Of the possible complications after a cephalic duodenopancreatectomy (CPD), the clinically relevant postoperative pancreatic fistula (PPF) is the most important, especially in patients with pancreas of a soft consistency. The main objective of this work is to analyze the different postsurgical complications, with special emphasis on the rate of PPF on soft pancreas with a risk of moderate/high PPF, and its incidence between the two different types of sutures used by our group (classic vs reinforced duct-mucosa anastomosis [REDMA]).
MethodsRetrospective observational study, between January 2017 and March 2020, of patients undergoing CPD in our unit after applying the inclusion and exclusion criteria. Analysis of preoperative, intraoperative factors and postoperative complications observed during follow-up.
ResultsSample of 34 patients; 67.6% (n = 23) of them under the classic protocol and 32.4% (n = 11) with REDMA. The only post-surgical complication in which we obtained statistical repercussion, without differences between cases and controls in terms of the risk of FPP, in favor of the REDMA anastomosis is that of FPP. Thanks to this surgical innovation, both the complications from stage IIIb, according to the Clavien-Dindo classification, and the mean hospital stay have also been reduced with statistical significance.
ConclusionsWhen REDMA reduces the rate of PPF in patients with moderate/high surgical risk of it, we consider it to be a useful alternative to consider in the reconstruction of transit after CPD.
De las posibles complicaciones tras una duodenopancreatectomía cefálica (DPC), la fístula pancreática postoperatoria (FPP) clínicamente relevante es la más importante, especialmente en enfermos con páncreas de consistencia blanda. El objetivo principal de este trabajo es analizar las diferentes complicaciones posquirúrgicas, haciendo especial hincapié en la tasa de FPP sobre páncreas blandos con riesgo de FPP moderado/alto, y su incidencia entre los dos tipos distintos de sutura empleados por nuestro grupo (clásica vs. anastomosis ducto-mucosa reforzada [REDMA]).
MétodosEstudio observacional retrospectivo, entre enero de 2017 y marzo 2020, de pacientes sometidos a DPC en nuestra unidad tras aplicar los criterios de inclusión y exclusión. Análisis de factores preoperatorios, intraoperatorios y de complicaciones postoperatorias observados durante el seguimiento.
ResultadosMuestra de 34 pacientes; el 67,6% (n = 23) de ellos bajo el protocolo clásico y el 32,4% (n = 11) con REDMA. La única complicación posquirúrgica en la que se obtuvo repercusión estadística, sin existir diferencias entre casos y controles en cuanto al riesgo de FPP, en favor de la anastomosis REDMA es el de FPP. Gracias a esta innovación quirúrgica, tanto las complicaciones a partir del estadio IIIb, según la clasificación de Clavien-Dindo, como la estancia media hospitalaria se han visto también reducidas con significación estadística.
ConclusionesAl reducir REDMA la tasa de FPP en pacientes con moderado/alto riesgo quirúrgico de la misma, consideramos que es una alternativa útil a tener en cuenta en la reconstrucción del tránsito tras una DPC.
The restoration of gastrointestinal continuity after pancreatoduodenectomy (PD) continues to be a major challenge today. Given the intrinsic characteristics of the pancreas, the complexity of the different reconstruction types is associated with a considerable percentage of complications1,2, which is the Achilles heel of this surgery. Clinically relevant postoperative pancreatic fistula (POPF) is the most significant of these complications2,3, especially in patients with a soft pancreas. This circumstance is what has led to a change in the anastomotic process used.
This article describes the modification of the reference technique (pancreaticojejunal duct-to-mucosa anastomosis with external catheter) performed in our unit. This innovation consists of a pancreaticojejunal suture and external stenting that is reinforced with biological mesh (Integra SurgiMend 1.0®, www.surgimend.com)4,5.
The main objective of this study is to analyze the postoperative complications, with special emphasis on the rate of POPF in a soft pancreas with moderate/high risk of POPF, and its incidence between the two suture types used (classic vs REDMA).
We call this type of anastomosis ‘reinforced duct-mucosa anastomosis’ (REDMA).
