Up to 45% anatomical variations are found in hepatic arterial system. Identifying these anatomical anomalies before or during surgery would prevent additional morbidity in performing a duodenopancreatectomy. They are routinely identified before surgery using CT imaging, but on certain occasions they are not reported and are only discovered during the surgical operation. The initial retroperitoneal access by the superior mesenteric artery (SMA) will avoid a fairly useless intervention if there is superior mesenteric artery invasion, and will identify the anatomical variations originating in the superior mesenteric artery. These anomalies acquire importance in that their unnoticed injury could lead to severe vascular compromise and/or perioperative bleeding.
ObjectivesTo analyze celiac–mesenteric anomalies of the hepatic artery before duodenopancreatectomy using the information from multidetector computed tomography (MDCT) using a non-standardized method, a standardized method with multidimensional reconstruction, and maximum intensity projection (MIP), after initial surgical access to the SMA.
Patients and methodsA retrospective study of the clinical, histopathological and surgical variables was conducted on patients with an indication for duodenopancreatectomy in our Department from 2008 until April 2010. A study was performed on the reports made after image acquisition by MDCT. A blind, three-dimensional, MIP reconstruction was performed on all the patients to identify arterial anomalies. A description is given of hepatic artery anomalies after initial access to the SMA.
ResultsA total of 61 patients were included in the study. The mean age was 65±11 years, with 33 (54%) males and 28 (46%) females. Vascular anomalies, right hepatic artery (RHA) (SMA) substitute (subst), 5 (8%); RHA (SMA) accessory (acc), 4 (7%); left hepatic artery (LHA) (left gastric artery) (LGA) acc 3 (5%); common hepatic artery (CHA) (SMA) subst 3 (5%); RHA (SMA) acc+LHA (LGA) acc 2 (3%); CHA (aorta) subst, 1 (2%); RHA+RGA+2 LHA (celiac trunk), 1 (2%); and CHA (SMA)+LHA (LGA) acc.
ConclusionOn being able to identify arterial anomalies with a mixture of preoperative radiological and methodological criteria, with three-dimensional reconstruction, MIP, and initially performing a dissection of the superior mesenteric artery could avoid duodenopancreatectomies that may not benefit the patient and compromise bleeding.
El sistema hepático arterial presenta variaciones anatómicas en hasta un 45%. La identificación pre o intraoperatoria de estas anomalías anatómicas, evitará morbilidad adicional a la realización de una duodenopancreatectomía. Rutinariamente son identificadas en el preoperatorio mediante tomografía computarizada (TC). En determinadas ocasiones no son informadas y solo se descubren durante la intervención quirúrgica. El acceso inicial retroperitoneal de la arteria mesentérica superior (AMS) evitará una intervención poco útil si existe invasión de arteria mesentérica superior, e identificará las variantes anatómicas con origen en arteria mesentérica superior. Estas anomalías adquieren importancia dado que su lesión inadvertida ocasionaría severo compromiso vascular y/o hemorragias perioperatorias.
ObjetivosAnalizar anomalías celiaco-mesentéricas de la arteria hepática ante una duodenopancreatectomía, mediante información de tomografía computarizada multicorte (TCMD), en régimen no protocolizado, Protocolizado y con reconstrucción multidimensional, proyección de intensidad máxima (MIP), Tras acceso quirúrgico inicial a AMS.
Enfermos y métodoEstudio restrospectivo de variables clínicas, anatomopatológicas y quirúrgicas de enfermos con indicación de duodenopancreatectomía en nuestro Servicio desde 2008 hasta abril de 2010. Estudio de informes realizados tras adquisición de imágenes mediante TCMC. Realizamos reconstrucción tridimensional, MIP, enmascarada, de todos los enfermos para identificación de anomalías arteriales. Descripción de anomalías arteriales hepáticas tras acceso inicial a AMS.
ResultadosLa población estudiada fue de 61 enfermos. La edad fue de 65±11 años. Varones 33 (54%) mujeres 28 (46%). Anomalías vasculares: arteria hepática derecha (AHD) (AMS) sustitutiva (sust.), 5 (8%); AHD (AMS) accesoria (acc.), 4 (7%); arteria hepática izquierda (AHI) (arteria gástrica izquierda [AGI]) acc., 3 (5%); arteria hepática común (AHC) (AMS) sust., 3 (5%); AHD (AMS) acc. + AHI (AGI) acc., 2 (3%); AHC (aorta) sust., 1 (2%); AHD + AGD + 2AHI (tronco celiaco), 1 (2%); AHC (AMS) + AHI (AGI) acc.
