The laparoscopic approach is not yet widely used in liver surgery, but has proven to be safe and feasible in selected patients even in malignant disease. The experience and results of a hepato-pancreato-biliary (HPB) surgery unit in the treatment of malignant liver disease by laparoscopic approach is presented.
Material and methodsBetween February 2002 and May 2011, 71 laparoscopic liver resections were performed, 43 for malignant disease (only patients with more than one year of follow-up were included). Mean age was 63 years old and 58% of the patients were male. Forty-nine percent of the lesions were located in segments ii–iii. Thirty segmentectomies were performed, 7 limited resections and 6 major hepatectomies.
ResultsThe median operative time was 163min. There were 3 conversions. Five cases (11%) required blood transfusion. The oral intake began at 32h and the median hospital stay was 6.7 days. There were no reoperations and there was one case of mortality. Nine patients (21%) had postoperative complications. The mean number of resected lesions was 1.2, with an average size of 3.5cm. All resections were R0. The median survival after resection of colorectal liver metastases (CLM) was 69% and 43.5% at 36 and 60 months, respectively, and 89% and 68% at 36 and 60 months, respectively, in hepatocellular carcinoma (HCC).
ConclusionThe laparoscopic liver resection in malignant disease is feasible and safe in selected patients. The same oncological rules as for open surgery should be followed. In selected patients it offers similar long-term oncological results as open surgery.
El abordaje laparoscópico no ha tenido una gran difusión en la cirugía hepática, aunque ha demostrado ser seguro y factible en pacientes seleccionados incluso en enfermedad maligna. Se presenta la experiencia y resultados en el tratamiento de la enfermedad hepática maligna por laparoscopia.
Material y métodoEntre febrero de 2002 y mayo de 2011 se realizaron 71 resecciones hepáticas laparoscópicas, 43 por enfermedad maligna (solo se incluyó a pacientes con más de un año de seguimiento). La edad media fue de 63 años y el 58% fueron varones. El 49% de las lesiones estaban situadas en los segmentos ii-iii. Se realizaron 30 segmentectomías, 7 resecciones limitadas y 6 hepatectomías mayores.
ResultadosEl tiempo operatorio fue de 163min. Hubo 3 conversiones. Cinco casos (11%) fueron transfundidos. La ingesta se inició a las 32h y la estancia hospitalaria fue de 6,7 días. No hubo reintervenciones y sí un caso de mortalidad. Nueve pacientes (21%) presentaron complicaciones. El número medio de lesiones resecadas fue 1,2, con un tamaño de 3,5cm. Todas las resecciones fueron R0. La supervivencia fue del 69 y del 43,5% a los 36 y 60 meses en metástasis hepáticas de cáncer colorrectal (MHCCR), y del 89 y 68% a los 36 y 60 meses en hepatocarcinoma (HCC).
ConclusionesLa resección hepática por laparoscopia en enfermedad maligna es factible y segura. Debe cumplir los mismos preceptos oncológicos que la cirugía abierta. En pacientes seleccionados ofrece resultados oncológicos a largo plazo similares a los obtenidos en cirugía abierta.
Laparoscopic liver surgery was performed for the first time in 1992,1 and in Spain in the year 2000.2 After nearly 15 years of technical and technological improvements, laparoscopic liver surgery is considered a feasible and safe technique, but its application is restricted in most cases to minor resections of lesions in peripheral segments.3
With the growing experience of liver surgery groups, the indications for laparoscopy have extended to major liver resections4,5 and include malignant disease in most series with similar oncologic results to those offered by the traditional approach.6,7
In addition to the typical advantages of laparoscopy (less pain, short hospital stay and esthetic benefit), this method also obtains better results in terms of blood loss.8 Today, however, there is still controversy about the benefits of laparoscopy in liver surgery compared to open surgery in malignant disease.