MethodsStudy subjectsWe present the initial results of a historical, observational and analytical study of a cohort consisting of cases (patients who had been treated under the new pancreaticojejunal reconstruction protocol) and control subjects (patients treated according to the classic protocol). Data were collected from medical records between January 2017 and March 2020 after applying the inclusion criteria (age >18 and <80 years, pathology [malignant or benign] in the head of the pancreas requiring Whipple-type PD, soft pancreas and moderate/high risk of POPF)6 as well as the exclusion criteria (hard pancreas) of the study. Next, the variables collected in the study were analyzed. Sociodemographic variables included: age, sex, body mass index (BMI), ASA classification, and risk of POPF6. Preoperative variables included: abdominal pain, diabetes mellitus (DM), constitutional syndrome, jaundice, preoperative drainage of the main bile duct, hemoglobin, albumin, CA 19.9, computed tomography (CT), endoscopic ultrasound (EUS), and fine-needle aspiration (FNA) of the pancreatic mass. Surgical variables were: surgical technique, intraoperative hemorrhage, consistency of the pancreas, size of the Wirsung duct, and type of anastomosis for the reconstruction of the gastrointestinal tract. Postoperative variables were: initiation of oral tolerance, pancreatic fistula, biliary fistula, stump pancreatitis, delayed gastric emptying (DGE), upper gastrointestinal bleeding (UGIB), intra-abdominal bleeding (IAB), intra-abdominal collection, surgical site infection, re-operation, hospital stay, death, histology, number of lymph nodes, and Clavien-Dindo classification for postoperative complications7.
Surgical techniqueThis novel technique (REDMA) is indicated for all patients undergoing PD with moderate/high risk of POPF and intraoperative finding of a soft pancreas. Since there still is no validated objective method for measuring the consistency of the pancreas at our hospital, we decided (based on the current bibliography) that the pancreatic texture would be evaluated subjectively by the surgical team during the procedure, which has been shown to adequately correlate with objective measures and the risk of the appearance of POPF8.