ConclusionesUna homogeneidad en criterios metodológicos radiológicos de forma preoperatoria, con reconstrucción tridimensional, MIP, y realización de disección de la arteria mesentérica superior de forma inicial evitaría duodenopancreatectomías que no benefician al enfermo y compromiso sanguíneo, al poder identificar las anomalías arteriales.
Pancreaticoduodenectomy (PD) is the only curative option for pancreatic and peripancreatic carcinomas, and it is also the only therapeutic option in inflammatory processes with an unfavorable clinical course or without a definitive diagnosis. The associated perioperative morbidity reaches 20%–30%. It is therefore important to identify any anomalies in the arterial anatomy that are not identified in up to 45% of the population and may increase associated perioperative morbidity.1
Usually, arterial variations are identified with pre-operative computed tomography (CT). However, identification is only possible during surgery in some cases, which may sometimes lead to unseen arterial injury that can cause hepatic ischemia or vascular compromise in bilioenteric anastomoses.
Because of the excessive workload in the health-care system, the lack of interest in some cases and the lack of consolidated multidisciplinary workgroups, necessary pre-operative anatomical information is often not available. In our study, we intend to describe this situation of deficient pre-operative radiological information, by initially dealing with the superior mesenteric artery (SMA). This circumstance could be definitively resolved with tridimensional reconstruction of images obtained with multi-slice CT (MSCT), which would improve the perioperative diagnostic strategy in cases of PD.
The initial dissection of the SMA as the first step before performing pancreatic resection during PD allows us to: (a) identify arterial variations and avoid additional perioperative morbidity; (b) identify neoplastic arterial infiltration that had not been identified pre-operatively, which avoids performing unnecessary surgery; (c) performing more extensive lymphadenectomies; and (d) avoid unnecessary bleeding by having access to pancreaticoduodenal vessels. We present the patients who were surgically treated in our department with an initial approach of the SMA during PD in order to describe anomalies of the arterial anatomy that had not been described pre-operatively.
We also created three-dimensional reconstructions with maximum intensity projection (MIP) of the images obtained from pre-operative MSCT in order to determine the power of this technique for its potential inclusion in the pre-operative extension study protocol.
Patients and MethodsStudy PopulationOur retrospective study includes patients treated surgically for pancreatic and peripancreatic disease from January 2008 to April 2010 by means of curative PD with initial access to the SMA.
Variables StudiedRadiological Anomalies of the Hepatic ArteryArterial anomalies and neoplastic infiltration were identified and described in the pre-operative MSCT report. For the diagnostic study and staging, we used multi-slice tomography (Brilliance CT by Philips) with tomographic slices of up to 0.9mm thickness. In 5 patients, 3D volume rendering (VR) reconstructions were also done, and in 12 patients we obtained multiplanar and curved reconstructions with MIP algorithm.
In the patients studied with MSCT, a slice thickness of 3mm was used with reconstruction at 0.9mm in the anatomical area of interest. Two 2 abdominal-pelvic acquisitions were taken at 25 and 50s (arterial and portal), after the injection of a bolus of iodine-based nonionic contrast (100ml infused at 2.5–3ml/s) through a peripheral venous catheter. A thoracic acquisition was taken to complete the tumor staging.
Oral neutral contrast was used with 1l of water/h for the proper extension through the gastric cavity, duodenum and jejunum.
Arterial anomalies and SMA infiltrations were identified in a blind, retrospective manner by means of a review of available pre-operative CT images with tridimensional reconstruction and MIP at the work station.
In order to classify the anatomic anomalies of the hepatic artery, we used the methods described by:
- -
Michels2: type 1, conventional anatomy; type 2, replaced left hepatic artery (LHA) originates from the left gastric artery (LGA); type 3, replaced right hepatic artery (RHA) originates from SMA; type 4, combination of types 2 and 3; type 5, accessory LHA with origin in LGA; type 6, accessory RHA with origin in SMA; type 7, combination of types 5 and 6; type 8, replaced RHA and accessory LHA from LGA; type 9, common hepatic artery originates from SMA; type 10, common hepatic artery originates from LGA.
- -
Hiatt et al.3: type 1: normal pattern (hepatic artery originates in the celiac artery, then divides into gastroduodenal artery and proper hepatic artery; distally, the proper hepatic artery divides into right and left hepatic branches); type 2: replaced or accessory LHA originates from LGA; type 3: replaced or accessory RHA originates from SMA; type 4: RHA originates from SMA and LHA from LGA; type 5: common hepatic artery originates from SMA; type 6: common hepatic artery from aorta.
