To describe the experience of the robotic approach for achalasia surgery in a tertiary center.
Material and methodsPatients with achalasia who underwent robotic surgery between May 2010 and April 2019 were analyzed. The study variables were collected in a prospective database and a descriptive analysis was performed.
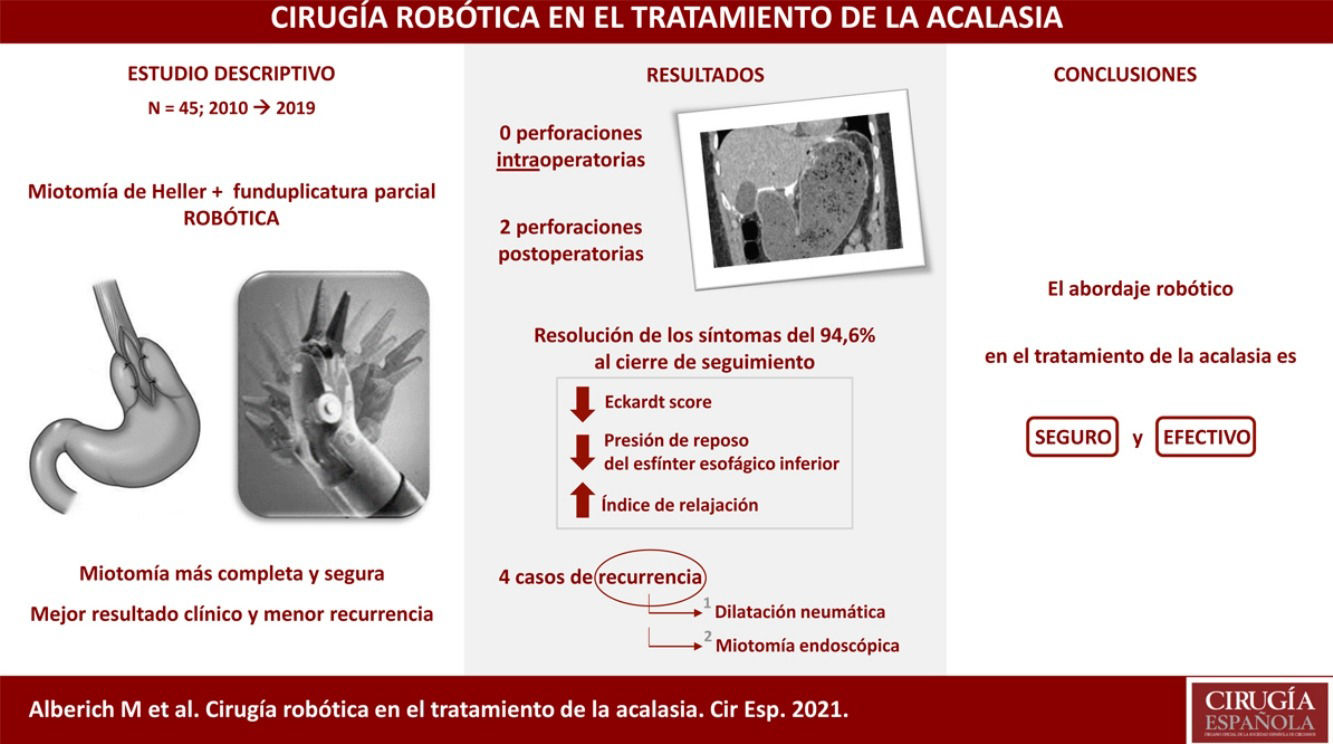
Results45 patients (55.6% male) with a mean age of 44 years were included. The main symptom at diagnosis was dysphagia. 19 patients (42.2%) received endoscopic treatment prior to surgery, mostly pneumatic dilation (84.2%). Heller's myotomy associated with Toupet fundoplication was the surgical technique of choice, with a mean operative time of 211 min. The average stay was 5 days. There were 2 postoperative perforations (4.4%). Perioperative mortality was 0%. The mean follow-up was 64 months. At 3 and 5 years, a significant decrease in the Eckardt score was observed and the manometric study showed a decrease in the lower esophageal sphincter pressure at rest of 58% and 70%, respectively, with persistence of hypomotility of the esophageal body. Pathological gastroesophageal reflux was diagnosed in two patients (5.4%) and 4 (10.8%) presented recurrence of symptoms, requiring endoscopic pneumatic dilations. In 2 cases, the dilations were not effective, so an endoscopic myotomy was considered.