SurgiMend® is an acellular dermal matrix. Therefore, it does not trigger an acute or chronic inflammatory response to a foreign body leading to degradation or rejection of the implant. Thus, it offers advantages over synthetic and other biological products for the reconstruction of soft tissue, such as the pancreatic gland. It is created from fetal or neonatal bovine dermis and is composed of type I and type III collagen, which gives it excellent mechanical resistance. In addition, the matrix allows for the sequestration of cells and growth factors, which favors its rapid vascularization for long-lasting reinforcement. Glubran 2® surgical glue is a class III surgical medical product (internal and external surgical use) that complies with the requirements of the EU Medical Device Directive 93/42/EEC and subsequent updates. Glubran 2® is a synthetic material with a cyanoacrylate base, thanks to which it has marked hemostatic and adhesive properties. Furthermore, once solidified, it creates an effective antiseptic barrier against the most frequent pathogens or infectious agents. In contact with living tissue and in a humid environment, it polymerizes rapidly (from 2 to 90 seconds), creating an elastic and waterproof film with high tensile strength that guarantees solid tissue adhesion. It is not damaged by blood or body fluids. Once solidified, the film can be easily pierced with a suture needle, since the polymerization of the product does not give rise to crystalline aggregates. Once solid, the glue no longer has any adhesive power.
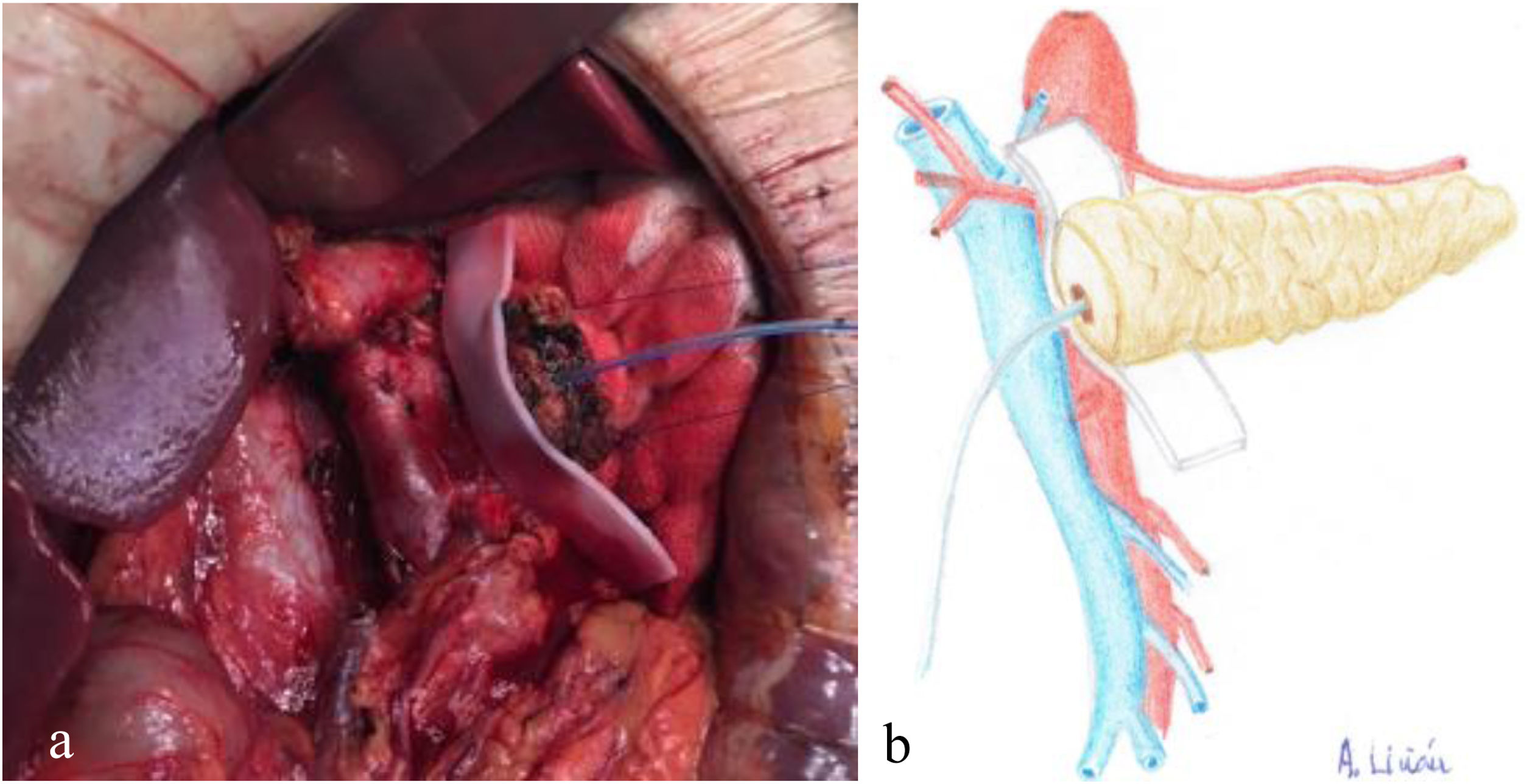
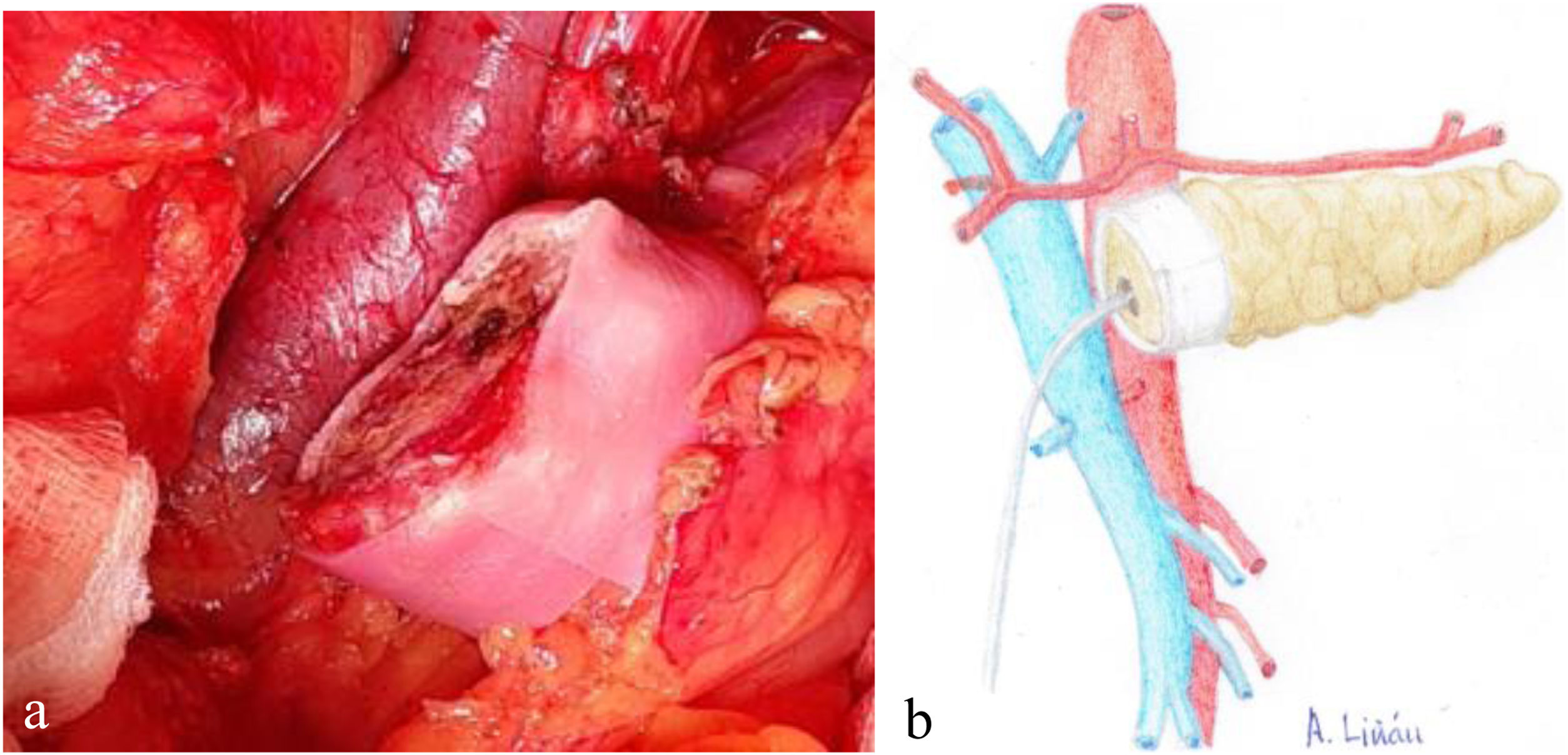
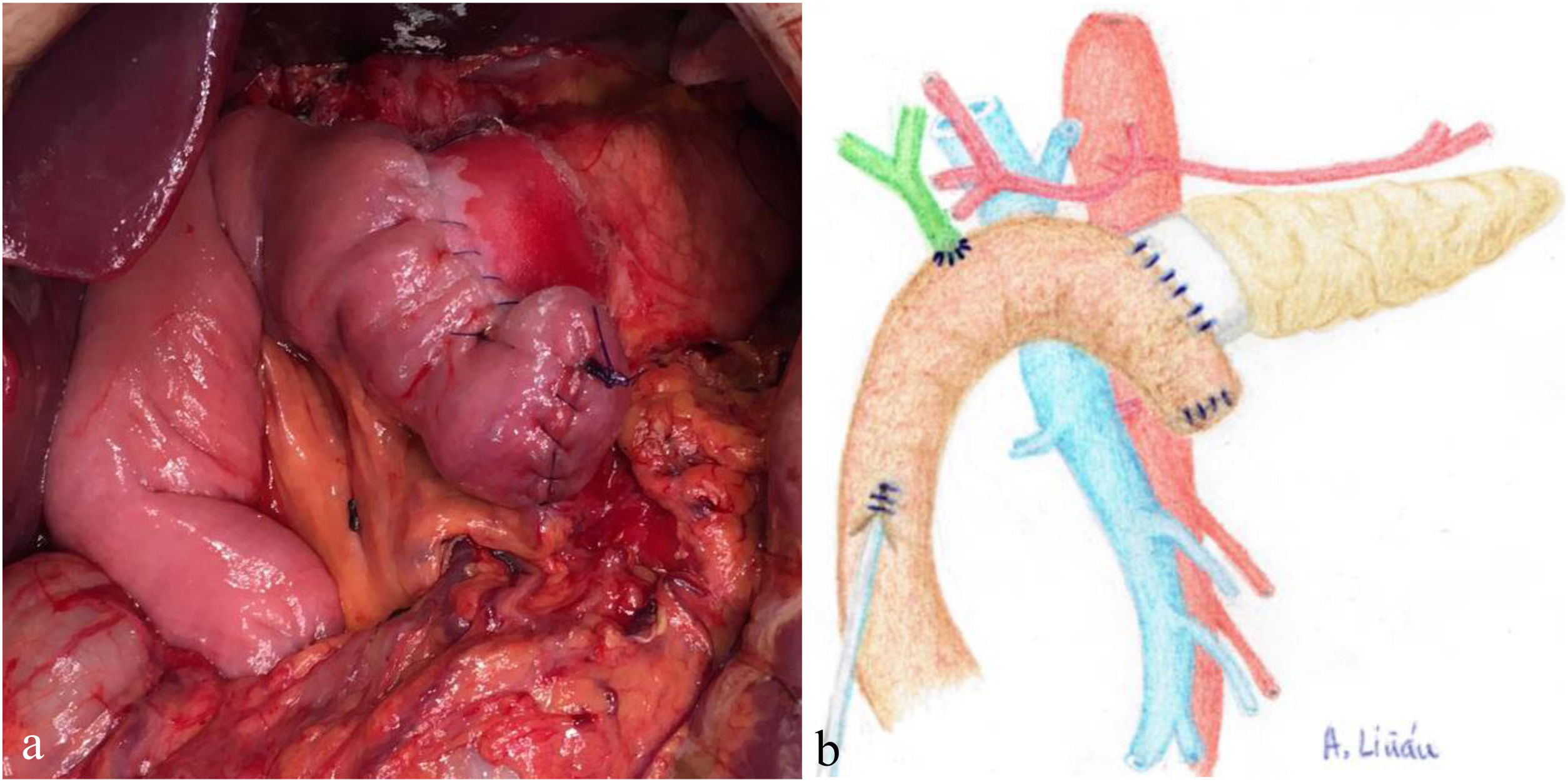
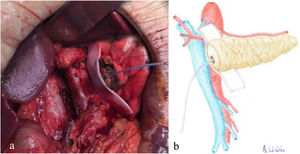
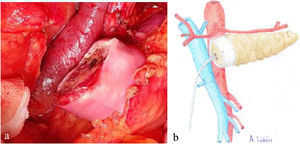
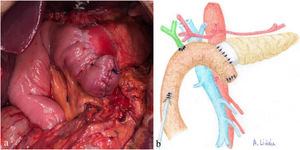