The aim of this paper is to present our experience in the treatment of malignant disease by laparoscopy and to analyze the results of operative time, hospital stay, morbi-mortality and survival.
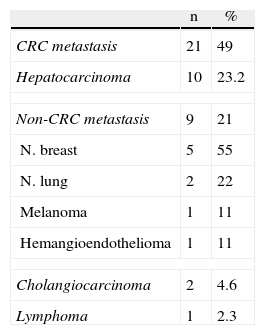
Material and MethodsIn the year 2000, laparoscopic liver surgery was started in our center, initially for benign disease. After 2002, the indications were extended to malignant disease. Between February 2002 and May 2011, 71 liver resections were performed in 71 patients. Of these, 43 were due to malignant disease; the diagnoses are listed in Table 1.
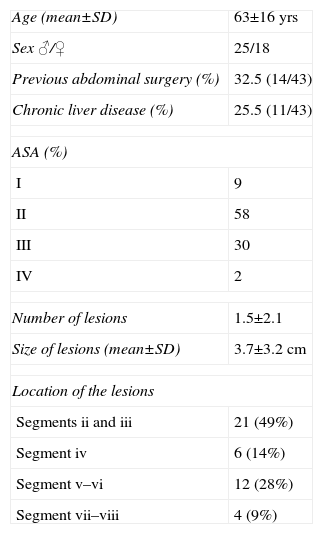
The characteristics of the patients for age, sex distribution, ASA classification, previous surgical history and preoperative localization and size of the lesions are summarized in Table 2. Thirty patients underwent segmentectomy, the most frequent of which was resection of segments II–III in 21 patients. Seven patients underwent limited resection and 6 cases had major hepatectomy (3 right and 3 left hepatectomies).
Patient Characteristics.
| Age (mean±SD) | 63±16yrs |
| Sex ♂/♀ | 25/18 |
| Previous abdominal surgery (%) | 32.5 (14/43) |
| Chronic liver disease (%) | 25.5 (11/43) |
| ASA (%) | |
| I | 9 |
| II | 58 |
| III | 30 |
| IV | 2 |
| Number of lesions | 1.5±2.1 |
| Size of lesions (mean±SD) | 3.7±3.2cm |
| Location of the lesions | |
| Segments ii and iii | 21 (49%) |
| Segment iv | 6 (14%) |
| Segment v–vi | 12 (28%) |
| Segment vii–viii | 4 (9%) |
In 3 patients, simultaneous laparoscopic resection was performed for a colorectal primary tumor and the liver metastases (left hemicolectomy, right hemicolectomy and abdominoperineal resection).
The interventions were performed under general anesthesia using propofol for induction and maintenance, with sevoflurane and remifentanil in perfusion. Patients with catheters were given methadone at the start of the intervention and local anesthetic (levobupivacaine) after liver resection
Patients were placed in supine decubitus with their legs spread or in the left lateral decubitus position to address lesions in the right posterior segments (VI and VII). We used 6 trocars (3 10–12mm and 3 5mm). The procedure was carried out at a pneumoperitoneum pressure of 10–12mmHg. The 30° optics were used through the umbilical port. Intraoperative ultrasound was performed routinely. During liver transection, central venous pressure (CVP) remained between 2 and 6mmHg. For hepatic transection, the monopolar scalpel was used to a depth of 0.5–1cm. After reaching this depth, vessels were dissected with the ultrasonic dissector and coagulated with a 5-mm Ligasure Atlas® (Valleylab, Tyco Healthcare, Boulder, CO, USA.) or Ultracision® (Ethicon Endo-Surgery, Johnson & Johnson Ltd., Cincinnati, OH, USA) combined with laparoscopic Tissuelink® (Salient Surgical Technologies, Portsmouth, NH, USA). The transection was performed in 90% of cases under continuous occlusion of the hepatic pedicle after ischemic preconditioning, with a mean clamping time of 31±14min. The portal segmental pedicles and suprahepatic veins were sectioned with GIA® linear endostaplers with vascular load.