ConclusionsIn our experience, robotic surgery is a safe and effective procedure for the treatment of achalasia.
Describir la experiencia del abordaje robótico en la cirugía de la acalasia en un centro de tercer nivel.
Materialy métodosSe analizaron los pacientes con acalasia intervenidos mediante cirugía robótica entre mayo de 2010 y abril de 2019. Las variables a estudio se recogieron en una base de datos prospectiva y se realizó un análisis descriptivo.
ResultadosSe incluyeron 45 pacientes (55.6% varones) con edad media de 44 años. El síntoma principal al diagnóstico fue la disfagia. 19 pacientes (42.2%) habían recibido tratamiento endoscópico previo a la cirugía, mayoritariamente dilatación neumática (84.2%). La técnica quirúrgica empleada fue la miotomía de Heller asociada a funduplicatura tipo Toupet, con un tiempo operatorio medio de 211 minutos. La estancia media fue 5 días. Se produjeron 2 perforaciones postoperatorias (4.4%). La mortalidad peroperatoria fue del 0%. El seguimiento medio fue de 64 meses. A 3 y 5 años se evidenció una importante disminución del Eckardt score y el estudio manométrico mostró una disminución de la presión del esfínter esofágico inferior en reposo media del 58% y del 70%, respectivamente, con persistencia de hipomotilidad del cuerpo esofágico. En dos pacientes (5.4%) se diagnosticó reflujo gastroesofágico patológico y 4 (10.8%) presentaron recurrencia de los síntomas, requiriendo dilataciones neumáticas endoscópicas. En 2 casos las dilataciones no fueron efectivas por lo que se planteó la realización de una miotomía endoscópica.
ConclusionesSegún nuestra experiencia, la cirugía robótica constituye un procedimiento seguro y efectivo para el tratamiento de la acalasia.
Achalasia is a rare oesophageal motility disorder of unknown aetiology, characterised by an inability of the lower oesophageal sphincter to relax and aperistalsis of the oesophageal body.1 Dysphagia is the main symptom and the surgical technique of choice for its treatment is Heller myotomy associated with laparoscopic partial fundoplication.1,2 Despite the proven long-term safety and efficacy of this approach, series published by experienced centres have reported a recurrence of 10% and up to 15% of intraoperative perforations.3–5 Recent studies suggest that these results could be improved by the better visualisation and greater range of motion provided by robotic surgery, allowing for a more complete and safer myotomy (0%–7% intraoperative perforations), with a consequent better clinical outcome and a tendency to a lower recurrence rate.3,6–10,14,15
The aim of this study is to present the outcomes of the robotic approach in achalasia surgery.
Material and methodsA review was carried out of patients with achalasia who underwent robotic surgery between May 2010 and April 2019 at the Esophagogastric Surgery Unit of the University Hospital of Bellvitge. Demographic, clinical, diagnostic, surgical and evolutionary variables were collected in a prospective database. The end of follow-up was September 2020.
The diagnosis was made on the basis of clinical, radiological (oesophagogram and sometimes also computed tomography) and endoscopic data, ruling out pseudoachalasia caused by tumours. In all cases, oesophageal manometry was confirmed (conventional type in the vast majority of patients, given that high-resolution manometry was not introduced in our centre until July 2020).
Symptoms were assessed using the Eckardt score on inclusion on the waiting list and in the outpatient controls after surgery. All patients were operated by the same surgical team. At 48 or 72 h post-surgery, depending on availability, an oral contrast imaging test was performed and if no leakages or other complications were detected, a liquid diet was started.