Once the pancreas has been divided at the neck, dissect the posterior side (with the splenic vein), the superior edge (with the splenic artery and gastric vein) and the inferior edge (retroperitoneum) of the pancreas for about 2 – 3 cm in length, ensuring correct hemostasis to avoid problems of poor adhesion of the biological mesh (Integra SurgiMend 1.0®). Once hydrated for 5 minutes in saline, a rectangle of the mesh is designed with the appropriate dimensions (the width will be between 1 and 3 cm). This will depend on the characteristics of each pancreas in order to cover its entire perimeter, like a scarf. Next, the Glubran 2® surgical glue is applied to the middle part of one of the sides of the mesh rectangle, which is then placed against the posterior side of the pancreas and pressed for 30 seconds (Fig. 1a, b). When the mesh has adhered to the body of the pancreas (Fig. 2a, b), we proceed with the end-to-side duct-to-mucosa pancreaticojejunal suture with external stenting (Fig. 3a, b), as follows:
- -
Continuous posterior side suture with non-absorbable 3/0 monofilament
- -
Duct-to-mucosa suture on the posterior side with interrupted stitches of absorbable 4-5/0 monofilament
- -
External stenting with a pediatric nasogastric tube; size determined by the diameter of the Wirsung duct
- -
Duct-to-mucosa suture on the anterior side with interrupted stitches of absorbable 4-5/0 monofilament
- -
Continuous anterior side suture with non-absorbable 3/0 monofilament
a) Intraoperative image of the placement of the biological mesh on the posterior side of the body of the pancreas; b) Diagram representing Fig. 1a.
a) Intraoperative image of the final position of the biological mesh around the pancreatic remnant; b) Diagram representing Fig. 2a.
a) Intraoperative image after completing REDMA; b) Diagram representing Fig. 3a.
The statistical analysis of the data was performed using the SPSS® program version 23.0 (SPSS Inc, Armonk, NY, USA). The level of statistical significance was established at 5% (P < .05). The descriptive analysis of the qualitative variables was carried out using frequencies and percentages. The quantitative variables were analyzed to verify their normality