The surgical specimen was extracted in a bag through an accessory incision. Sixty-five percent of patients received intravenous postoperative analgesic treatment and 32% through the catheter.
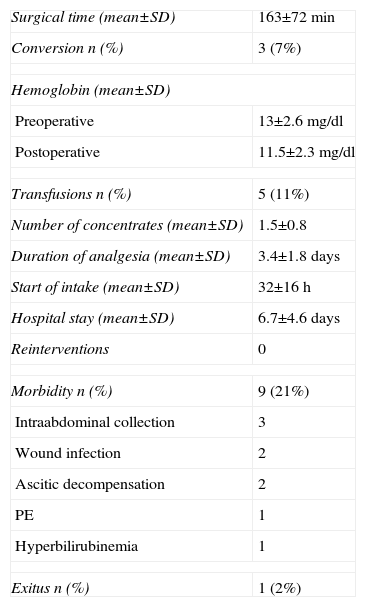
ResultsThe results in terms of operative time, conversion rate, analgesia requirements, start of oral intake and hospital stay are shown in Table 3.
Results.
| Surgical time (mean±SD) | 163±72min |
| Conversion n (%) | 3 (7%) |
| Hemoglobin (mean±SD) | |
| Preoperative | 13±2.6mg/dl |
| Postoperative | 11.5±2.3mg/dl |
| Transfusions n (%) | 5 (11%) |
| Number of concentrates (mean±SD) | 1.5±0.8 |
| Duration of analgesia (mean±SD) | 3.4±1.8 days |
| Start of intake (mean±SD) | 32±16h |
| Hospital stay (mean±SD) | 6.7±4.6days |
| Reinterventions | 0 |
| Morbidity n (%) | 9 (21%) |
| Intraabdominal collection | 3 |
| Wound infection | 2 |
| Ascitic decompensation | 2 |
| PE | 1 |
| Hyperbilirubinemia | 1 |
| Exitus n (%) | 1 (2%) |
Five patients (11%) required red blood cell concentrate transfusion. Three patients were transfused due to intraoperative hemorrhage and the other 2 were cases of simultaneous liver metastases of colorectal cancer (LMCRC) and primary colorectal resection.
There were no reoperations and 9 patients (21%) had postoperative complications, defined according to the Dindo–Clavien classification as 2 grade I complications, 4 grade II complications, 3 grade IIIa complications and one grade V complication (progressive hyperbilirubinemia with renal failure in a patient who underwent right hepatectomy due to LMCRC and died 90 days after surgery), which was the only case of mortality recorded (2%).
In the pathologic analysis of the specimens, the mean number of resected lesions per patient was 1.2±0.6, with an average size of 3.5±1.2cm and a distance to the resection margin of 10.6±9.8mm. All resections were R0.