Following surgery, clinical follow-up was carried out in consultations at 1 month, 6 months and annually thereafter. Endoscopic, manometric and ph-metric controls were performed at 3 and 5 years.
Disease recurrence was defined as the need for intervention to treat symptoms after surgery.
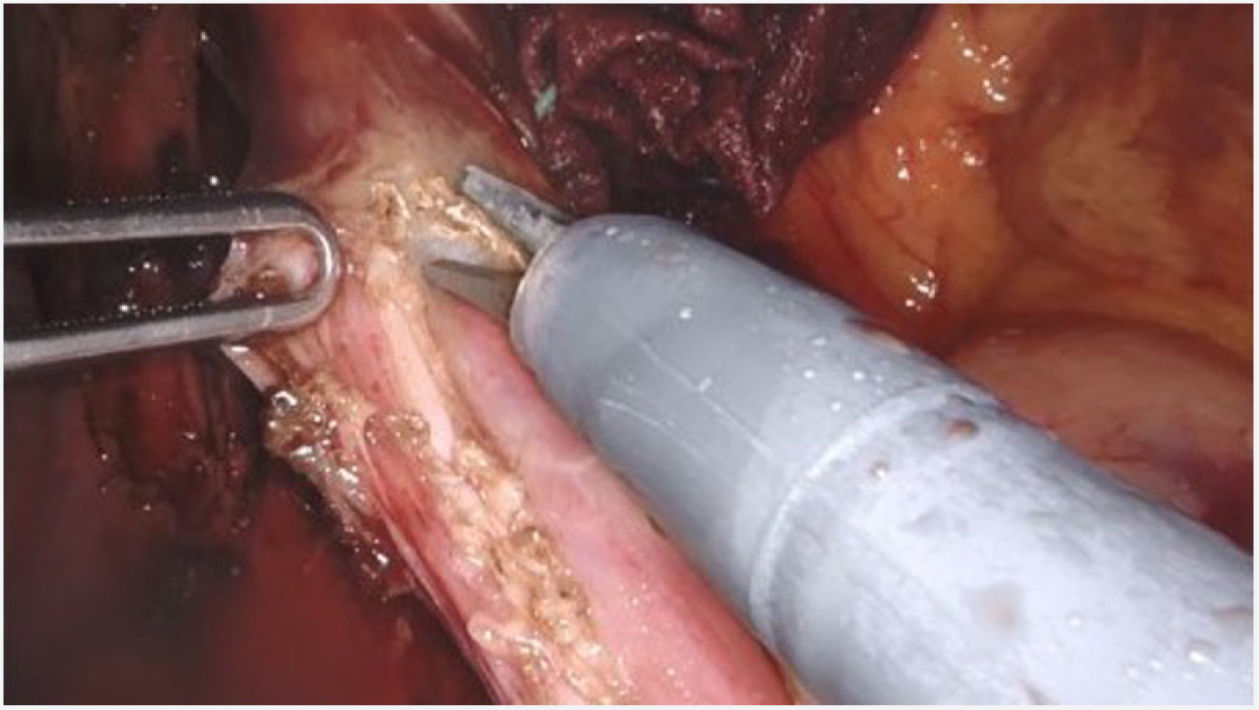
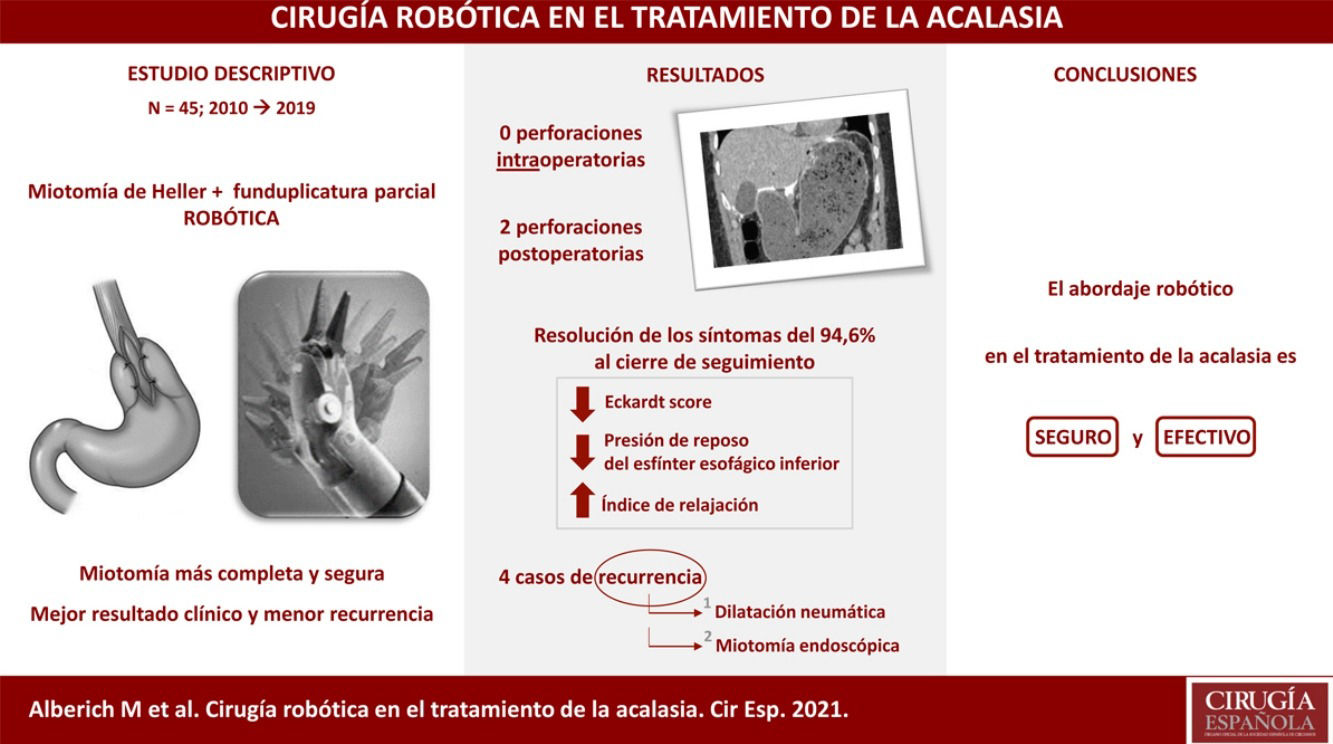
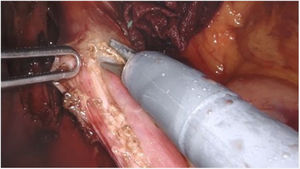
Surgical techniqueThe Si version of the Da Vinci surgical system was used, with a four-arm trolley, three of which were used. The patient was positioned in the supine position. Pneumoperitoneum was performed with a Veress needle in the left hypochondrium. Five trocars are used: one epigastric trocar of 8 mm, two right and left paramedian trocars of 8 mm and two lateral trocars of 5 mm. The robot was then attached. The procedure began with the opening of the lesser omentum, followed by dissection of the diaphragmatic pillars and the intra-abdominal oesophagus, preserving the vagal branches. The short vessels were sectioned. A longitudinal myotomy 10 cm in length was performed, extending 8 cm proximally from the cardia and 2 cm distally towards the stomach, with preservation of the submucosal plane (Fig. 1). The anti-reflux technique of choice is the Toupet-type partial fundoplication, with four simple stitches on each side between the myotomy margin and the gastric fundus, the first of which is also fixed to the ipsilateral diaphragmatic pillar. If necessary, e.g., if there were doubts about the integrity of the mucosa, partial Dor-type fundoplication was performed. An aspiration drain was left in place.
Statistical analysisA descriptive analysis of data was performed, expressed as percentages for categorical variables and as mean ± standard deviation for continuous variables.