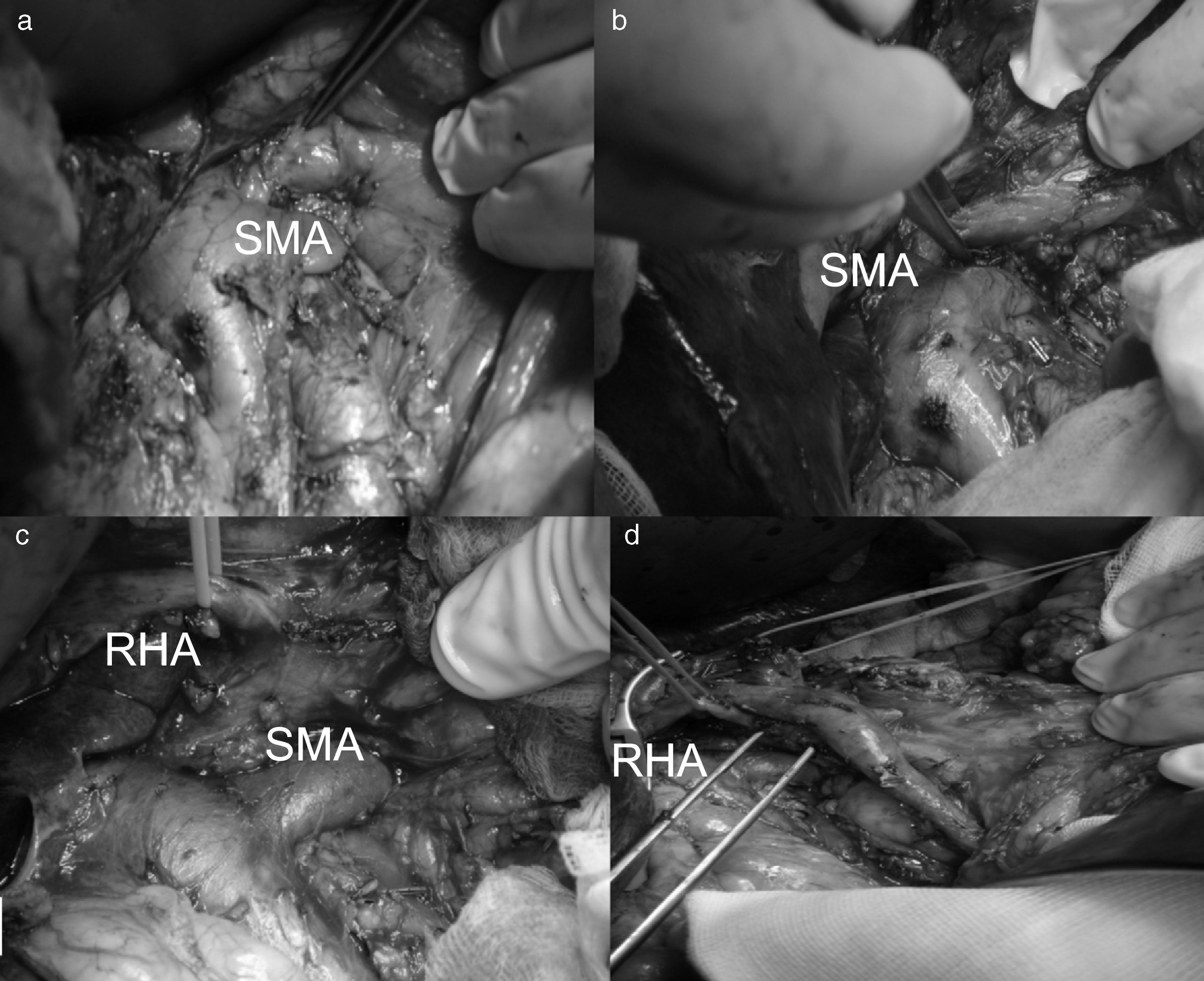
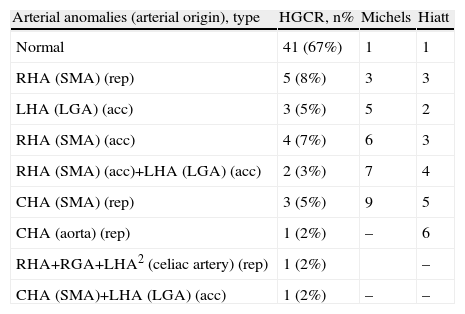
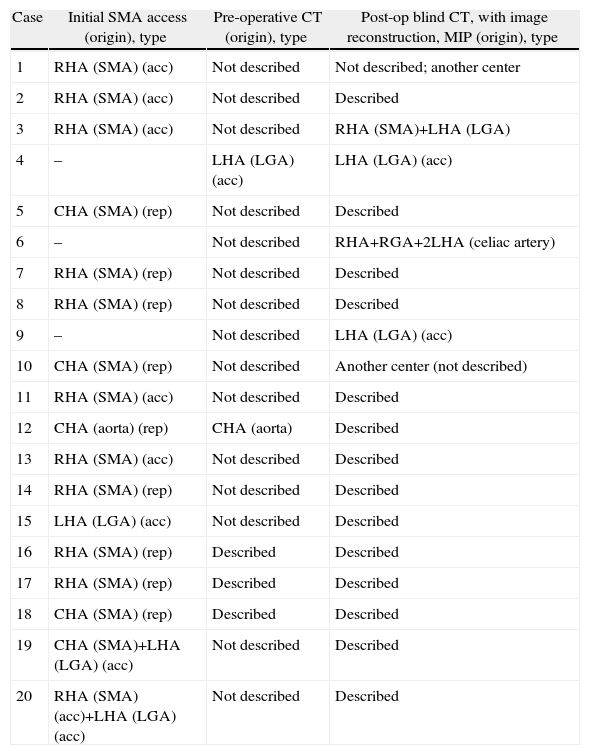
All patients underwent bilateral subcostal laparotomy. After examination of the abdominal cavity to rule out the existence of hepatic metastases that would contraindicate continuing the process, the mobilization of the hepatic flexure of the colon and transverse colon, together with an extensive Kocher maneuver was carried out. This allowed us to free the ventral region, duodenum and head of the pancreas from the retroperitoneum in order to expose the large vessels up to the left lateral margin of the aorta and proceed with interaortocaval lymphadenectomy with the proximal limit at the celiac artery and distal limit at the inferior mesenteric artery. We identified the SMA at its origin at an angle made up by the left lateral region of the inferior vena cava and upper edge of the left renal vein. The SMA was dissected in order to isolate it from the portal vein and pancreatic region behind the pancreas to the distal pancreatic region, with ligation of the inferior pancreaticoduodenal artery, and fatty tissue was removed. Infiltration >50% of the circumference of the celiac artery or SMA and the occlusion of the superior mesenteric vein or mesentericoportal confluence without the possibility for resection or reconstruction would contraindicate surgical resection (Fig. 1).