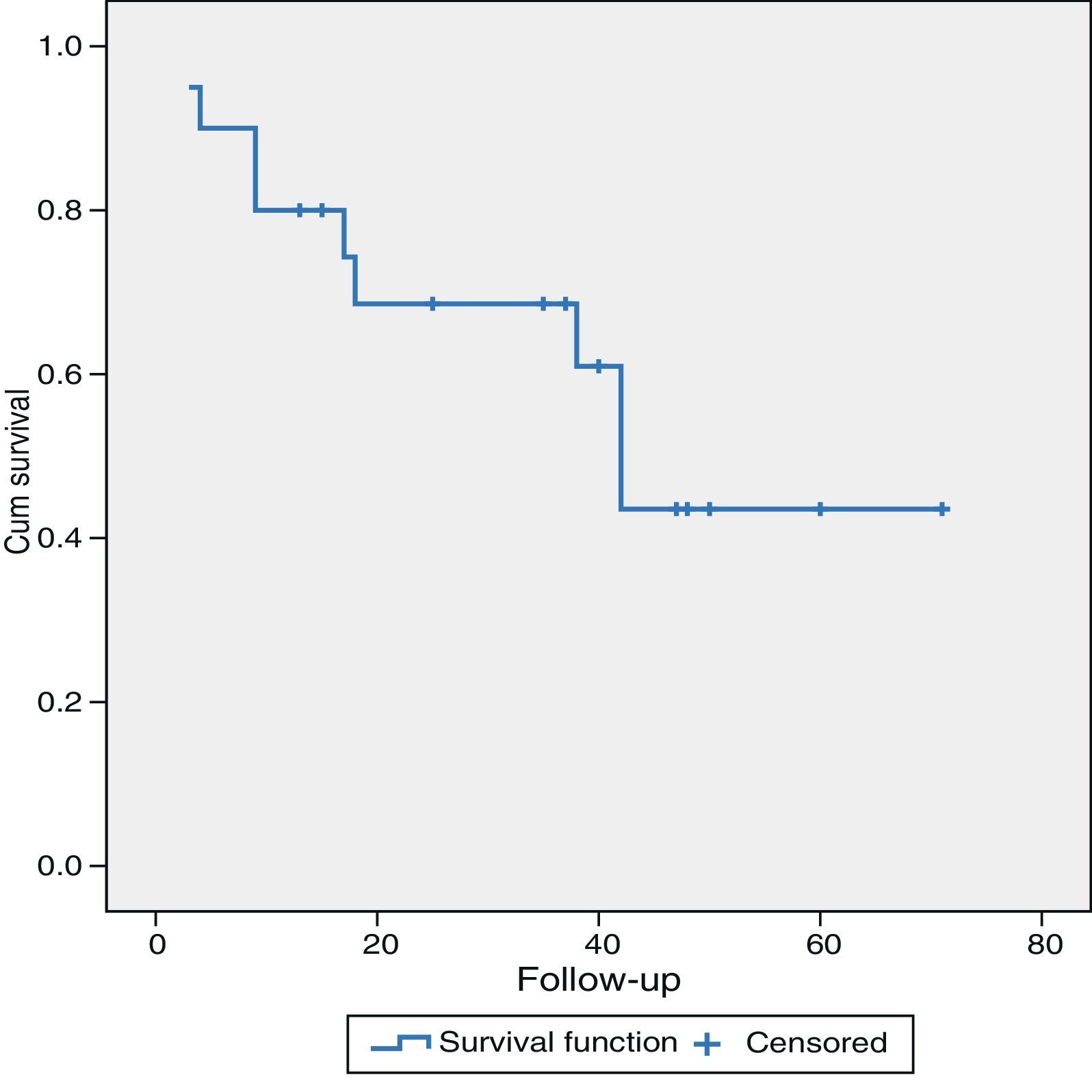
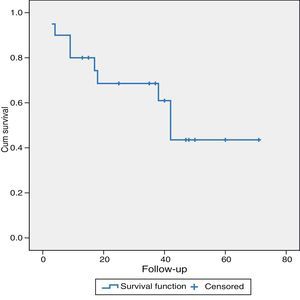
As for the long-term results, patients with LMCRC (21 cases), with a mean follow-up of 31±19.5 months presented a mean survival of 69% and 43.5% at 36 and 60 months, respectively. Eleven patients (52%) were alive without recurrence of their disease, one patient (5%) was alive but with disease progression and 9 patients (43%) had died: one of them was disease-free and had died of another cause, 7 patients due to disease progression and one patient died in the postoperative period (Fig. 1).