ResultsThe sample comprised 45 patients, 25 men and 20 women, with a mean age of 44 years (range 20–65 years). The majority of patients presented a body mass index within the normal interval (68.9% <24.9 kg/m2) and a grade II anaesthetic risk (64.5%) according to the American Society of Anesthesiologists’ classification. No patient had a history of supramesocolic abdominal surgery.
The time period from onset of symptoms to diagnosis was less than 5 years in 82.2% of patients. Preoperatively the mean Eckardt score was 4.26. The main symptom at diagnosis was dysphagia (93.3% of patients) which appeared daily (42.2%) or even with every meal (31.1%). Twenty-four patients (53.3%) had weight loss, being >10 kg in 10 cases. Other frequent symptoms were chest pain and regurgitation (33.3% both). Pathological gastro-oesophageal reflux was detected in 2 of the 12 patients who had preoperative pH-metry (DeMeeste > 14.7).
The mean lower oesophageal sphincter pressure at rest was 27.25 mmHg and the mean residual pressure after swallowing was 12.05 mmHg, with a mean relaxation index of 56.35%. At the level of the oesophageal body, manometry showed aperistalsis, except in 3 cases corresponding to vigorous achalasia in which hypertensive waves were detected.
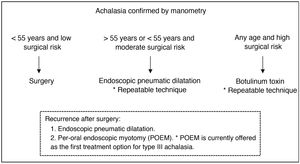
Nineteen patients (42.2%) had received endoscopic treatment prior to surgery, 14 (73.7%) underwent pneumatic dilatation, 3 (15.8%) received botulinum toxin injection and 2 (10.5%) received both treatments. Of the 16 patients undergoing pneumatic dilatation, 12 (75%) had required 2 or more sessions. Fig. 2 shows the treatment algorithm used in our centre. In the patients in the series who received botulinum toxin, this was indicated as a bridge therapy to surgery in the context of the need for admission due to absolute dysphagia.
