First described in 2002, the use of robotic liver surgery has not spread widely due to its high cost and the lack of a standardized training program. While still considered a technique ‘under development’, it has the potential to overcome the traditional limitations of the laparoscopic approach in liver surgery.
MethodsWe analyzed the postoperative outcomes of 10 patients who had undergone robotic partial resection of the caudate lobe (Spiegel lobe) from March 2014 to May 2016 in order to evaluate the advantages of the robotic technique in the hands of a young surgeon.
ResultsThe mean operative time was 258min (150–522) and the estimated blood loss 137ml (50–359); in none of the cases was blood transfusion required. No patients underwent conversion to open surgery; the overall morbidity was 2/10 (20%), and none of the complications (biliary fistula and pleural effusion) required surgical revision. At histological examination, the mean tumor size was 2.63cm, and we achieved an R0-resection rate of 100%. The 90-day mortality rate was null. The 1-year overall and disease-free survival rates were 100% and 80%, respectively.
ConclusionsDespite several concerns regarding cost-effectiveness, fully robotic partial resection of the caudate lobe is an advantageous, implementable technique that provides promising short-term postoperative outcomes with an acceptable benefit–risk profile.
La cirugía hepática robótica descrita por la primera vez en 2002 no se ha extendido amplialmente debido a su alto costo y a la falta de un programa de entrenamiento estandarizado. Aún siendo considerada como una técnica de “desarrollo en progreso”, tiene, sin embargo, potencial para superar las limitaciones tradicionales del abordaje laparoscópico en las intervenciones hepáticas.
MétodosSe analizaron los resultados postoperatorios de 10 pacientes sometidos a resección robótica de lóbulo caudado (lóbulo de Spiegel) desde Marzo de 2014 hasta de Mayo 2016 para evaluar las ventajas de la técnica robótica.
ResultadosEl tiempo medio de operación fue de 258min (150-522) y la pérdida estimada de sangre 137ml (50-359), en ninguno de los casos una transfusión de sangre fue necesaria. Ningun paciente se sometía a una conversión a cirugía abierta; la morbilidad global fue de 2/10 (20%) y todas las complicaciones (fístula biliar y derrame pleural) fueron clasificadas como menores. En el examen histológico, el tumor diametro medio fue de 2.63cm y se logró realizar la resección R0 en 100% de casos (10/10). La tasa de mortalidad a los 90 días fue nula. Las tasas de supervivencia general y de supervivencia libre de enfermedad a 1 año fueron de 100% y 80%, respectivamente.
ConclusionesA pesar de varias preocupaciones con respecto a la rentabilidad, la resección robótica del lóbulo caudado es una técnica ventajosa y aplicable, que proporciona prometedores resultados postoperatorios a corto plazo con un perfil de riesgo-beneficio aceptable.
Firstly described in 1992, the introduction of minimally invasive surgery (MIS) for liver resections has been slower than in other surgical fields. An impressive meta-analysis including 31 publications and 2473 patients1 has demonstrated superior results of the laparoscopic approach for hepatic procedures in terms of estimated blood loss, transfusion rate, post-operative pain, shortened length of hospital stay and enhanced cost-effectiveness in comparison to open approach with similar morbidity and mortality rates showing similar findings as previously published by other authors.2,3
These advantages can be potentially beneficial with regard to the overall survival ensuring a faster postoperative return to normal activities and faster adjuvant chemotherapy start time.4,5
Considering serious clinical and surgical challenges in patients presented with hepatocellular carcinoma (HCC), the potential advantages of MIS technique in liver interventions are even more significant with regard to an opportunity to preserve the abdominal wall integrity and the function of diaphragm. In fact, by this approach a better collateral venous drainage is maintained leading to a less risk of postoperative ascites and lower number of post-operative adherence.6,7
Despite no oncologic disadvantages of MIS with respect to open technique in terms of resection margin infiltration, local recurrence, 5-year overall survival and mortality,8,9 the implementation of the above approach is still confined to highly specialized centres.
However, while the number of worldwide laparoscopic resections reported per year increased from 1471 procedures in 2009 to 1908 in 2014, the rate of complex hepatectomies performed in a minimally invasive fashion still remains low.