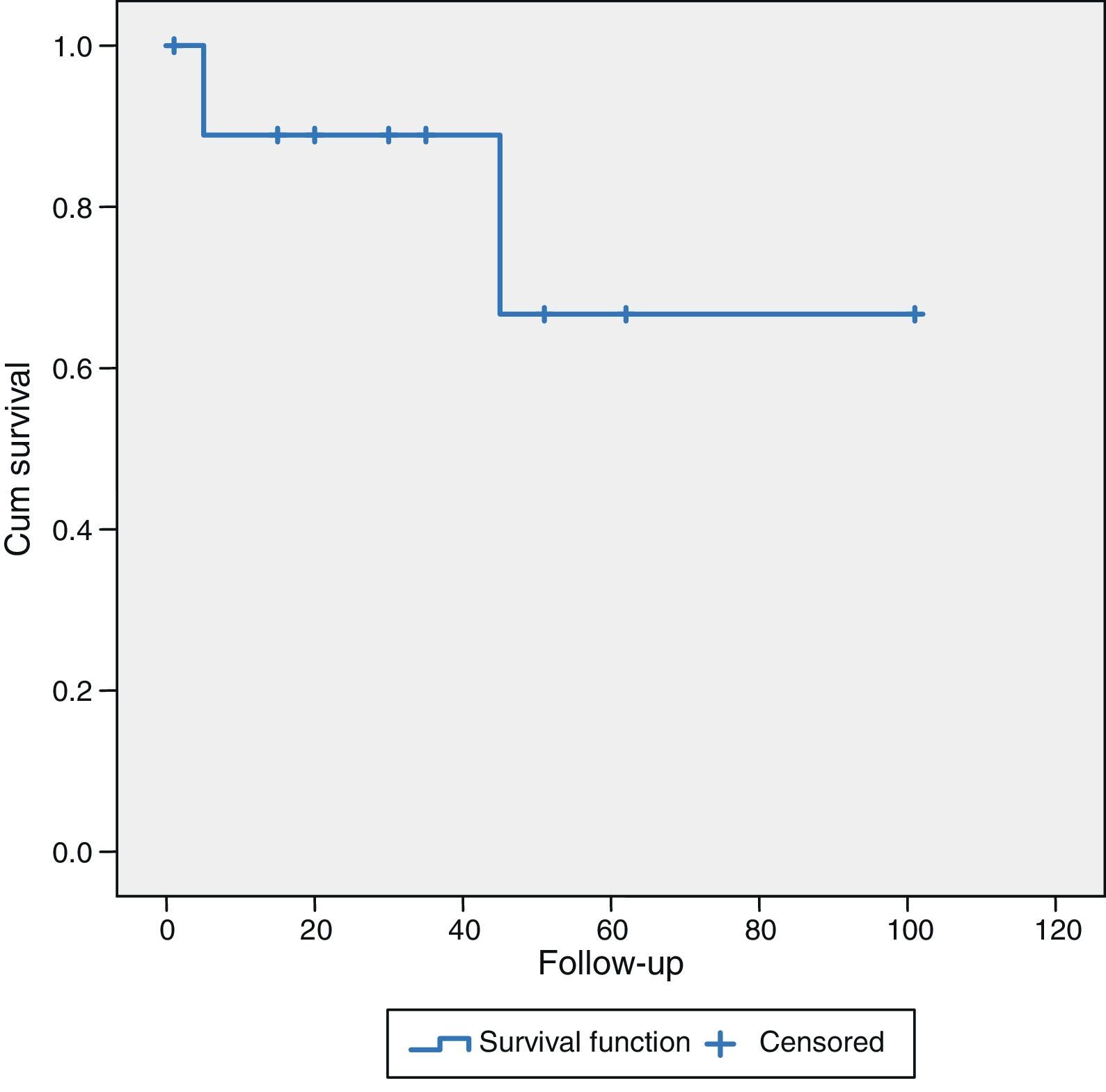
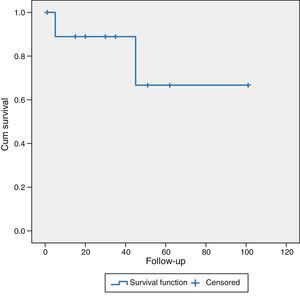
Patients undergoing surgery for hepatocellular carcinoma (HCC) (10 cases), with a mean follow-up of 38.8±28 months, had a mean survival of 89% and 68% at 36 and 60 months, respectively. Five patients (50%) were alive without recurrence of their disease, 3 cases (30%) were alive but with liver recurrence and 2 patients (20%) had died due to progression of the chronic liver disease (Fig. 2).