ResultsFrom 2008 until April 2010, 61 patients were included in our program to study pancreaticoduodenectomy with an initial retroperitoneal approach of the SMA. Age was 65±11 years, with 33 males (54%) and 28 females (46%).
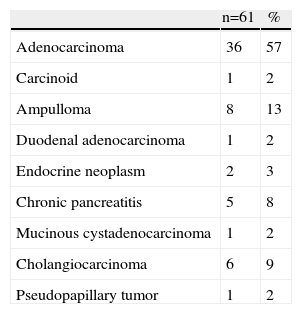
The histologic diagnoses were: 28 cases of pancreatic adenocarcinoma (44%); 1 carcinoid tumor (2%); 8 ampullomas (13%); 1 duodenal adenocarcinoma (2%); 2 malignant endocrine neoplasms (3%); 5 chronic pancreatitis (8%); 6 cholangiocarcinomas (9%); 1 mucinous cystadenocarcinoma (2%); 1 solid pseudopapillary tumor (2%) (Table 1).
The hepatic arterial distribution was normal in 41 patients (67%). Vascular anomalies included: 5 cases of replaced right hepatic artery from SMA (8%); 4 accessory RHA from SMA (7%); 3 accessory left hepatic artery from LGA (5%); 3 replaced common hepatic artery from SMA (5%); 2 accessory RHA from SMA+accessory LHA from LGA (3%); 1 CHA from aorta (2%): 1 RHA+RGA+2LHA from the celiac artery (2%); 1 CHA from SMA+accessory LHA from LGA (2%) (Table 2).