It is worthy of note that the use of the laparoscopic equipment is hampered by the presence of many already well-known drawbacks: the compromised dexterity, the limited degrees of motion (only 4) and the fulcrum effect associated with a physiological tremor. The mentioned features are potential deterrents to the widespread adoption of the minimally invasive laparoscopic liver surgery.10
Laparoscopic isolated caudate lobectomy is considered a particularly risky and difficult procedure that has been reported rarely. In fact, only few case series of S1-resection have been described, mainly in the context of technically dyshomogeneous series.11–13
Caudate lobe liver resection is a challenging procedure because of the unique and complicated anatomy of the lobe (deep location and proximity to great vessels due to the position between the major vascular structures with the IVC posteriorly, the portal triad inferiorly and the hepatic venous confluence superiorly). Furthermore, the variability of portal and arterial flow as well as the complex venous and biliary drainage system oblige surgeons to perform a meticulous vascular control.
Even though the isolated robotic resection of hepatic segment I has been reported in a context of small series, there is still a lack of systematic robotic technical descriptions of the procedure provided along with the analysis of postoperative outcomes in the literature.
The aim of this work is to describe our technique for robotic isolated partial caudate lobectomy (Spiegel lobe resection) by left-sided approach and our initial experience as well as technical considerations in a series of patients, providing a retrospective analysis of our case-series short-term outcomes associated with the above surgery.
MethodsBetween March 2014 and May 2016, 10 consecutive patients underwent robotic isolated partial caudate lobe resection. A single surgeon, both expert in open and mini-invasive surgery, performed all surgeries by left-sided approach, employing the da Vinci Si surgical system (Intuitive Surgical, Inc., Sunnyvale, CA) at General Hospital of Palermo (Italy).
The maintained databases were retrospectively reviewed to evaluate the short-term outcomes and analyze the feasibility and safety of this intervention.
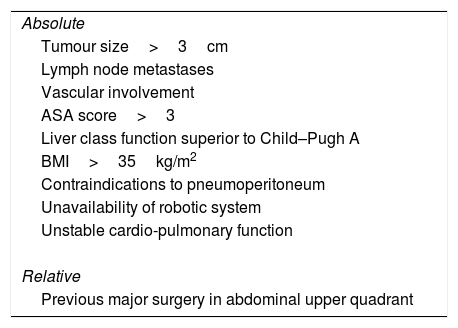
All clinical cases were discussed on a pre-operative multidisciplinary meeting conference, during which we selected the best surgical approach for individual patient (robotic versus open). In one case, a diagnostic uncertainty regarding asymptomatic lesion, which appeared to enlarge in size on the consecutive imaging procedures, was considered an indication for surgery; the following histopathology report revealed the signs of focal nodular hyperplasia. We excluded from this study all patients requiring a simultaneous procedure. Contraindications for robotic surgery are depicted below (Table 1). Patients were informed of the innovative nature of the procedure, and written consent was obtained before surgery.
Contraindications to Robotic-Assisted Caudate Lobectomy.
| Absolute |
| Tumour size>3cm |
| Lymph node metastases |
| Vascular involvement |
| ASA score>3 |
| Liver class function superior to Child–Pugh A |
| BMI>35kg/m2 |
| Contraindications to pneumoperitoneum |
| Unavailability of robotic system |
| Unstable cardio-pulmonary function |
| Relative |
| Previous major surgery in abdominal upper quadrant |
BMI, body mass index; ASA, American Society of Anaesthesiologists.
The preoperative work-up included a whole-body contrast-enhanced computed tomography (CT), liver gadoxetic acid-enhanced magnetic resonance imaging (MRI), abdominal ultrasound (US) tumor markers assessment (AFP, CEA, Ca-19.9), and routine blood examination. Hepatitis panel was requested in the case of suspicion, while if a hydatid cyst was suspected, the patient underwent serological tests for echinococcosis. A preoperative evaluation of the liver function was carried out to assess the retention rate of indocyanine green at 15min after administration.
A biliary leakage was diagnosed in case when a bilirubin concentration in the drainage fluid was at least 3-fold higher than that of the serum.
A positive resection margin was defined as the presence of tumor cells at the line of transection due to microscopic involvement of the main tumor, venous permeation, or microsatellite nodules.
All patients were followed-up monthly for the first year after the operation, and then quarterly with a CT scan or abdominal ultrasonography; in case of recurrence suspicion, a MRI or a tissue samplings were performed.
Technical DescriptionUnder general anesthesia, the patient is placed in a supine, 25°-reverse Trendelenburg position with the arms tucked to the sides and legs apart; the table is slightly tilted to the right side where the Ultrasound system is located.
The scrub nurse stands at the left side of the patient, while the assistant surgeon is positioned between the patient's legs.
