episodes of 0.69 (0.70–0.85) in Stage 2–3 patients, and with a Number Needed to Treat (NNT) of 24 (19–32); or of 0.78 (0.63–0.96) in Stage 4 patients, with an NNT of 36 (19–330). Meanwhile, in Stage 5, depending on whether the patient is undergoing dialysis, the relative risk of suffering new cardiovascular episodes would be 0.93 (0.86–1.00) or 0.82 (0.60–1.11), with an NNT of 46 (25–257).32,33 As regards the effect of statins on the progression of kidney disease (25% GFR reduction, doubling the level of serum creatinine, or evolution to end stages), there were few benefits found (RR 0.95 [0.90–1.01]),33 although benefits were observed in some clinical trials with some of the statins.32
All of this leads us to the question of whether it is necessary to treat dyslipidaemia in CKD, whether to treat it less intensively in the early stages (LDL-C<100mg/dl) and whether to intensify treatment in the late stages, or even whether to suspend treatment in the terminal stages of CKD. These are doubts that need to be elucidated in the course of daily clinical practice. Even though there is no evidence in this regard, it would seem logical that taking intensive action (LDL-C<70mg/dl) as of the first stages of CKD would be more effective in treating cardiovascular disease than a less intensive treatment (LDL-C<100mg/dl). It would thus be desirable to keep the patient's LDL-C<70mg/dl throughout the entire evolution, adapting the pharmacological treatment to the patient's characteristics, and bearing in mind that intestinal absorption of cholesterol and phytosterols will increase in parallel with the advance of the CKD.34
Dyslipidaemia in chronic kidney diseaseRegardless of the patients’ characteristics, the onset and advance of their CKD will cause changes in their lipid profile.
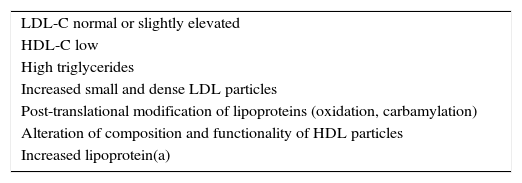
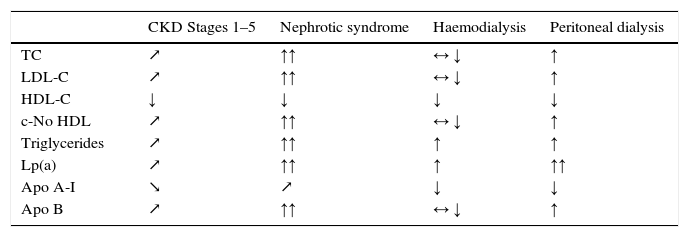
Dyslipidaemia in CKD is characterised by normal or slightly elevated LDL-C levels, low HDL-C, high triglycerides, higher proportions of small and dense LDL-C particles, and increased lipoprotein(a) [Lp(a)]35,36 (Table 4). These modifications are related to the degree of renal affection, the primary aetiology of the CKD, the presence of nephrotic syndrome, and the dialysis technique used as substitute kidney treatment.37 These changes in lipoprotein profiles are clear even in children with moderate CKD, and they are associated with more drastic decreases in GFR and nephrotic-range proteinuria, as well as age and the presence of obesity.38
Primary characteristics of lipid alterations in chronic kidney disease.
| LDL-C normal or slightly elevated |
| HDL-C low |
| High triglycerides |
| Increased small and dense LDL particles |
| Post-translational modification of lipoproteins (oxidation, carbamylation) |
| Alteration of composition and functionality of HDL particles |
| Increased lipoprotein(a) |
The mechanisms behind these modifications in the patients’ lipid profiles change as their illness advances, and depending on their substitute kidney treatment. Triglycerides accumulate due to the excess production of lipoparticles rich in triglycerides and due to the decrease of triglyceride catabolism caused by a reduction of lipoprotein lipase (LPL) and hepatic lipase activity. This is due to an increase in apoC-III levels that causes an increase in the apoC-III/apoC-II ratio, and a decrease in LPL synthesis secondary either to hypoparathyroidism or decreased insulin levels.