Arterial Anomalies According to the Michels and Hiatt Classification Systems.
| Arterial anomalies (arterial origin), type | HGCR, n% | Michels | Hiatt |
| Normal | 41 (67%) | 1 | 1 |
| RHA (SMA) (rep) | 5 (8%) | 3 | 3 |
| LHA (LGA) (acc) | 3 (5%) | 5 | 2 |
| RHA (SMA) (acc) | 4 (7%) | 6 | 3 |
| RHA (SMA) (acc)+LHA (LGA) (acc) | 2 (3%) | 7 | 4 |
| CHA (SMA) (rep) | 3 (5%) | 9 | 5 |
| CHA (aorta) (rep) | 1 (2%) | – | 6 |
| RHA+RGA+LHA2 (celiac artery) (rep) | 1 (2%) | – | |
| CHA (SMA)+LHA (LGA) (acc) | 1 (2%) | – | – |
HGCR: Hospital General Universitario de Ciudad Real.
In 6 patients, infiltration was reported in the mesenteric artery after the initial SMA approach. These infiltrations were not described in the initial MSCT reports in any of these cases. After the retrospective study with MSCT and MIP reconstruction, only one patient, whose pre-operative CT images had been done in another center, had no pre-operative diagnosis.
DiscussionHepatic Artery AnomaliesAnomalous anatomic distribution of the hepatic artery occurs in up to 45% of patients. It would be advisable to have prior knowledge of a patient's distribution in order to avoid problems when performing pancreaticoduodenectomy because the morbidity of this procedure is already high without adding the possibility of episodes of perioperative hemorrhage and ischemia. In normal situations, the celiac artery is the origin of the LGA, common hepatic artery and splenic artery. The common hepatic artery divides into the gastroduodenal artery and the proper hepatic artery. The proper hepatic artery divides into the RHA and LHA.4–8
Anatomic anomalies of the hepatic artery can be accessory anomalies, which are those that originate in a different place than normal and are present with the vessel in its classic situation. There may also be replacement anomalies, which originate in a different place than normal and there is no normally placed vessel.
Different classification systems have been developed to identify the anatomical variations of the hepatic artery by authors such as Michels,2 after a study of 200 autopsies, or later modifications by Hiatt,3 in a study of 1000 liver transplants. In addition, isolated unconventional anatomical situations have been described.8