The pneumoperitoneum is induced through a Veress needle inserted in the Palmer's point, and the abdomen is insufflated with gas. The intraabdominal pressure is maintained at 12mmHg. The central venous pressure is maintained low (5cm H2O) to decrease the risk of blood loss during the liver transection.
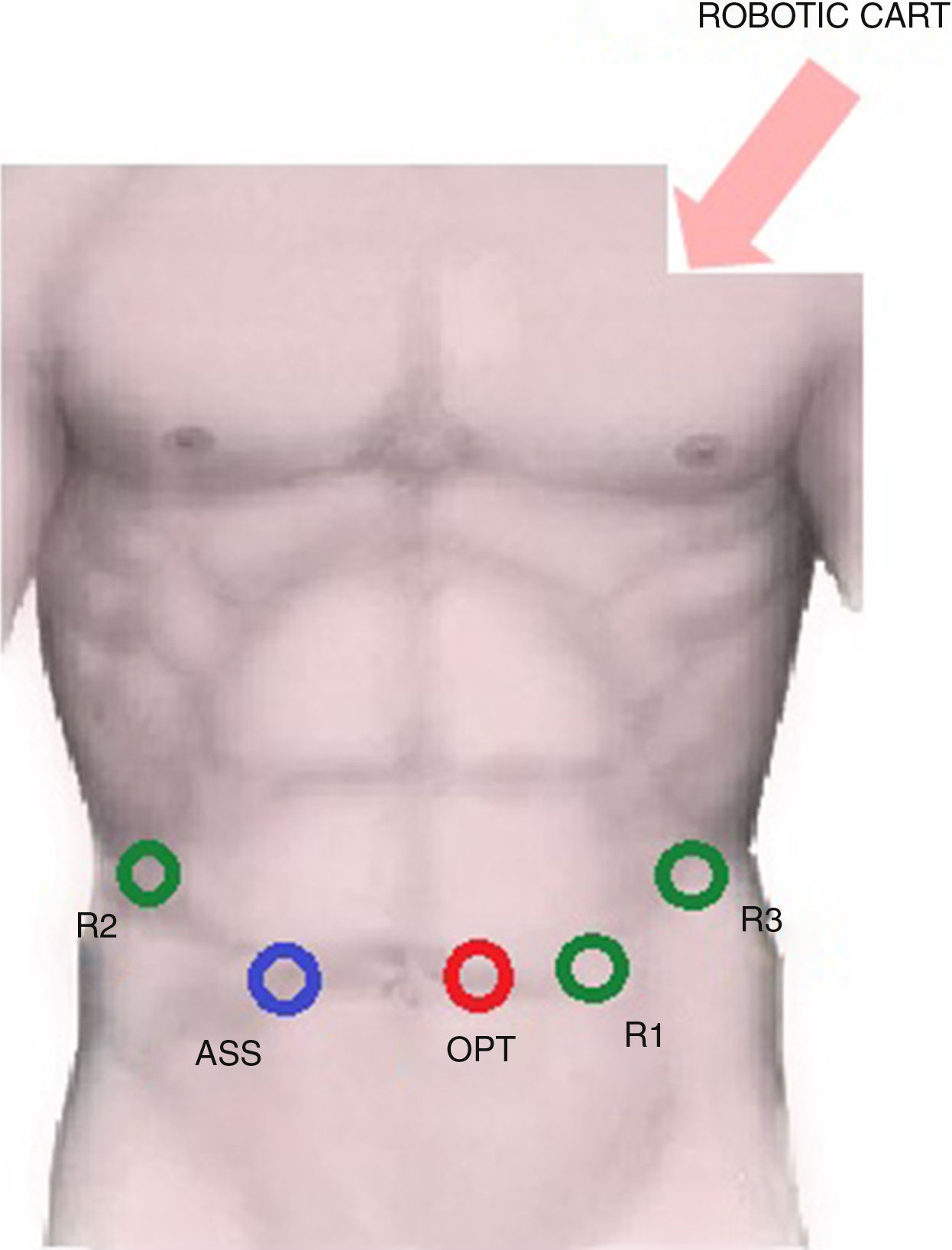
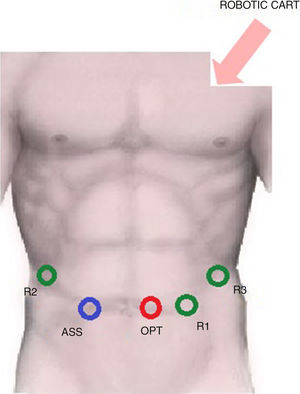
The trocar layout is showed in Fig. 1.