One mechanism that can affect the increase of cardiovascular risk in CKD patients would be post-translational modifications of LDL particles in CKD that make them more atherogenic. The oxidative stress associated with uraemia can contribute to the atherosclerosis process by oxidising and carbamylating LDLs. LDL carbamylation occurs due to the spontaneous non-enzymatic chemical modification of apolipoprotein B (a protein component of LDLs), by the isocyanic acid derived from the urea.39 The decrease in the levels of apoA-I and lecithin:cholesterol acyltransferase (LCAT) associated with CKD leads to quantitative changes, with a decrease in HDL-C concentration, and qualitative changes with transformation to malfunctioning HDL particles. In addition to all of this, the activity of the paraoxonase present in the HDLs decreases, diminishing their antioxidant and anti-inflammatory capacity.40,41
The HDLs of patients in haemodialysis present diverse alterations in proteomic and lipidomic composition that are related to the modification of their capacity to accept cholesterol. The HDLs of uraemic patients are rich in albumin, apoC-III, and apoA-IV, and in pro-inflammatory proteins such as serum amyloid A (SAA) and phospholipase A2 associated with lipoproteins (Lp-PLA2), and a decrease in apoA-I and A-II. Changes in the HDL lipid composition of dialysis patients occur by way of a decrease in phospholipids and free cholesterol, in addition to increased triglycerides.42 All of this impairs the ability of the HDL to assist in transporting macrophages back to the liver.43 In short, the quantitative and qualitative alterations of HDLs in CKD reduce their atheroprotective properties and may contribute to the increased cardiovascular mortality in CKD patients, although the effect of advanced CKD on the composition and function of HDLs is not fully understood.
High levels of Lp(a) have also been reported in these patients, which are associated with an increased cardiovascular risk in both the terminal and early stages of CKD, probably due to a decrease in the patients’ renal catabolism.44 This early increase in Lp(a) will preferably occur in patients with larger isoforms Lp(a). These are the patients who present lower Lp(a) levels under normal conditions. High Lp(a) levels are especially influenced by the severity of the patients’ proteinuria and not by the aetiology of their kidney disease, and they partially revert when patients receive a kidney transplant.44
These changes in lipid metabolism change as the patients’ CKD advances under the influence of their various clinical situations (Table 5) and reflecting the increased risk of them developing atherosclerosis and its complications.
Changes in lipid profile in different chronic kidney disease stages.
| CKD Stages 1–5 | Nephrotic syndrome | Haemodialysis | Peritoneal dialysis | |
|---|---|---|---|---|
| TC | ↗ | ↑↑ | ↔ ↓ | ↑ |
| LDL-C | ↗ | ↑↑ | ↔ ↓ | ↑ |
| HDL-C | ↓ | ↓ | ↓ | ↓ |
| c-No HDL | ↗ | ↑↑ | ↔ ↓ | ↑ |
| Triglycerides | ↗ | ↑↑ | ↑ | ↑ |
| Lp(a) | ↗ | ↑↑ | ↑ | ↑↑ |
| Apo A-I | ↘ | ↗ | ↓ | ↓ |
| Apo B | ↗ | ↑↑ | ↔ ↓ | ↑ |
↗: increasing or ↘: decreasing depending on GFR; ↑: increasing, ↓: decreasing, ↑↑: extremely increased, ↓↓: extremely decreased versus subjects without uraemia; ↔: normal.
Source: Adapted from Kwan et al.40
Less known is the role that the intestinal absorption of cholesterol and phytosterols plays in cardiovascular risk, even though a rare autosomal recessive disease called familial sitosterolemia (characterised by high plasma levels of phytosterols) contributes to the early appearance of cardiovascular episodes.45 Data exists on the increased intestinal absorption of cholesterol and phytosterols in CKD patients. Thus, diabetic CKD patients not treated with statins have been observed to have high levels of campesterol (an intestinal sterol absorption marker), directly related to higher albumin:creatinine ratio values and inversely related to lower GFR levels,34 and this is especially notable in haemodialysis patients.46 These data would justify, at least in part, the findings of the post hoc analysis of the 4D study (German Diabetes and Dialysis Study) that compared the effects of atorvastatin 20mg to a placebo in dialysis patients, showing that treatment with atorvastatin was only beneficial in subjects who absorbed less, while hyper-absorbing patients saw no benefits.47
Based on all of this, it is advisable to run routine lipid profile screens in all CKD patients, determining their total cholesterol, triglycerides, HDL-C and LDL-C, defining the exact therapeutic measures that will allow them to achieve their recommended control targets, personalising their treatment, and adjusting it to each patient and how their disease progresses.48
Treatment of dyslipidaemia in chronic kidney diseaseAs noted above, dyslipidaemia in chronic kidney disease is characterised by increased plasma triglycerides, low plasma HDL-C concentrations, and normal or slightly elevated LDL-C levels with increased Lp(a) levels, and it is associated with increased cardiovascular morbidity and mortality and a greater impairment of kidney function.49 Strictly controlling the various risks factors is crucial for reducing the high cardiovascular risk of these patients.