- -
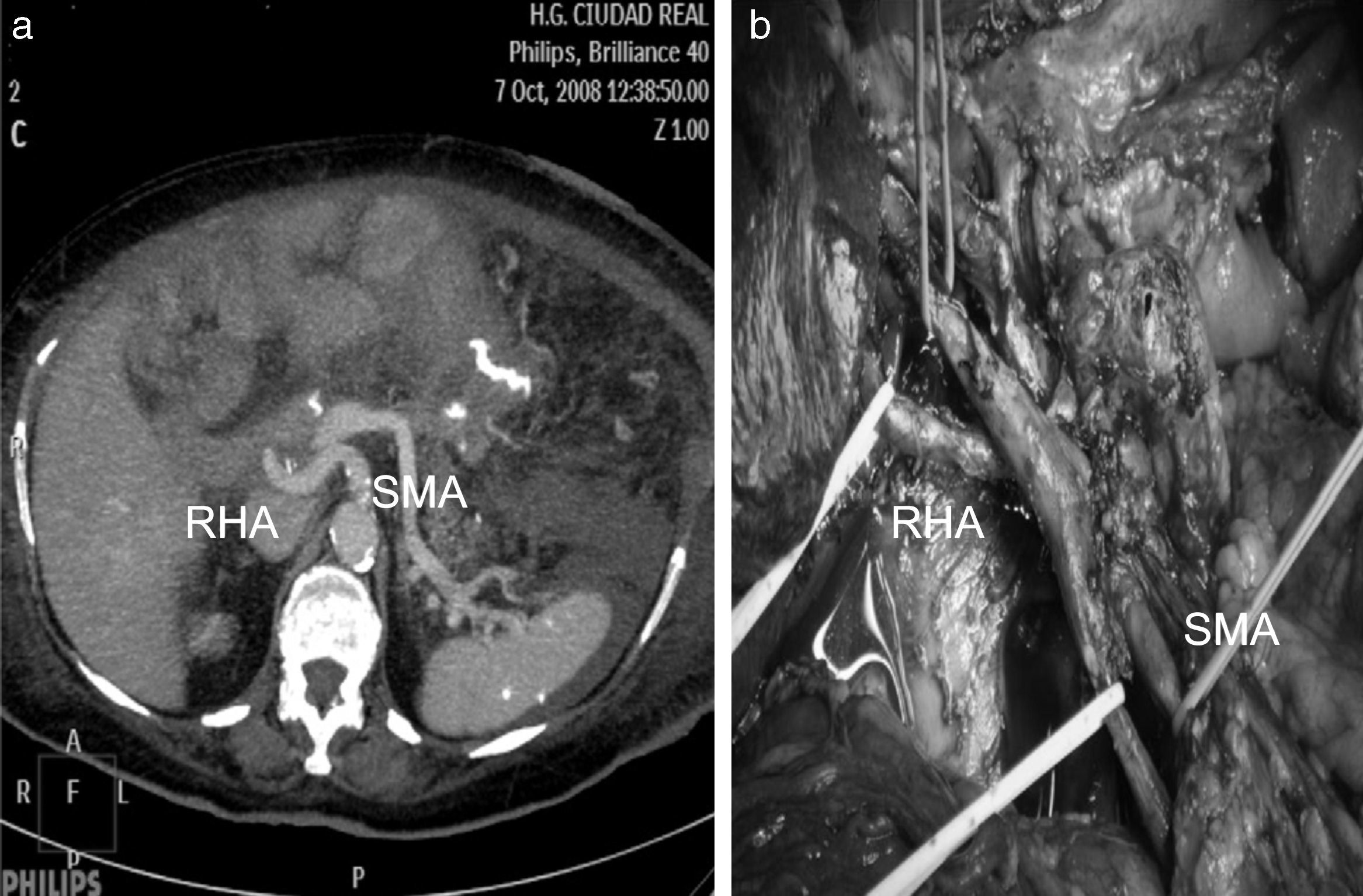
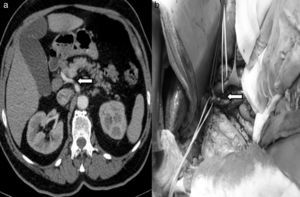
Replaced right hepatic artery. This is the most common anatomical variation of the hepatic artery (11%–21%). It originates in the SMA. In our sample, this occurred in 5 patients (8%). In all cases, its path was retropancreatic and retroportal with entry to the hepatoduodenal ligament posterolateral to the bile duct (type 1). But it can also run through the head of the pancreas (type 2) or between the head of the pancreas and the portal vein (type 3).4 Its perioperative identification avoids damage such as intra- or post-operative hemorrhage and ischemic phenomena, with risk of complications in the bilioenteric anastomosis (Fig. 2).
- -
Accessory RHA and LHA. This occurs when there are RHA from SMA and LHA from LGA in addition to the normally located RHA and LHA. It occurs with a frequency of 0.8%–8%, and in our series there were 2 cases (3%).
- -
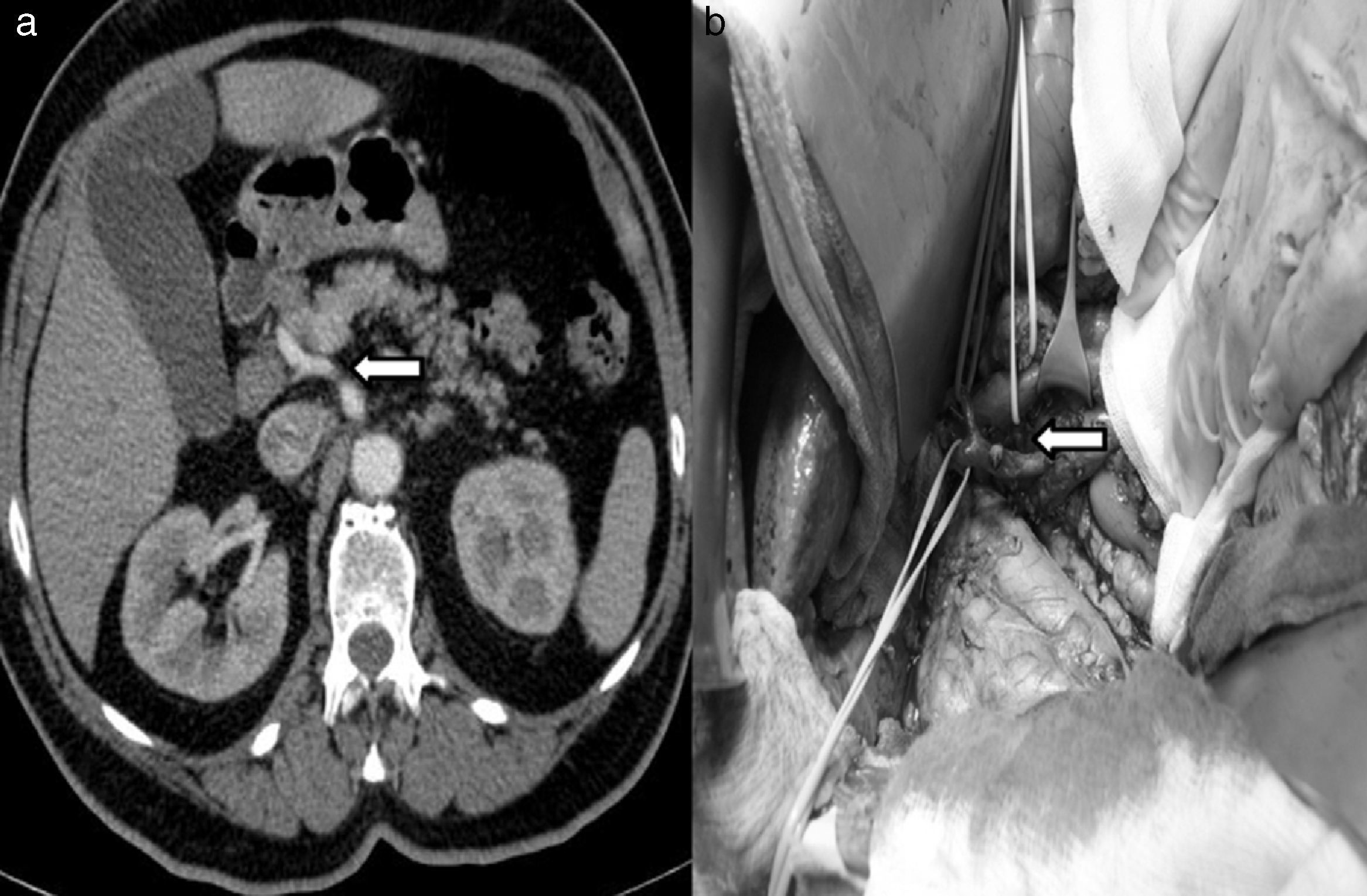
Replaced common hepatic artery, or hepatomesenteric artery. This occurs in 0.4%–4.5% patients. The common hepatic artery originates in the SMA. Its proximal pathway can be ventral to the pancreatic surface, through the pancreatic parenchyma or behind the head of the pancreas ascending medially to the bile duct in the normal location of the gastroduodenal artery. This occurred in 3 of our patients (5%) (Fig. 3).
- -
Common hepatic artery from the aorta. Described as an anatomic variation according to Hiatt (type 6, 0.2%), which occurred in one of our patients (2%).
- -
Accessory LHA from LGA. This corresponds with Michels type 5 anatomical variation (8%–18%), and Hiatt's type 2 (9.7%). Three of our patients presented this anomaly (5%).
- -
RHA, gastroduodenal artery, 2 LHA originating in the celiac artery. Anatomic variation that happened in one case (2%); not described in the classification systems studied.
- -