- -
R1: 8mm trocar in the epigastrium, 4cm to the left of xipho-umbilical line,
- -
R2: 8mm trocar in the right hypochondrium,
- -
R3: 8mm trocar in the left hypochondrium,
- -
Optic port: 12mm trocar in the periumbilical area,
- -
Assistant: 12mm laparoscopic trocar in right pararectal area.
The robotic patient cart is placed between the patient's head and his left shoulder for the docking phase.
We employ a 30-degree laparoscope in the optic port; the monopolar scissors – in arm number 1, while in the arm number 2, we insert the bipolar forceps; finally, the Prograsp forceps is used in the arm number 3 as a stable liver retractor.
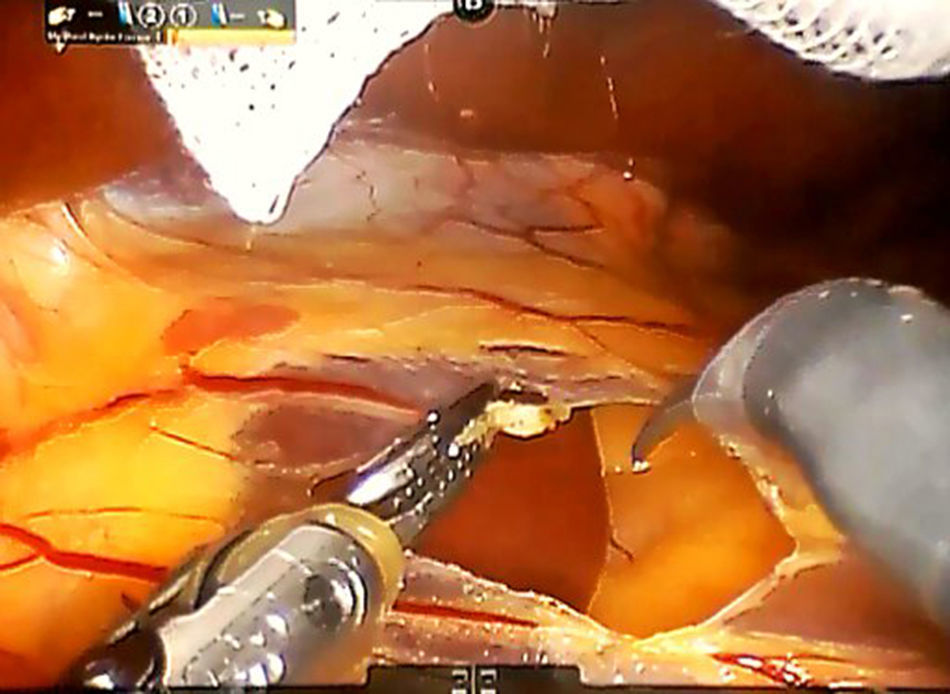
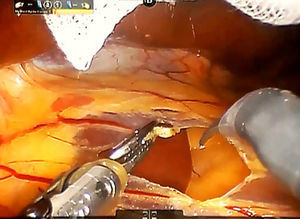
Step 1: We dissect the round and falciform ligaments of the liver and perform an intraoperative ultrasonography to confirm the presence of tumour and its relationship with the main vascular structures. After the division of the left triangular and coronary ligaments, the mobilization of the left liver lobe is performed to provide a more effective exposure. The pars flaccida of the lesser omentum is incised, while the third arm retracts the left lobe upward, exposing the caudate lobe (Fig. 2).
An umbilical tape is inserted around the hepatic hylum in order to allow a retraction of the hepatic hylum since we do not perform routinely a Pringle's maneuver.