Operative and postoperative resultsThe surgical technique used was Heller myotomy associated with Toupet-type partial fundoplication, except in 2 cases in which Dor-type partial fundoplication was associated, due to intimate contact between the stomach and spleen in one patient and difficulty in dissection due to significant fibrosis in relation to previous dilatations in another. There were no intraoperative perforations and no need for conversion to open surgery.
The mean operative time was 211 min, including robot docking time. In the first 8 cases in the series, intraoperative endoscopy was performed to assess completeness of myotomy and possible perforations, with the mean operative time amounting to 294 min vs. 193 min (range 122–290) in the remaining cases. In patients who underwent pneumatic dilatation prior to surgery, the mean operative time was only increased by 2 min.
The median hospital stay was 5 days (range 3–17 days), with 62.2% of patients being discharged within the first 4 days.
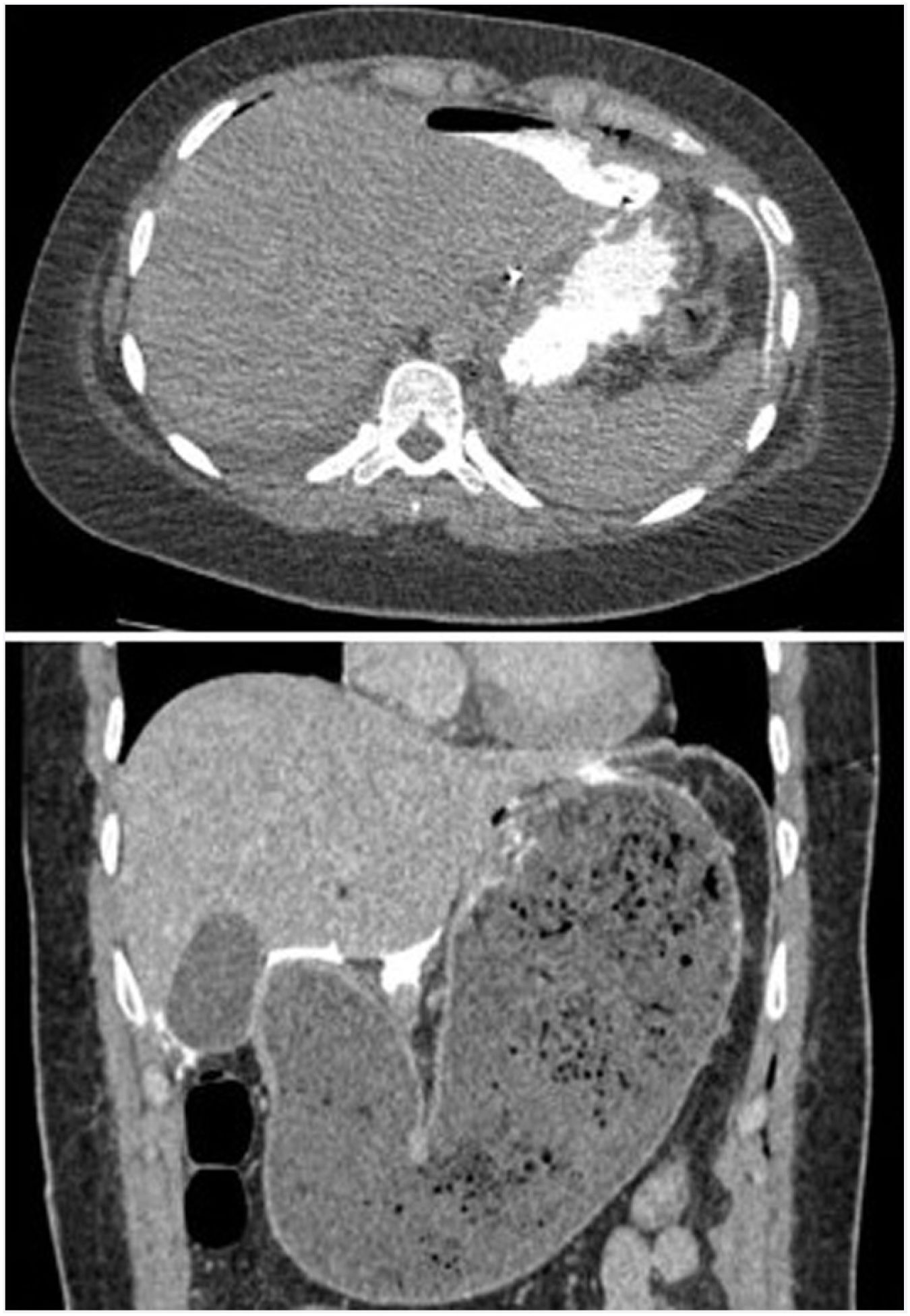
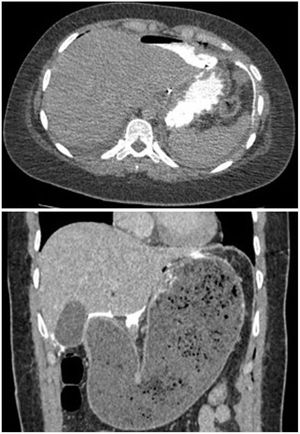
There were two postoperative perforations (4.4%); one on the third day in a patient with Allgrove syndrome (congenital adrenal insufficiency, achalasia and alacrhythmia) and another on the fourth day in a patient who had been discharged on the third day with an imaging test without leakage and correct oral tolerance, and who after diastetic transgression consulted the emergency department for vomiting and abdominal pain with evidence of gastroparesis and oral contrast leakage in the emergency scan (Fig. 3). Both were re-intervened by mid laparotomy, with evidence of perforation in the gastric aspect of the myotomy and primary suturing of the defect and placement of drains, with good postoperative evolution. The patient with triple A syndrome presented a partial epileptic seizure in the postoperative period after the reoperation. Another minor complication was acute urinary retention, which occurred in 3 patients. The operative and postoperative data are shown in Table 1.