Common hepatic artery originating from the SMA, together with LHA from LGA. Anatomic variation that occurred in one case (2%); also not described in the classification systems studied.
Therefore, due to the presence of these arterial anomalies seen in 45% of patients, their pre- or intra-operative diagnosis is important in order to avoid complications. The methods used that allowed us to identify these anatomical variations were:
Multi-slice Computed Tomography: With Later Tridimensional Reconstruction and Maximum Intensity ProjectionMSCT has a higher quality compared with helical CT for the diagnosis and staging of periampullary lesions. The advantages it provides include the acquisition of volumes, study speed, better use of contrast and the possibility to create tridimensional reconstructions. There are several tridimensional reconstruction techniques, such as shaded surface display (SSD), VR and MIP.
MIP is a tridimensional representation technique that evaluates each unit of volume, voxel, and selects points of maximum intensity. High-intensity materials like intra-arterial calcium or vascular prostheses can hide endovascular contrast information.
The greater precision for evaluating the tumor resectability of MSCT is due to its higher resolution and ability to create multiplanar reconstructions. The disadvantages it presents include the need for work stations, training for radiologists and sufficient time (which is often impossible due to the excessive amount of work) to create multiplanar and volumetric reconstructions.9–12
In our study, we reviewed the pre-operative radiological information; there was no exhaustive staging protocol for periampullary lesions (although there was a protocol for image acquisition); therefore, information about the hepatic artery variations was not always included. Reconstructions were only done in a series of patients. In 5 patients, VR reconstructions were also done, and in 12 patients we obtained multiplanar and curved plane reconstructions with MIP algorithm. This explains why anomalies of the hepatic artery were reported in only 2 patients (11%).