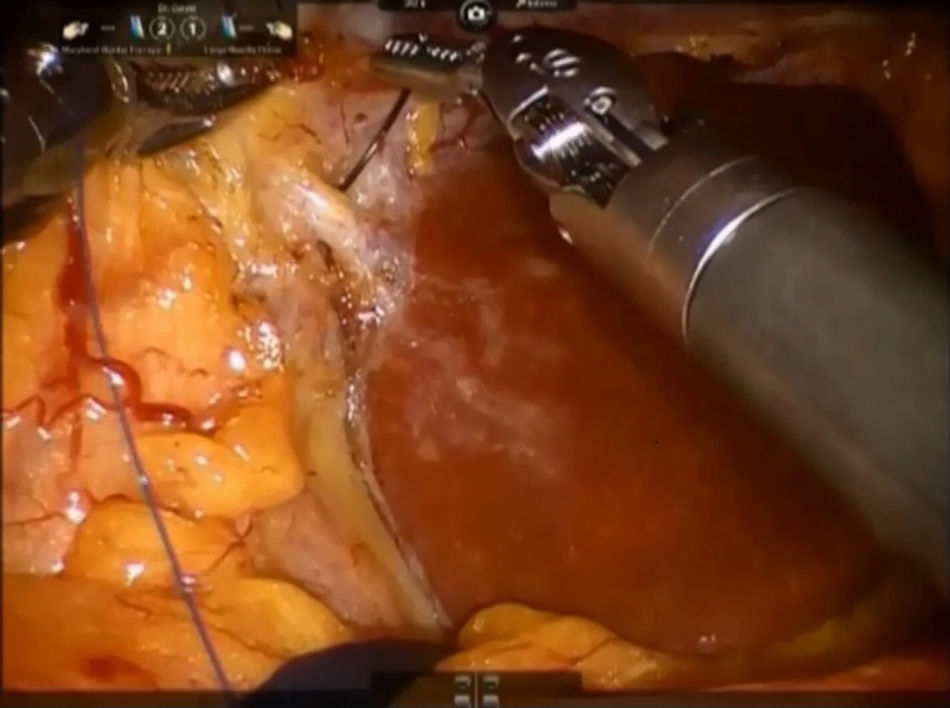
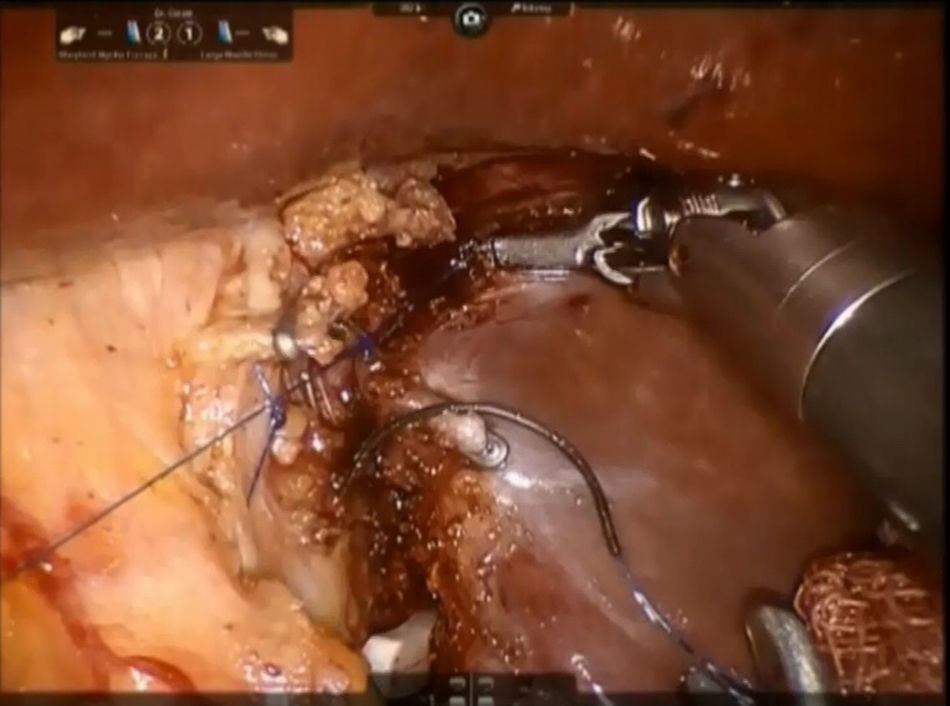
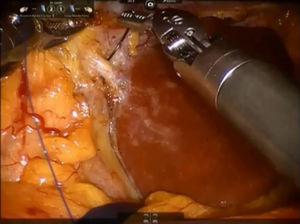
Step 2: The caudate lobe is retracted toward the left side, and the caudal branches from the left portal vein are sutured and divided with employment of stitches or metallic clips (Fig. 3). The Arantius’ ligament (which represents the left boundary of the caudate lobe) is dissected in cranial direction and to the right in order to expose the upper part of caudate lobe and the posterior Glissonean pedicle.
A stay suture is placed on the Spiegel lobe and retracted ventrally by the third arm.