using the Shapiro-Wilks test and described using measures of central tendency, such as mean and standard deviation or median and range, as appropriate. To study the relationship between a dichotomous nominal qualitative variable and a normal quantitative variable, we used the t test for comparison of means in independent groups, based on the Student-Fisher law. Non-parametric tests were used (Mann-Whitney U in independent groups) when the distribution of a variable violated the assumptions of normality and equality of variances. In the bivariate analysis, the independence test was used to compare proportions observed in independent groups (chi-squared, association between two qualitative variables), verifying the application conditions (predicted no less than 5, or no less than 3 using the Yates correction); when they were not met, Fisher’s exact test was used.
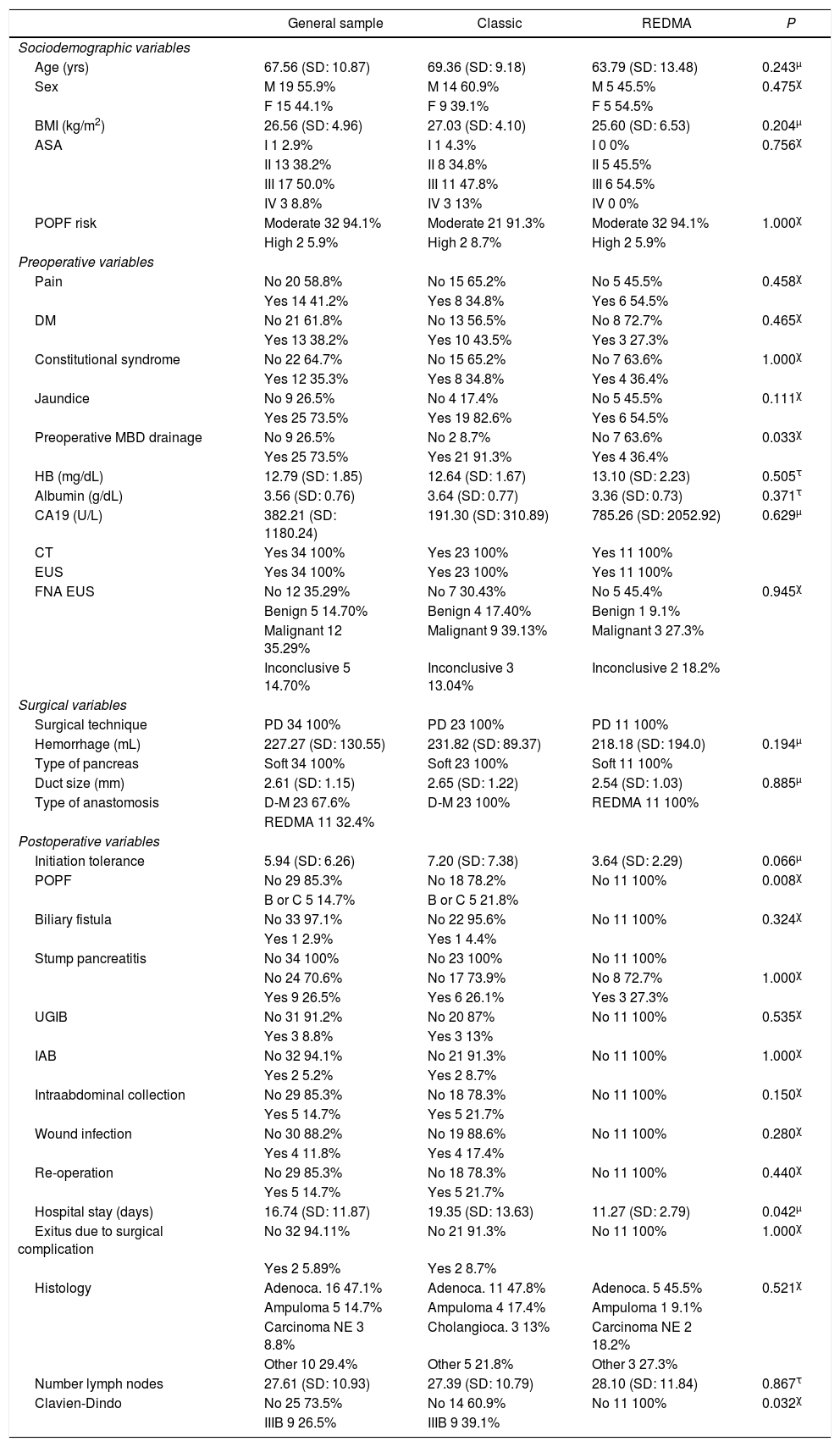
ResultsA sample of 34 patients was obtained; 67.6% (n = 23) were treated under the classic protocol, and 32.4% (n = 11) following REDMA. In the general sample, 55.9% (n = 19) of the patients were men. The mean age of the general sample was 67.56 years (SD: 10.87), not following a normal distribution (P = .011). The most frequently expressed symptoms at diagnosis were jaundice 73.5% (n = 25) and pain 41.2% (n = 14). The most frequent definitive pathological diagnosis was adenocarcinoma of the head of the pancreas (47.1%; n = 16), followed by intra-ampullary adenocarcinoma (14.7%; n = 5). In all cases, PD was performed using a laparotomic Whipple-type approach with Roux-en-Y reconstruction. Only one patient required venous resection, and 100% (n = 34) of the patients had a soft pancreas (Table 1).
Characteristics of the general sample, the group treated with the classic anastomosis and the group treated with REDMA. χ, τ, μ Comparisons of cases and controls.
| General sample | Classic | REDMA | P | |
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age (yrs) | 67.56 (SD: 10.87) | 69.36 (SD: 9.18) | 63.79 (SD: 13.48) | 0.243μ |
| Sex | M 19 55.9% | M 14 60.9% | M 5 45.5% | 0.475χ |