Operative and postoperative data (n = 45).
| Length of hospital stay (days)±s (range) | 5 ± 3 (3–17) |
| Surgical technique n (%) | |
| Myotomy + fundoplication | |
| - Toupet | 43 (95.6) |
| - Dor | 2 (4.4) |
| Time in surgery (min)±s | 211.75 ± 60.17 |
| Conversion to open surgery n (%) | 0 (0) |
| Intraoperative perforation in (%) | 0 (0) |
| Postoperative perforation n (%) | 2 (4.4) |
| Re-intervention n (%) | 2 (4.4) |
| Perioperative mortality n (%) | 0 (0) |
Mean follow-up was 64 months (s ± 34). Eight patients were excluded. Seven were lost to follow-up and one died from unknown causes at 32 months follow-up. Out of the remaining 37, 10 have reached 3 years follow-up, 19, 5 years follow-up and 2, 10 years follow-up.
At the end of follow-up, 94.6% of patients had resolution of symptoms. There was a significant decrease in the Eckardt score at 3 and 5 years compared to preoperative values of .5 and 1, respectively (Table 2).
Clinical and manometric outcomes (n = 37).
| Preoperative | Postoperative | ||
|---|---|---|---|
| 3 years | 5 years | ||
| Eckardt score ±s | 4.26 ± 2.51 | .5 ± .77 | 1 ± 1.85 |
| LOS mean resting pressure (mmHg) ± s | 27.16 ± 10.81 | 11.32 ± 6.02 | 8.07 ± 3.67 |
| LOS mean residual pressure (mmHg) ± s | 12.06 ± 7.76 | 6.56 ± 3.26 | 5.45 ± 3.70 |
| Relaxation index (%) ± s | 56.35 ± 29.04 | 59.76 ± 14.41 | 62.54 ± 20.49 |
LOS : lower oesophageal sphincter.
Postoperative manometric results were available in 14 patients at 3 years and in 14 at 5 years (Table 2). A reduction in both mean resting pressure (58% and 70% at 3 and 5 years, respectively) and mean residual pressure (45% and 54% at 3 and 5 years, respectively), as well as an increase in the relaxation index were evident. Aperistalsis of the oesophageal body persists in all patients. Additional follow-up examinations at 10 years follow-up are not yet available.
In follow-up endoscopies, performed in 25 patients at 3 years and in 14 at 5 years, 7 cases of oesophagitis were detected. There were two cases (5.4%) of pathological gastro-oesophageal reflux demonstrated by pH-metry. In the absence of symptoms and normal pH-metry, proton pump inhibitors are withdrawn.
There have been 4 cases (10.8%) of recurrence: two at one year after surgery, one at two years and one at six years. The diagnosis was clinical in all 4 cases, 2 of which also had manometry. All have undergone treatment with endoscopic pneumatic dilatation with good subsequent evolution in two cases after 2 and 3 dilatations, respectively. The remaining 2 cases are awaiting evaluation of oral endoscopic myotomy as rescue treatment.