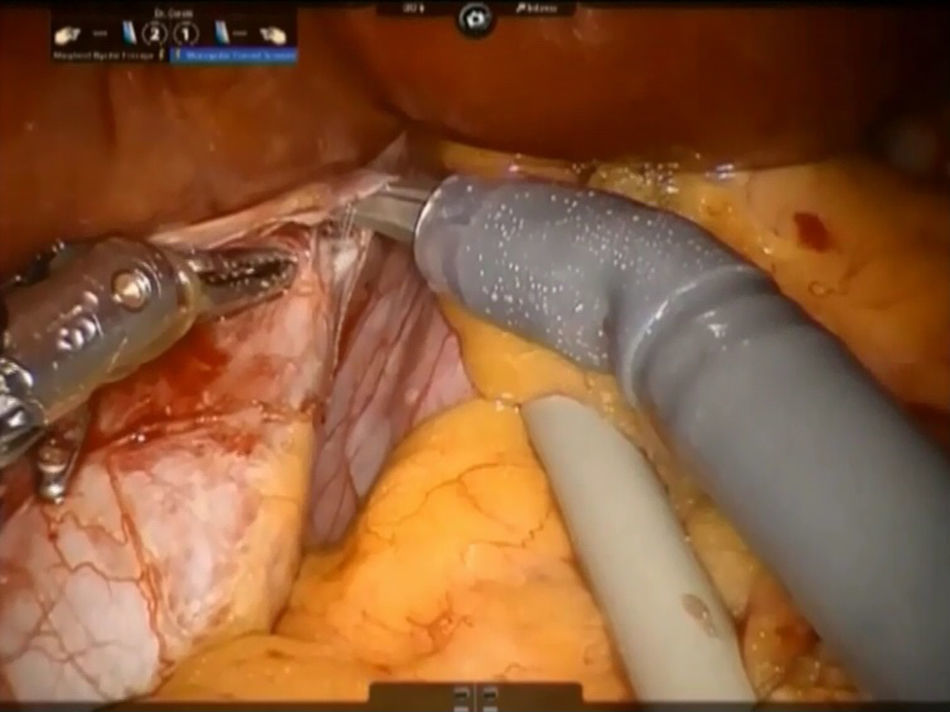
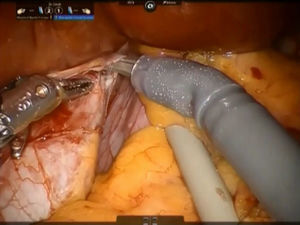
The left caval ligament underneath the caudate lobe is opened and the short accessory hepatic veins are dissected with Maryland forceps and scissors, sutured with 4-0 polypropylene or controlled with metallic clips and next divided, freeing completely the anterior surface of IVC from the Spiegel lobe (Fig. 4). This step is performed from the left side to the right and in caudo-cranial direction.
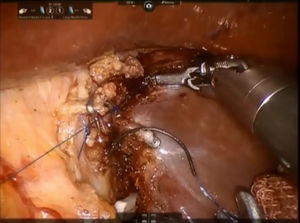
Step 3: The parenchyma of the pericaval portion is transected by bipolar forceps or monopolar scissors with clipping and division of all short glissonian branches by simple stitches or Hem-o-lock® (TFX Medical Ltd., RTP, Durham, NC, USA) clips (Fig. 5). All biliary structures are clipped by laparoscopic metallic clips. During this step, the caudate process is kept on the right side while the caudate lobe is pulled leftwards. A control of the bile leakage and haemostasis on the resection surface is achieved by suturing with a 3/0 monofilament polypropylene suture. A fibrin glue is placed on the resection margin and a drainage tube is inserted into the Winslow's foramen. The specimen is placed inside a plastic bag and the extraction is performed trough the assistant's trocar site or Pfannenstiel incision.
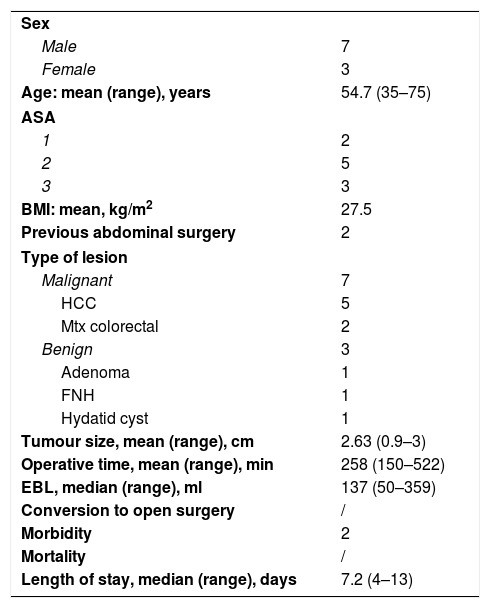
ResultsIn our series of 10 patients (mean age 54.7, male/female ratio 7:3), 7 malignant conditions (including 5 cases of hepatocellular carcinomas (HCC), 2 cases of metastatic colon cancer) and 3 benign conditions were confirmed by pathologists. The demographic aspects and the perioperative outcomes are summarized in Table 2.