| F 15 44.1% | F 9 39.1% | F 5 54.5% | ||
| BMI (kg/m2) | 26.56 (SD: 4.96) | 27.03 (SD: 4.10) | 25.60 (SD: 6.53) | 0.204μ |
| ASA | I 1 2.9% | I 1 4.3% | I 0 0% | 0.756χ |
| II 13 38.2% | II 8 34.8% | II 5 45.5% | ||
| III 17 50.0% | III 11 47.8% | III 6 54.5% | ||
| IV 3 8.8% | IV 3 13% | IV 0 0% | ||
| POPF risk | Moderate 32 94.1% | Moderate 21 91.3% | Moderate 32 94.1% | 1.000χ |
| High 2 5.9% | High 2 8.7% | High 2 5.9% | ||
| Preoperative variables | ||||
| Pain | No 20 58.8% | No 15 65.2% | No 5 45.5% | 0.458χ |
| Yes 14 41.2% | Yes 8 34.8% | Yes 6 54.5% | ||
| DM | No 21 61.8% | No 13 56.5% | No 8 72.7% | 0.465χ |
| Yes 13 38.2% | Yes 10 43.5% | Yes 3 27.3% | ||
| Constitutional syndrome | No 22 64.7% | No 15 65.2% | No 7 63.6% | 1.000χ |
| Yes 12 35.3% | Yes 8 34.8% | Yes 4 36.4% | ||
| Jaundice | No 9 26.5% | No 4 17.4% | No 5 45.5% | 0.111χ |
| Yes 25 73.5% | Yes 19 82.6% | Yes 6 54.5% | ||
| Preoperative MBD drainage | No 9 26.5% | No 2 8.7% | No 7 63.6% | 0.033χ |
| Yes 25 73.5% | Yes 21 91.3% | Yes 4 36.4% | ||
| HB (mg/dL) | 12.79 (SD: 1.85) | 12.64 (SD: 1.67) | 13.10 (SD: 2.23) | 0.505τ |
| Albumin (g/dL) | 3.56 (SD: 0.76) | 3.64 (SD: 0.77) | 3.36 (SD: 0.73) | 0.371τ |
| CA19 (U/L) | 382.21 (SD: 1180.24) | 191.30 (SD: 310.89) | 785.26 (SD: 2052.92) | 0.629μ |
| CT | Yes 34 100% | Yes 23 100% | Yes 11 100% | |
| EUS | Yes 34 100% | Yes 23 100% | Yes 11 100% | |
| FNA EUS | No 12 35.29% | No 7 30.43% | No 5 45.4% | 0.945χ |
| Benign 5 14.70% | Benign 4 17.40% | Benign 1 9.1% | ||
| Malignant 12 35.29% | Malignant 9 39.13% | Malignant 3 27.3% | ||
| Inconclusive 5 14.70% | Inconclusive 3 13.04% | Inconclusive 2 18.2% | ||
| Surgical variables | ||||
| Surgical technique | PD 34 100% | PD 23 100% | PD 11 100% | |
| Hemorrhage (mL) | 227.27 (SD: 130.55) | 231.82 (SD: 89.37) | 218.18 (SD: 194.0) | 0.194μ |
| Type of pancreas | Soft 34 100% | Soft 23 100% | Soft 11 100% | |
| Duct size (mm) | 2.61 (SD: 1.15) | 2.65 (SD: 1.22) | 2.54 (SD: 1.03) | 0.885μ |
| Type of anastomosis | D-M 23 67.6% | D-M 23 100% | REDMA 11 100% | |