In a later blind, protocolized and multidisciplinary study about the acquisition of images and findings necessary for reporting, the hepatic arterial anatomy was evaluated with coronal and sagittal images in combination with axial images by means of MIP reconstruction techniques. This homogenization allowed us to identify anomalies in 18 out of the 20 patients. The 2 unidentified cases were due to the lack of possibility for reconstruction with images from other hospitals (Table 3).
Description of Peripancreatic Arterial Anomalies According to the Information Methods Available.
| Case | Initial SMA access (origin), type | Pre-operative CT (origin), type | Post-op blind CT, with image reconstruction, MIP (origin), type |
| 1 | RHA (SMA) (acc) | Not described | Not described; another center |
| 2 | RHA (SMA) (acc) | Not described | Described |
| 3 | RHA (SMA) (acc) | Not described | RHA (SMA)+LHA (LGA) |
| 4 | – | LHA (LGA) (acc) | LHA (LGA) (acc) |
| 5 | CHA (SMA) (rep) | Not described | Described |
| 6 | – | Not described | RHA+RGA+2LHA (celiac artery) |
| 7 | RHA (SMA) (rep) | Not described | Described |
| 8 | RHA (SMA) (rep) | Not described | Described |
| 9 | – | Not described | LHA (LGA) (acc) |
| 10 | CHA (SMA) (rep) | Not described | Another center (not described) |
| 11 | RHA (SMA) (acc) | Not described | Described |
| 12 | CHA (aorta) (rep) | CHA (aorta) | Described |
| 13 | RHA (SMA) (acc) | Not described | Described |
| 14 | RHA (SMA) (rep) | Not described | Described |
| 15 | LHA (LGA) (acc) | Not described | Described |
| 16 | RHA (SMA) (rep) | Described | Described |
| 17 | RHA (SMA) (rep) | Described | Described |
| 18 | CHA (SMA) (rep) | Described | Described |
| 19 | CHA (SMA)+LHA (LGA) (acc) | Not described | Described |
| 20 | RHA (SMA) (acc)+LHA (LGA) (acc) | Not described | Described |
acc: accessory; RGA: right gastric artery; LGA: left gastric artery; CHA: common hepatic artery; RHA: right hepatic artery; LHA: left hepatic artery; SMA: superior mesenteric artery; MIP: maximum intensity projection; rep: replaced; CT: computed tomography.
In 2003, Pessaux et al.13 reported this modification in the resection of the retroportal lamina during pancreaticoduodenectomy, which allowed for more extensive lymphadenectomy and the identification of arterial anomalies. In addition, perioperative bleeding is reduced with the control of the lower pancreaticoduodenal vessels. Furthermore, useless resections are avoided in cases of infiltration of the SMA. Its application may be enormously helpful when there is suspicion of SMA infiltration, RHA originating from the SMA and also when there is infiltration of the portal vein and SMV, and the periampullary neoplasm extends to the body of the pancreas. Traditionally, after the Kocher maneuver, palpation has been used to try to identify neoplastic infiltration as well as the existence of arterial anomalies, although this maneuver is not very precise. With the initial access of the SMA, we can reach an exact diagnosis.14–17
All the anomalies of the hepatic artery originating in the SMA were identified. RHA originating from the SMA was the most frequent finding (8%), each of which was type 1. In cases with artery variations in the parenchyma or neoplastic infiltration of the anomalous hepatic artery, reconstruction is recommended to avoid ischemic problems.
ConclusionsThe excessive workload of the Radiology Department may explain why on many occasions image reconstruction, such as three-dimensional reconstruction or MIP, could not be done in our study population, even though this would have provided preoperative vascular and vascular invasion information.
The creation of multidisciplinary teams that unify study criteria and demand protocolized radiological testing in patients who are scheduled for pancreaticoduodenectomy would provide surgeons with adequate preoperative information and staging.18,19
In all cases, it would be recommendable to use an initial SMA approach during PD to identify arterial variants in order not to damage them and thus avoid hepatic parenchymal ischemia or perianastomotic vascular failure. Perioperative bleeding can also be reduced with initial access of the SMA and pancreaticoduodenal arterial vessels. Lastly, aggressive surgery is also avoided with the identification of any neoplastic infiltration of the SMA that had not been identified preoperatively.13–17
Conflict of InterestsThe authors declare no conflict of interests.
Parts of this manuscript were presented at the 2010 Spanish National Surgery Congress in Madrid.
Please cite this article as: Padilla Valverde D., et al. Métodos radiológico-quirúrgicos para la identificación de anomalías celiaco-mesentéricas de la arteria hepática ante una duodenopancreatectomía. Cir Esp. 2013;91:103–10.