Demographic Characteristic and Perioperative Outcomes of Robotic Partial Caudectomy Cohort (No.=10 Patients).
| Sex | |
| Male | 7 |
| Female | 3 |
| Age: mean (range), years | 54.7 (35–75) |
| ASA | |
| 1 | 2 |
| 2 | 5 |
| 3 | 3 |
| BMI: mean, kg/m2 | 27.5 |
| Previous abdominal surgery | 2 |
| Type of lesion | |
| Malignant | 7 |
| HCC | 5 |
| Mtx colorectal | 2 |
| Benign | 3 |
| Adenoma | 1 |
| FNH | 1 |
| Hydatid cyst | 1 |
| Tumour size, mean (range), cm | 2.63 (0.9–3) |
| Operative time, mean (range), min | 258 (150–522) |
| EBL, median (range), ml | 137 (50–359) |
| Conversion to open surgery | / |
| Morbidity | 2 |
| Mortality | / |
| Length of stay, median (range), days | 7.2 (4–13) |
BMI, body mass index; ASA: American Society of Anaesthesiologists; HCC, hepatocarcinoma; FNH, focal nodular hyperplasia; MTX, colorectal liver metastasis; EBL, estimated blood loss.
The operative time ranged from 180 to 522min, mean time was 258min. Regarding the estimated blood loss, it ranged from 50 to 359ml with mean value of 136.7ml. There was no need to perform blood transfusions in any of the cases. The mean tumour diameter was 2.63cm and the mean length of hospital stay was 7.2 days.
None of the patients underwent conversion to laparotomy, while the complications occurred in 2 patients (20%). Among them, one biliary leakage was diagnosed at postoperative day 7 and the patient was treated by percutaneous drainage placement; another patient developed a pleural effusion with a rapid onset of fever, which was successfully managed with antibiotic therapy. The mortality rate in the presented case series was null.
The mean follow-up was 23 months (range 18–36 months). Two patients (20%) developed recurrences. In the first case, a new colorectal cancer metastasis was found in the segment 8 at 12 months after surgery, for which the patient underwent surgical re-intervention. Another patient also was diagnosed with a recurrent metastatic lesion in the segment 7 after 9 months following surgery, which was treated with radiofrequency ablation. Both patients are still alive. The 1-year overall and disease free-survival rates were 100% and 80%, respectively.
DiscussionLaparoscopic liver surgery enables to reduce the blood loss, surgical trauma during operation and is associated with lower morbidity and shorter hospital stay; however, this approach requires high proficiency of surgeon and is widely applicable only for a small range of hepatic resection procedures. Long and complex surgeries actually oblige surgeons to work in poor ergonomic conditions during many crucial steps of the operation that enhances its difficulty. The use of rigid laparoscopic instruments forces to perform major straight-line liver resections even for small nodules and neglect the concept of ‘parenchymal-sparing surgery’, increasing the difficulties encountered during the operation, the fear of mismanaging a major bleeding and compromising a gentle handle of the deep structures.11 The robotic surgery is an interesting technology, which overcomes the traditional limits of laparoscopy and allows performing more complex resections of the liver, especially in case of lesions located in the proximity of the hilar structures and large blood vessels, providing high ergonomics and offering a possibility of ‘curved-shape’ non-anatomic resections.
Since its first description in 2003 by Giulianotti et al.,14 the robotic surgery has presented some promising benefits (EndoWrist instrumentation with seven degrees of freedom, a stable high definition 3-D camera platform, and an improved ergonomy) which can overcome the traditional limitations of laparoscopic surgery. Nevertheless, until now the robotic liver resection is still considered a ‘development in progress’ technique due to the poor data available and the unavailability of all necessary robotic liver surgery equipment.
Therefore, the adoption of robotic liver surgery has been long time hampered by its high cost, the difficulties associated with the anatomic variations of the liver, the absence of a standardized training program and concerns related to the lack of tactile feedback. However, though the robotic approach failed to demonstrate its significant superiority to other MIS techniques, it allowed to increase the rate of major and complex (superior-posterior segments) hepatectomies that can be performed in a minimally invasive fashion following the parenchymal-preserving principle and providing further extension of the indication for MIS liver resections in the near future.