| REDMA 11 32.4% | ||||
| Postoperative variables | ||||
| Initiation tolerance | 5.94 (SD: 6.26) | 7.20 (SD: 7.38) | 3.64 (SD: 2.29) | 0.066μ |
| POPF | No 29 85.3% | No 18 78.2% | No 11 100% | 0.008χ |
| B or C 5 14.7% | B or C 5 21.8% | |||
| Biliary fistula | No 33 97.1% | No 22 95.6% | No 11 100% | 0.324χ |
| Yes 1 2.9% | Yes 1 4.4% | |||
| Stump pancreatitis | No 34 100% | No 23 100% | No 11 100% | |
| No 24 70.6% | No 17 73.9% | No 8 72.7% | 1.000χ | |
| Yes 9 26.5% | Yes 6 26.1% | Yes 3 27.3% | ||
| UGIB | No 31 91.2% | No 20 87% | No 11 100% | 0.535χ |
| Yes 3 8.8% | Yes 3 13% | |||
| IAB | No 32 94.1% | No 21 91.3% | No 11 100% | 1.000χ |
| Yes 2 5.2% | Yes 2 8.7% | |||
| Intraabdominal collection | No 29 85.3% | No 18 78.3% | No 11 100% | 0.150χ |
| Yes 5 14.7% | Yes 5 21.7% | |||
| Wound infection | No 30 88.2% | No 19 88.6% | No 11 100% | 0.280χ |
| Yes 4 11.8% | Yes 4 17.4% | |||
| Re-operation | No 29 85.3% | No 18 78.3% | No 11 100% | 0.440χ |
| Yes 5 14.7% | Yes 5 21.7% | |||
| Hospital stay (days) | 16.74 (SD: 11.87) | 19.35 (SD: 13.63) | 11.27 (SD: 2.79) | 0.042μ |
| Exitus due to surgical complication | No 32 94.11% | No 21 91.3% | No 11 100% | 1.000χ |
| Yes 2 5.89% | Yes 2 8.7% | |||
| Histology | Adenoca. 16 47.1% | Adenoca. 11 47.8% | Adenoca. 5 45.5% | 0.521χ |
| Ampuloma 5 14.7% | Ampuloma 4 17.4% | Ampuloma 1 9.1% | ||
| Carcinoma NE 3 8.8% | Cholangioca. 3 13% | Carcinoma NE 2 18.2% | ||
| Other 10 29.4% | Other 5 21.8% | Other 3 27.3% | ||
| Number lymph nodes | 27.61 (SD: 10.93) | 27.39 (SD: 10.79) | 28.10 (SD: 11.84) | 0.867τ |
| Clavien-Dindo | No 25 73.5% | No 14 60.9% | No 11 100% | 0.032χ |
| IIIB 9 26.5% | IIIB 9 39.1% | |||
BMI: body mass index; ASA: American Society of Anesthesiologists; POPF: postoperative pancreatic fistula; DM: diabetes mellitus; PD: pancreaticoduodenectomy; MBD: main bile duct; HB: hemoglobin; CA: carbohydrate antigen; CT: computed tomography; EUS: endoscopic ultrasound; FNA: fine-needle aspiration; DGE: delayed gastric emptying; UGIB: upper gastrointestinal bleeding; IAB: intra-abdominal bleeding.