Treatment with statins in chronic kidney diseaseStatin therapy is essential for cardiovascular prevention in patients with high and very high cardiovascular risk (including CKD patients), so that they can achieve their proposed LDL-C targets and reduce their cardiovascular risk.34 An analysis of the subgroups in the statin studies that included CKD patients showed benefits that were similar in patients with and without CKD.50 Yet there are discrepancies in the results for patients treated with statins that depend on which stage of CKD the patients are in. A meta-analysis that included 21,295 participants from 11 clinical trials concluded that statin treatment reduced overall mortality (p<0.0001)and cardio- and cerebrovascular episodes (p=0.0001 and p=0.0022, respectively) in CKD patients who do not require dialysis.51 Conversely, the use of statins in CKD patients undergoing dialysis reduced their cardiac mortality and their cardiovascular episodes (p<0.05 in both), but with an insignificant effect on their overall mortality and on cerebrovascular episodes.
Early intensive intervention with statins in CKD patients has been shown to benefit cardiovascular risk. The findings in the subgroup of patients with mild to moderate CKD who were included in the follow-up of 5801 Japanese patients who had a stent inserted and were treated with high-intensity statins (atorvastatin, pitavastatin or rosuvastatin), showed a reduced risk of cardiovascular mortality versus those who received a lower intensity statin (pravastatin, simvastatin, fluvastatin).52 This would support the argument for higher-intensity lipid-lowering treatment using statins from the initial stages of CKD, with no need to wait for further impairment in kidney function in order to intensify the treatment.
Another meta-analysis concluded that statin therapy in patients with light to moderate CKD reduced their cardiovascular disease by 24%, their risk of cardiovascular mortality by 23%, and their risk of overall mortality by 21%. The findings were also favourable for the risk of both MI (a 34% decrease) and cerebrovascular accidents (a 30% decrease), but with insignificant effects on cardiovascular disease in patients with baseline creatinine levels over 1.5mg/dl.53
A follow-up of a cohort of 14,706 patients after coronary revascularisation showed no benefits of statins in CKD patients on haemodialysis.54 Another systematic review and meta-analysis that included 31 clinical trials with over 48,000 CKD patients researched the effects of statins on cardiovascular morbidity and mortality and found that statin therapy reduced the risk of cardiovascular episodes at the various levels of kidney function. Major cardiovascular episodes decreased by 23% (p<0.001), including an 18% reduction in coronary episodes. In this meta-analysis, the adverse effects of statins were not found to increase in CKD patients, which confirms that they are safe for use in this population.33 One finding that bears emphasising from this study is that the effect of treatment with statins was significantly modified by the patients’ kidney function. An analysis of the sub-groups showed that the relative reduced risk of episodes was significantly lower in late-stage CKD patients (p<0.001), although the risk reductions were comparable. It would thus seem that CKD patients benefit from statin treatment, but these relative benefits diminish as their CKD becomes more severe.32 It is nevertheless important to emphasise that the absolute reductions in risk were only slightly less in patients with late-stage CKD, which suggests that statin treatment might still provide major benefits to these individuals.33
The ALERT study included 2102 kidney transplant recipients who were treated with fluvastatin (a statin with a lower degree of renal elimination) who presented an insignificant 17% reduction in the combined primary objective, while their cardiac-caused mortality and MI showed a significant reduction.55,56 A post hoc analysis found that the early introduction of fluvastatin in post-kidney transplant treatment presents a higher benefit.57
When the efficacy of 20mg/day of atorvastatin was compared to a placebo in 1255 Type-2 diabetics in haemodialysis, there was no significant reduction in the cardiovascular mortality, non-lethal MI or stroke, in spite of the similar cholesterol-lowering effect compared to non-dialysis patients.58 Similarly, a study that evaluated the benefit of rosuvastatin 10mg/day in haemodialysis patients did not obtain a significant effect on the final primary objective of cardiovascular death, non-fatal MI or cerebrovascular accidents.59 A meta-analysis of 25 studies that included 8289 dialysis patients likewise did not find any benefit from treatment with statins in cardiovascular episodes, cardiovascular mortality, mortality from all causes or MI, in spite of the reduction in cholesterol levels.60 Conversely, in the SHARP study on CKD patients, the use of simvastatin in combination with ezetimibe significantly reduced cardiovascular episodes compared to the use of a placebo in a test group that included a significant number of dialysis patients,61 although a sub-group analysis showed that there was no significant benefit in the dialysis patients, presenting only one trend in the reduction of episodes.