Of the patients undergoing surgery for breast tumor metastasis (5 cases), 2 died of disease progression at 17 and 20 months, respectively. Two are still alive without recurrence at 12 and 52 months. One patient has recently been operated on for a single liver recurrence 41 months after laparoscopic resection.
DiscussionThe laparoscopic approach to liver surgery offers advantages over conventional surgery in terms of blood loss, analgesia requirements and hospital stay.9,10 Despite this, its application is not as widespread as in other areas of abdominal disease due to the prolonged learning curve, risk of difficult-to-control intraoperative hemorrhage, difficulty to completely examine the liver and the risk of air embolism.7 Despite these limitations, most liver surgery groups have progressively introduced the use of laparoscopy.11,12
Currently, laparoscopic resection of benign or malignant liver tumors located in peripheral liver segments is well established and is even considered the treatment of choice for resecting segments II and III.13–15 Performing larger liver resections is technically much more demanding and, although it is feasible and safe when done by highly-experienced groups, its use is not as widespread.4,5,16–18
In our series, there were three cases that required conversion to laparotomy. Two of these patients had hepatocellular carcinoma (HCC) in whom, during liver mobilization, omental vessel lesions led to bleeding that was difficult to control by laparoscopy, and possibly related to some degree of portal hypertension. The remaining case was a patient with LMCRC in the left sector in whom intraoperative ultrasound detected another lesion located deep in segment V. Laparoscopic left lateral segmentectomy was carried out with later assistance by laparotomy to complete the resection of the metastasis in segment V.
Intraoperative bleeding is a serious problem during laparoscopic liver surgery. To minimize this risk, the CVP is maintained low and in most cases hilar clamping is done. Unlike open surgery, in laparoscopic liver resection the CVP is kept higher (about 4mmHg) to reduce the gradient between the intraabdominal pressure and PVC, reducing the risk of air embolism.19
Furthermore, in most cases (90%) continuous hilar clamping was used after ischemic preconditioning during liver transection. Clamping of the hepatic hilum during transection is controversial, and possible postoperative complications have been described in association with prolonged clamping.20 Ischemic preconditioning with 10min of clamping followed by 10min unclamped could have a protective effect against later ischemia-reperfusion injuries.21,22 In the series, reasons why hilar clamping is used with systematic preconditioning include: (1) absence of side effects observed in our series and other studies published after clamping both in laparoscopic as well as open surgery; (2) clamping maintains as bloodless a field as possible, which is of great importance because the presence of blood in the field absorbs light, darkens the image and makes it difficult to identify structures during liver transection; and (3) blood loss and subsequent transfusion of red blood cell concentrates in patients undergoing liver resection for malignant disease may be a prognostic factor for long-term survival.23,24
In a recent randomized prospective study comparing 2 groups of patients who underwent open liver resection for LMCRC, one with systematic hilar clamping and the other without clamping, the long-term results in terms of overall survival, disease-free survival and hepatic recurrence rate were similar.25
As for the duration of surgery, most groups with experience report operating time results that are shorter than in open surgery.26,27 In the series presented, the average operative time was 163min, although it is noteworthy that a large proportion of the resections were left lateral segmentectomies, which is a standardized technique that allows for short operating times. The association of simultaneous resections of the colon or rectum or performing major liver resections has caused an increase in operating time in our experience.