Because there were no statistical differences between cases and controls in terms of the risk of POPF using the Callery clinical score6, we present the specific complications of this surgery for each of the groups. For the classical technique, the clinically relevant POPF rate was 21.8% (n = 5), specifically 8.8% type B (n = 2) and 13% type C (n = 3). In contrast, for the REDMA technique, the clinically relevant POPF rate was 0%, with 9.1% (n = 1) of the biochemical fistula type (Table 1).
The only postoperative complication in which a significant statistical impact was obtained in favor of the REDMA anastomosis, after applying the bivariate analysis, was POPF, with a P = .008 (P < .05). As a result of this, we observed that both postoperative complications from stage IIIb (based on the Clavien-Dindo classification) as well as hospital stay were reduced thanks to this innovation in a statistically significant manner, P = .032 and .042 (P < .05), respectively. The rates dropped from 39.1% (n = 9) to 0% in the case of complications, and from 19.35 (SD: 13.63) days to 11.27 (SD: 2.79) (Table 1 ).
DiscussionPancreatoenteric anastomosis is notoriously complex and carries a risk of dehiscence and POPF that ranges between 9.9% and 28.5%, depending on the series and the definition of fistula used1,2. Furthermore, when this percentage is analyzed only in patients with soft pancreas, it rises considerably and even surpasses 40% in some studies2,9,10. In recent years, there have been many attempts to reduce its incidence, but none has managed to minimize it or become the standard anastomosis. Therefore, there are different techniques for performing the pancreatoenteric anastomosis to limit the risk of immediate complications and improve functional results. However, recent clinical trials and meta-analyses comparing these techniques have not been able to demonstrate a clearly superior efficacy of one over the others1,2. Thus, there is no anastomotic technique that completely eliminates the risk of POPF. The current evidence available on the use of fibrin sealants (regardless of the glue or mesh) to reinforce the pancreatojejunal anastomosis after PD is uncertain, since after their analysis their use cannot be ensured to significantly decrease POPF or the postoperative death rate11.
Regardless of the anastomotic technique used, a precise and meticulous procedure is essential to achieve good results. It is practically impossible to reduce the POPF rate to zero, especially in patients with soft pancreas, but it would be an achievement to approach the rate of patients with hard pancreas. According to recent studies, the soft consistency of the pancreas is probably the most notable determining factor for POPF6,12–14. For this reason, our technique pursues the idea of intraoperatively transforming (as previously described in the surgical technique section) a soft pancreas into a hard one by following a simple and reproducible technical step, with no additional morbidity or mortality. It is generally accepted that, in addition to the improvement in surgical techniques to reduce the incidence and morbidity and mortality of this complication, various strategies are necessary to obtain better results in high-risk patients.
The limitations of this study are clear. First of all, the sample size is small; second, the sample is retrospective; third, it is a single-center study; fourth, 3 different surgeons performed the surgery; and fifth, the consistency of the pancreas is a subjective parameter. However, we feel it is necessary to inform others about REDMA due to the low morbidity and mortality rates observed in patients with a soft pancreas and a moderate/high risk of POPF, despite all the limitations listed above.
In conclusion, after analyzing the results of postoperative complications between the classical technique vs REDMA, there are statistically significant differences in terms of morbidity and mortality that favor the latter. This suggests that REDMA could become a useful alternative for reconstruction after PD in a pancreas with a soft consistency and moderate/high risk of POPF. However, these results should be interpreted with caution, since either the completion of the study with the sample size for which it was designed or a randomized clinical trial could provide more information to confirm or refute the superiority of this surgical innovation and thus be able to independently and objectively assess these impressions.
FundingThis study has received no funding of any kind.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Valero Liñán AS, Miota De Llama JI, González Masiá JA, Conde Inarejos B. Anastomosis ducto-mucosa reforzada (REDMA). Nueva alternativa tras la duodenopancreatectomía cefálica. Cir Esp. 2022;100:95–101.