According to the clinical evidence available, the relative effect of statins on cardiovascular morbidity and mortality is more modest in late-stage CKD than in early-stage CKD. This can be explained by the substantial proportion of cardiovascular episodes in populations with terminal CKD on a haemodialysis programme, which result from alterations not related to atherosclerosis, but rather to left ventricular hypertrophy, heart failure and sudden death.62,63 Given all of this, the recommendation in the 2013 KDIGO Guidelines64 for adult CKD patients on dialysis is to not start treatment with statins or combined treatment with statins and ezetimibe, although they do not recommend suspending this treatment in patients who are already receiving it when they begin dialysis.
While there is a degree of controversy, statins may have beneficial effects on proteinuria and kidney function. In the CARDS study, a beneficial effect of atorvastatin 10mg/day on GFR was observed, especially in patients with albuminuria.65 Meanwhile, in the SHARP study, in the group that received a combination of ezetimibe and simvastatin, the advance of CKD was not delayed, nor was any significant beneficial impact on GFR observed.66 A meta-analysis that included 41 studies with a total of 88,523 participants showed that GFR significantly reduced in the patients who received a placebo treatment compared to the group treated with statins, while the group treated with statins presented a delay in the advance of their proteinuria compared to the placebo group. The group of patients that received high-intensity statins maintained GFR levels significantly higher than those of the patients who used medium-intensity statins (95% CI: 0.08–0.16; p=0.00001).67
In the TNT study,68,69 treatment with atorvastatin improved renal function in Stage-3 CKD patients. The increased GFR levels achieved with atorvastatin 80mg/day were significantly higher than with 10mg/day (9.9% versus 6.6%, respectively; p<0.005), while the CKD patients also presented a reduction in their cardiovascular risk when undergoing the intensive treatment. In the LIVES study, pitavastatin increased CKD patients’ GFR by 5.4ml/min/1.73m2 after 104 weeks of treatment (GFR<60ml/min/1.73m2), which translated into a 10.5% improvement in filtration.70 Pitavastatin treatment helped type-2 diabetics with mixed dyslipidaemia and moderate CKD to significantly improve their GFR.71 Even so, not all statins yielded the same results, as in the PLANET I (diabetes patients) and PLANET II (non-diabetes patients) studies, treatment with rosuvastatin 40mg/day was accompanied by an impairment of renal function.72 The heterogeneity of the impact of statins in renal function and albuminuria can also be observed in patients with diabetic nephropathy, for whom atorvastatin 10mg/day had a renoprotective effect, since, compared to a group that received pravastatin 10mg/day, their albuminuria decreased significantly, and they also had the best GFR of all the patients treated with atorvastatin.73 Other statins with lower renal elimination, such as pitavastatin at a dose of 2mg/day, were also compared to pravastatin 10mg/day in CKD patients with Type-2 diabetes. Pitavastatin was more effective than pravastatin at reducing these patients’ albuminuria.74 A sub-analysis of the SAGE study showed that intensive dyslipidaemia control in elderly patients (ages 65–85) with stable coronary disease could provide benefits for renal function. This study examined the effect of treatment with a high-intensity statin (atorvastatin 80mg/day) versus a medium-intensity statin (pravastatin 40mg/day) in 893 randomised patients over 12 months (418 of the patients had CKD). Their GFR increased with atorvastatin and it remained stable with pravastatin (2.38 versus 0.18ml/min/1.73m2, respectively; p<0.0001). The increased GFR in non-CKD patients was significantly higher with atorvastatin (2.08ml/min/1.73m2), whereas it decreased with pravastatin (−1.04ml/min/1.73m2).75
Elsewhere, statins seem to play a role in protecting against the kidney injuries induced by administrating contrast agents when performing coronary angiograms. When the use of statins in diabetic CKD patients subject to angiograms was specifically examined, the group of patients treated with statins presented a reduced risk of acute kidney failure induced by the use of contrast agents.76 A meta-analysis of 15 trials that assessed the effect of statins administered prior to coronary angiograms revealed a significant decrease in acute renal injury in patients treated with high-intensity statins compared not only with the control group treated with placebo, but also with patients who received statins at low doses.77