DiscussionThe treatment of achalasia seeks to alleviate the symptoms derived from the lack of relaxation of the lower oesophageal sphincter (LOS) by reducing the pressure at the level of the LOS, thus facilitating the passage of food from the oesophagus to the stomach. Compared to other treatments, surgery achieves the most durable results3,5,11 while treating the gastro-oesophageal reflux that frequently occurs in these patients due to LOS disruption.8,14 Heller myotomy associated with partial fundoplication is the surgical technique of choice. Since its introduction in the early 1990s, the laparoscopic approach has been considered the gold standard, proving to be effective and safe while providing less morbidity, shorter hospital stay and less pain compared to open surgery.2,10,11 However, the percentage of oesophageal perforations and recurrence of symptoms remained high and even comparable to those of the open technique.3,5 In 2001 Melvin et al. described the robotic approach for the treatment of achalasia,12 and in the series published over the years it has emerged as a comparable, if not better, option to laparoscopy.5–10,13 Kim et al. describe a trend towards better clinical outcome and lower recurrence in the robotic-assisted group compared to the laparoscopic group in relation to a longer and more complete myotomy into the stomach due to the better visualisation and greater range of motion offered by the robot,7 which in turn makes the robotic procedure safer, resulting in a lower percentage of intraoperative perforations3,15 and, if any, less conversion to open surgery for repair.5 The main disadvantage of robotic technology is its cost, although in hospitals where it is shared by several specialties this is mitigated8and will probably be reduced over time, like that of laparoscopy.10,15 Nevertheless, prospective randomised clinical trials in large series of patients are needed to give definitive conclusions on the best surgical approach for the treatment of achalasia.3,9
Since its introduction in 2010 in our centre, robotic surgery has been used in the treatment of patients with achalasia by performing Heller myotomy associated with Toupet-type partial fundoplication. Posterior fundoplication is preferred to anterior fundoplication because it is considered to help keep the myotomy margins open and less fibrosis occurs in this area. Dor-type partial fundoplication is used if necessary, either for technical reasons or if there are doubts about the integrity of the mucosa. In our series there were no intraoperative perforations, but there were two postoperative perforations (4.4%) located on the gastric side of the myotomy and in patients undergoing pneumatic dilatation prior to surgery. Horgan et al. describe that perforations are more frequent at the oesophagogastric junction and in the proximal stomach, probably due to the greater difficulty in developing the submucosal plane due to the change in direction of the muscle fibres from the oesophagus to the stomach and to a greater tendency to bleed in these areas.5 They also report that other factors, and not only previous dilatations, seem to be related to the frequency of this complication.5 Four patients in the series (10.8%) had recurrence of symptoms. All of them underwent several balloon dilatations, which were successful in two cases, while the remaining two are awaiting evaluation of endoscopic per-oral endoscopic myotomy (POEM), which in our centre would have its main indication as a treatment for recurrence after robotic myotomy. The 2018 clinical guidelines of the International Society for Diseases of the Esophagus recommend pneumatic dilatation as the first treatment option after failed myotomy, and POEM is considered an appropriate treatment for recurrence after laparoscopic myotomy.14 The robot may also have an application in reoperation for incomplete laparoscopic myotomy.7
POEM has been shown to be an effective and safe technique in the short and medium term,15,16 superior to laparoscopic myotomy13 especially in the treatment of type III achalasia as it allows a greater extension of the myotomy proximally towards the spastic thoracic oesophagus.17 Our group believes that the robot is also suitable in this scenario. Kashab et al. describe significantly longer myotomies by POEM than by robot (11.6 cm vs. 8.6 cm, P < .0001), but with equal efficacy and safety for both techniques.18 The main drawback of POEM versus surgery is the high incidence of gastro-oesophageal reflux and oesophagitis associated with it.15,16 To address this, Ione et al. presented a pilot study with 21 patients in which an endoscopic fundoplication was added to the standard POEM.19 Will POEM eventually replace surgery? Randomised studies comparing long-term effectiveness of POEM, laparoscopic and robotic Heller myotomy will be needed to elucidate this.
Patients with achalasia require long-term follow-up; early success after treatment does not indicate that this result will be maintained over time.6 In our centre, we propose follow-up with manometric, endoscopic and pH-metric studies at 3, 5 and 10 years in order to objectively validate the long-term results of the implementation of robotic myotomy. These are patients with a chronic disorder in which the motor disorder of the oesophageal body cannot be restored, so we must not only assess the possibility of pathological gastro-oesophageal reflux but also of symptoms caused by stasis of material in the distal oesophagus.6 It should not be forgotten that patients with achalasia carry an increased risk of developing squamous cancer of the oesophagus 10 years or more after treatment.14
ConclusionsIn our experience, the robotic approach in the treatment of achalasia is safe and effective. Endoscopic myotomy is reserved for cases of recurrence after surgical treatment that do not improve with pneumatic dilatation.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Alberich Prats M, Bettonica Larrañaga C, Miró Martín M, Aranda Danso H, Estremiana García F, Farran Teixidor L. Cirugía robótica en el tratamiento de la acalasia. Cir Esp. 2022;100:410–415.