The morbidity recorded in the series is mostly due to grade I and II Dindo–Clavien complications28 in 5 patients. Three patients required percutaneous drainage to treat collections in the liver resection bed (grade IIIa) and one patient developed progressive postoperative hyperbilirubinemia after right hepatectomy that led to his death 3 months after surgery (grade V). This patient was a male with a history of diabetes mellitus being treated with metformin who underwent surgery for LMCRC. Initially, the patient presented a correct postoperative course, but after the seventh day post-op he showed a progressive elevation of serum bilirubin that reached 36mg/dl with no altered prothrombin time. Magnetic resonance cholangiography ruled out biliary alterations in the left bile duct and demonstrated correct hypertrophy of the left lobe of the liver. Doppler ultrasound confirmed correct venous and arterial vascular flow to the left lobe. The pathology study of the healthy liver parenchyma showed no abnormalities. All drugs were suspended to rule out the toxic etiology. During liver resection, hilar clamping was not performed as the right portal pedicle was initially controlled with an extra-Glissonian approach. The patient died suddenly 3 months after surgery, but the cause of death was not determined with certainty.
The use of laparoscopy for the treatment of malignant liver disease is controversial, which has contributed negatively toward extending its use. In this series, mean patient follow-up was 36 months and we only included those patients who had at least one year of monitoring because it is during the first year when most recurrences occur. Recent studies on the treatment of metastatic colorectal cancer using laparoscopy show that: (1) there are no differences with regards to obtaining negative resection margins29–32; (2) there is no evidence for the presence of metastases in the trocar wounds or a higher rate of recurrence of liver disease26; and (3) the oncologic results do not differ in the series when comparing the laparoscopic and open approaches.6,32–35
Nevertheless, candidates for laparoscopic resection are selected in relation to the number, size and location of the lesions and therefore have a more favorable presentation of the disease, which could affect results in greater long-term survival.36
Despite the fact that liver surgery groups currently have greater expertise in the laparoscopic approach, the extended indications for resection of LMCRC means that the percentage of patients eligible for laparoscopic hepatectomy remains stable while the total number of patients treated by these groups increases.37,38
In our series of patients with laparoscopic resection of LMCRC, we have not observed any case of recurrence in the trocar region after a long follow-up. In addition, the pathology analysis of the specimens demonstrated negative resection margins in all cases. The routine use of laparoscopic intraoperative ultrasound may have been an influence.
The 5-year survival of the patients with LMCRC in this series was 43.5%, which is similar to the survival obtained in our overall series of liver resections due to LMCRC in 209 patients (included in the LiverMetSurvey database), which was 43%.
In the surgical treatment of HCC in cirrhotic patients, laparoscopy offers a lower rate of decompensated liver disease (mainly ascites), with less parietal aggression, which preserves collateral venous circulation.27,39,40 There are doubts about the risk of tumor dissemination and margin invasion in laparoscopic resections of HCC.41–43 The routine use of intraoperative laparoscopic ultrasound could minimize this risk.44,45 The long-term results have shown no differences in 5-year survival between patients with HCC resected either laparoscopically or in open surgery.27,46–48 Transfusion of blood products is a negative prognostic factor for disease-free survival in patients with HCC resection.24,49 Laparoscopy obtains better results in terms of blood loss,17,31,46,50 and this could have a beneficial effect in these patients. In the series, the 5-year survival results are similar to those reported by other groups with larger series.
In conclusion, laparoscopy is a feasible, safe alternative to open surgery for the treatment of malignant liver disease, provided it is done in selected patients and by groups with experience in this type of surgery. It offers long-term oncologic outcomes equivalent to those of conventional surgery, while providing the advantages of this minimally invasive approach.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Cugat Andorrà E, Herrero Fonollosa E, García Domingo MI, Camps Lasa J, Carvajal López F, Rodríguez Campos A, et al. Resultados tras resección hepática laparoscópica: una opción adecuada en patología maligna. Cir Esp. 2013;91:510–516.
Part of the information contained in the manuscript was presented at the 28th National Congress of Surgery held in Madrid on November 8–11, 2010.