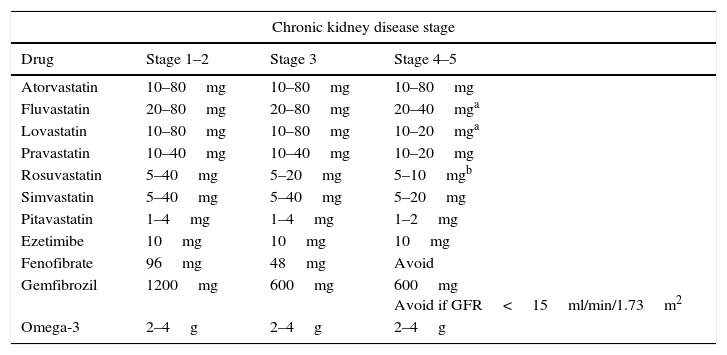
In short, treatment with statins can reduce the risk of cardiovascular disease and delay the advance of CKD, with substantial benefits in mild and moderate CKD. Nevertheless, the debate persists with regard to advanced stage CKD, especially in patients subject to haemodialysis. Recent studies suggest that the benefits of the various statins on the kidneys may be heterogeneous, with greater benefits when cholesterol-lowering intensity is maximised. Since CKD patients are complex and subject to various medications, it is important to assiduously evaluate how safe statins are in their full context. The statins recommend by the European guidelines for treating dyslipidaemia in CKD patients78 are those with the lowest renal excretion, such as fluvastatin, atorvastatin and pitavastatin. Some statins need their doses adjusted in CKD patients: in Stages 4 and 5, the doses of atorvastatin, pitavastatin and fluvastatin should be adjusted (Table 6).79,80 It is therefore reasonable to carefully determine the statin doses whenever there is a greater impairment of kidney function.
Daily doses of statins and other lipid-lowering medications for managing dyslipidaemia in chronic kidney disease.
| Chronic kidney disease stage | |||
|---|---|---|---|
| Drug | Stage 1–2 | Stage 3 | Stage 4–5 |
| Atorvastatin | 10–80mg | 10–80mg | 10–80mg |
| Fluvastatin | 20–80mg | 20–80mg | 20–40mga |
| Lovastatin | 10–80mg | 10–80mg | 10–20mga |
| Pravastatin | 10–40mg | 10–40mg | 10–20mg |
| Rosuvastatin | 5–40mg | 5–20mg | 5–10mgb |
| Simvastatin | 5–40mg | 5–40mg | 5–20mg |
| Pitavastatin | 1–4mg | 1–4mg | 1–2mg |
| Ezetimibe | 10mg | 10mg | 10mg |
| Fenofibrate | 96mg | 48mg | Avoid |
| Gemfibrozil | 1200mg | 600mg | 600mg Avoid if GFR<15ml/min/1.73m2 |
| Omega-3 | 2–4g | 2–4g | 2–4g |
It is always important to bear in mind that the statins that are metabolised in the cytochrome P450 3A4 pathway (lovastatin, simvastatin and atorvastatin) may cause adverse reactions when they interact with other medications that are common in these types of patients.23
Treatment with fibrates in chronic kidney diseaseFibrates are medications used in dyslipidaemia treatment because of their ability to reduce triglycerides and increase HDL-C, two of the components present in CKD dyslipidaemia. A meta-analysis of CKD patients corroborated these effects on the lipid profile.81
The VA-HIT secondary prevention study82 showed that gemfibrozil increased serum creatinine compared to the placebo (5.9% versus 2.8%; p=0.02). Nevertheless, while its use can be recommended at doses of up to 600mg/day in CKD patients who have severe hypertriglyceridemia (triglycerides above 500mg/dl), so long as their GFR is not below 15ml/min/1.73m2,78,83 it is not recommended to administer it jointly with statins, as this can significantly increase the risk of myopathy.23
Fenofibrate causes a sustained acute increase in plasma creatinine38 that can be reversed when treatment is suspended, but extreme caution is required in CKD patients. For those who have GFR levels of 60–90ml/min/1.73m2, a 50% dose decrease is recommended, and for those with levels under 60ml/min/1.73m2 the daily dose should not exceed 48mg. Its use is contraindicated in patients with GFR levels below 15ml/min/1.73m2.83 Nevertheless, fenofibrate can reduce the risk of albuminuria progressing in diabetic patients,84,85 and it is associated with a reduction in severe cardiovascular episodes, cardiovascular death, stroke and mortality from any cause in CKD patients with moderately impaired renal function, but not in those with GFR>60ml/min/1.73m2. Thus, even though fibrates increase serum creatinine, which seems to be due more to a decrease in a tubular secretion of creatinine than a decrease in GFR, there is a potential cardiovascular and renal benefit that allows them to be used in CKD, albeit with caution. However, the KDIGO Guidelines,57 recommend not using them, and the dyslipidaemia guidelines of the European societies23 restrict the use of fenofibrate to patients with GFR levels above 50ml/min/1.73m2, although they do allow gemfibrozil to be used in patients with lower glomerular filtration rates due to their lower renal elimination, but they do not recommend using it in conjunction with statins.