Nine series comparing robotic and laparoscopic liver surgery have shown that robotic approach is a feasible and safe option in well-selected patients providing similar results in terms of perioperative and short-term oncologic outcomes with no increase of complications and mortality rate, however, a longer operative time and higher cost have been reported in the robotic arms.15
The caudate lobe represents one of the most challenging segments for a surgical approach due to its unique and variable anatomy and a close relationship with relevant vascular structures. The isolated caudatectomy requires a high skilled performance even in the traditional open approach regarding the intrinsic anatomy and the presence of several pitfalls: complexity of the radical lobe resection, difficult exposure and the absence of clear boundary. According to Kumon's classification, the caudate lobe consists of three sections: the Spiegel lobe (Couinaud's segment I), the paracaval portion (Couinaud's segment IX), and the caudate process.16 The first part is covered by the pars flaccida of the lesser omentum and lies to the left of the Arantius’ ligament along the left part of inferior vena cava (IVC). The paracaval portion is located to the right of the Spiegel lobe in front of the intrahepatic portion of the IVC. The last portion is the smallest one lying between the IVC and the portal vein on the right of the previous parts.
The caudate lobectomy is classified as complete and partial resection, and also as isolated or combined.
Regarding the paucity of data, now it is impossible to draw definitive conclusions on the safety and oncologic efficacy of the above procedure performed with robotic approach. The initial data reported by several authors suggest the technical feasibility of robotic caudatectomy and show encouraging results in terms of morbidity rate and oncologic adequacy.17,18
Di Benedetto et al. provided a report of one case of totally robotic caudate-lobe resection for hydatid disease showing the advantages, safety and effectiveness of this technique also for this challenge setting.19
In our series, the findings (mean estimated blood loss 137ml, no conversion and null mortality rate) are comparable with other reports19,20 and the encouraging results confirm the potentiality of the robotic technique to overcome the limits of the laparoscopic approach. The relatively long mean operative time in our report was associated with large sizes of the lesions and the need to perform a careful R0-resection. The length of hospitalization was determined by a specific patient management strategy established in our department, when during extra days, an observation aimed to exclude surgery-related complication development was being performed.
The Endowristed instrumentation allowed us to carry out a gentle and precise dissection around the important vascular structures and the HD-3D magnified vision was crucial to perform a meticulous haemostasis. The easier suturing and the opportunity to handle fine structures safely enabled us to provide a hand-sewn control of delicate vascular structures reducing the number of clip application and thus the risk of post-operative clip slippage.
The Motion-scaling modulation of robotic instrument movements according to the choice of operator is essential for a meticulous and safer dissection in case of complex anatomy.
Though this retrospective series is relatively small, herein we have described the largest case series of fully robotic isolated partial caudate lobe resection reported in the literature.
The robotic hepato-pancreato-biliary (HPB) surgery is a promising field, which offers interesting results, as we showed previously,21,22 embracing the complex fields of transplantation and vascular reconstruction. It offers outcomes that are at least not inferior to other approaches for minor liver resections but are relevant in more complex liver interventions. We believe it is necessary for surgeons to establish a correct patient selection, to work in a multi-disciplinary environment point of view following the principle of cooperation, and especially to undergo an adequate specific training to face the potential pitfalls and troubleshooting related to the robotic surgery aiming to increase the rate of robotic major liver resections cases.23
This technology represents a new perspective option, which can be easily integrated with image-guided surgery and fluorescence that are truly essentials in case of unclear and complex anatomy, mainly in the initial phase of learning curve.
In conclusion, the robotic partial caudatectomy is an advantageous, implementable technique providing promising short-term postoperative outcomes with acceptable benefit–risk profile. Our results demonstrate the clear benefits of the robotic system employment in terms of perioperative outcomes suggesting the possibility to extend the indications for hepatic MIS even for hardly accessible anatomic areas.
Contribution of AuthorsMarino MV: Study design and manuscript preparation.
Glagolieva A: Acquisition of data and analysis of results.
Guarrasi D: Critical revison and approval of the final version of manuscript.
Conflicts of InterestThe authors have no conflict of interest to declare.
Please cite this article as: Marino MV, Glagolieva A, Guarrasi D. Resección robótica del lóbulo hepático caudado: descripción técnica y consideraciones iniciales. Cir Esp. 2018;96:162–168.