Treatment with niacin in chronic kidney diseaseNiacin has great potential as a therapeutic renoprotective agent, even though its joint administration with laropiprant was discontinued in January 2013 because of the results of the HPS2-THRIVE study86 in which it did not demonstrate a significant effect in reducing vascular episodes, whereas niacin treatment in combination with laropiprant was associated with a higher incidence of side-effects. Treatment with niacin presents favourable effects on HDL-C, triglyceridemia, oxidative stress and inflammation, and endothelial function. It can also decrease serum phosphorous levels by reducing absorption in the gastrointestinal tract.87 A sub-study that examined the combined use of niacin and laropiprant in CKD patients with dyslipidaemia found that treatment with niacin led to an average 11% decrease in serum phosphorous, with similar changes in patients with GFR levels above or below 60ml/min/1.73m2.88 These effects may delay GFR impairment and prevent cardiovascular risk, especially in the later stages of CKD.
Niacin is theoretically safe in CKD patients because it is not eliminated through the kidneys. It has been widely studied in clinical studies on cardiovascular disease prevention, but not specifically in CKD patients.89 The clinical trials that evaluated the combination of statins with niacin did not find any additional benefits for cardiovascular risk reduction compared to the use of statins in monotherapy. There were also no significant differences in GFR changes between the participants in the two study groups, but the overall mortality was significantly higher in the group that received niacin,89 so this combination is no longer recommended.90 The use of niacin also showed no cardiovascular or renal benefits when the study was stratified by renal function, according to the findings of a post hoc study. Of the 3414 participants who were studied (505 of whom presented Stage 3 CKD at the start of the trial), the appearance of cardiovascular episodes was similar to that of the participants with CKD, regardless of whether they were treated with a statin or a statin combined with niacin.91 Thus, in spite of its potential benefits, niacin is not recommended for use in combination with statins in CKD patients.
Treatment with Omega-3 fatty acids in chronic kidney diseaseOmega-3 fatty acids can be used in cases of hypertriglyceridemia and altered lipid profiles, which are commonly found in CKD. Aside from their lowering of triglycerides at doses of 2–4g/day, a meta-analysis with low-dose Omega-3 supplement did not find any evidence of cardiovascular prevention.92 There is little data as to their usefulness in CKD, although there is evidence of possible benefits in terminal CKD, including anti-inflammatory and anti-arrhythmic effects, in addition to platelet stabilisation and improved endothelial function.93 In haemodialysis patients, treatment with an Omega-3 supplement of 2g/day did not result in a reduction in cardiovascular episodes or mortality.94 Conversely, in a recent meta-analysis, the group of patients in haemodialysis who were given a fish oil supplement seem to have reduced their risk of cardiovascular episodes and improved their secondary hyperparathyroidism and hypertriglyceridemia.95 If Omega-3 fatty acids are used, there is no need for the dose to be adjusted in CKD patients37 and they can be used in cases of hypertriglyceridemia or mixed dyslipidaemia, either in monotherapy or in combination with statins, or they can be an alternative to using fibrates.
Treatment with bile acid sequestrants in chronic kidney diseaseIon-exchange resins can be used to lower patients’ cholesterol, either in monotherapy if patients have a contraindication or intolerance to statins, or in combination with ezetimibe and/or statins to try to achieve the adequate lipid control recommended in the guidelines.23 However, their use in CKD is restricted because they can increase triglyceride levels. If they are used, there is no need to adjust doses in patients with mild to moderate CKD, although there are no data confirming their safety and effectiveness in terminal kidney disease.80
Treatment with ezetimibe in chronic kidney diseaseEzetimibe inhibits the absorption of both dietetic and bile cholesterol in the small intestine by acting on the Niemann-Pick C1 like 1 protein, which has a greater expression in certain circumstances, such as CKD, especially in its later stages. It can reduce LDL-C up to 20%, but with an individualised response, and this can help achieve the strict lipid control targets recommended for CKD patients.23 Ezetimibe does not require a dose adjustment in CKD patients, and its use was evaluated in the SHARP study, in which the treatment of chronic nephropathy patients with statins and ezetimibe was compared to a placebo. The combined therapy achieved a reduction in cardiovascular morbidity and mortality.61 The IMPROVE-IT study,96 which included 18,444 post-acute coronary syndrome patients (one exclusion criterion was creatinine clearance under of 30ml/min), showed that a combination of ezetimibe 10mg/day with simvastatin 40mg/day yielded a modest (yet significant) reduction in the primary objective of the study: cardiovascular death, MI, unstable angina, coronary revascularisation and stroke (a relative reduction of 6.4%; p=0.016). An analysis of the sub-groups showed a special benefit among diabetic patients.
The combined statin/ezetimibe treatment was shown to reduce monocyte chemotactic protein (MCP-1) levels in diabetic CKD patients, who frequently experience an increase in inflammatory activity. This effect may be beneficial with regard to the progress of arteriosclerosis and diabetic nephropathy.97
ConclusionsIn summary, CKD patients present a high/very high cardiovascular risk, and the importance of reducing their cholesterol levels in cardiovascular prevention has been demonstrated from the early stages of CKD. Table 7 shows the specific characteristics of treatment of CKD patients with lipid-lowering medications, where treatment with statins has been shown to be safe and effective in decreasing LDL-cholesterol, and in the reduction of cardiovascular events in individuals with CKD, or after renal transplant, although there is less evidence in the case of dialysed patients. An early and intensive intervention seems to be a priority before a significant decrease in kidney function occurs.98
Lipid-lowering medications in chronic kidney disease.
| The most appropriate statins are those that have a lower renal excretion: atorvastatin, fluvastatin and pitavastatin |
| Statins that are metabolised in the cytochrome P450 3A4 pathway (lovastatin, simvastatin and atorvastatin) may cause adverse reactions due to their higher risk of interaction with other medications that are common in these types of patients. |
| If the LDL-C target is not reached with statins in monotherapy at the maximum tolerated dose, then ezetimibe is indicated |
| In cases of severe hypertriglyceridemia, Omega-3 fatty acids can be used at doses of 2–4g/day. Fibrates should only be used with extreme caution, monitoring any pharmacological interactions. The use of gemfibrozil can be recommended at doses of up to 600mg/day for patients with GFR levels of no less than 15ml/min/1.73m2, but combining it with statins is contraindicated. The use of fenofibrate in CKD patients is restricted to certain specific cases, and the dose needs to be adjusted and the patient's renal function needs to be closely monitored |
Patients who cannot tolerate or who have contraindications against statin therapy may receive some benefits from other lipid-lowering medications. In spite of the high frequency of the treatment with statins, only one third of CKD patients reach their LDL-C targets. If they do not reach their LDL-C target, the addition of ezetimibe is indicated. Fibrates and/or Omega-3 fatty acids can be used in cases of isolated hypertriglyceridemia.
It is important to implement an intensive plan for treating dyslipidaemia in CKD patients from the early stages in order to increase their ability to reach their LDL-C target, and thus reduce the cardiovascular morbidity and mortality in this population.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments were conducted on human beings or animals for this research.
Data confidentialityThe authors declare that patient details do not appear in this article.
Right to privacy and informed consentThe authors declare that patient details do not appear in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pascual V, Serrano A, Pedro-Botet J, Ascaso J, Barrios V, Millán J, et al. Enfermedad renal crónica y dislipidemia. Clin Invest Arterioscler. 2017;29:22–35.