Surgical site infection (SSI) is associated with prolonged hospital stay, increased morbidity, mortality and sanitary costs, and reduced patients’ quality of life. Many hospitals have adopted guidelines of scientifically validated processes for prevention of surgical site and central-line catheter infections and sepsis. Most of these guidelines have resulted in an improvement in postoperative results. A review of the best available evidence on these measures in abdominal surgery is presented. The best measures are: avoidance of hair removal from the surgical field, skin decontamination with alcoholic antiseptic, correct use of antibiotic prophylaxis (administration within 30–60min before incision, use of 1st or 2nd generation cephalosporins, single preoperative dose, dosage adjustments based on body weight and renal function, intraoperative re-dosing if the duration of the procedure exceeds 2 half-lives of the drug or there is excessive blood loss), prevention of hypothermia, control of perioperative glucose levels, avoidance of blood transfusion and restriction of intraoperative liquid infusion.
La infección del sitio quirúrgico (ISQ) se asocia a prolongación de la estancia hospitalaria, aumento de la morbimortalidad y gasto sanitario. La adherencia a paquetes sistematizados de medidas de prevención consigue disminuir la tasa de infección. Se presenta un análisis crítico de las medidas de profilaxis de la ISQ en cirugía abdominal. Cuentan con mayor grado de evidencia: no eliminación del vello del campo quirúrgico, descontaminación de la piel con soluciones alcohólicas antisépticas, aplicación correcta de la profilaxis antibiótica sistémica (inicio 30–60min antes de la incisión, uso preferente de cefalosporinas de 1.a y 2.a generación en monodosis, ajuste de la dosis con relación al peso y a la función renal, administración de dosis intraoperatoria en hemorragia > 1.500ml o duración > 2 veces la vida media del antibiótico), mantenimiento de la normotermia, control de la glucemia perioperatoria, limitación de las transfusiones sanguíneas y restricción del aporte intravenoso intraoperatorio.
Surgical site infection (SSI) is one of the most frequent complications in abdominal surgery. It is associated with prolonged hospital stay, a compromised quality of life and an increase in morbimortality and in costs.1,2 The published variability of SSI incidence depends, among other factors, on its definition.3,4 The centers for disease control (CDC) define SSI as the infection which presents in or near the surgical incision, during the first 30 days, or up to one year, if an implant is inserted.5 The SSI appears when the bacterial inoculum exceeds the immune system's ability to control it. Contamination in abdominal surgery originates from the skin or organs that have been targeted during surgery.6,7
The determinant factors for infection are the surgeon, the pathogen and the patient. The surgeon plays a principal role in surgical infection. His or her experience and skill may reduce the inoculum to dimensions which the body's own defences are able to control. Correct surgery involves the careful management of tissues, good haemostasis, no unnecessary prolongation of surgery and minimised extravasation of intraluminal content. The patient-dependent factors include comorbidities, obesity, smoking habits and advanced age. The standard organisms causing infection have not changed over the last few decades, but the numbers of antibiotic-resistant bacteria have risen.3,4,8
Both pharmacological and non-pharmacological measures are available to the surgeon to reduce bacterial contamination at the surgical site and the incidence of SSI. The aim of this review was to update the best evidence available on preventive measures for SSI in abdominal surgery.
Shower Before SurgeryIt has been demonstrated that a shower before surgery with chlorhexidine results in greater reduction of bacterial contamination of the skin.9,10 However, several meta-analyses have been unable to correlate this reduction in colonisation with a lower incidence of SSI.11,12 Although a pre-operative shower is recommended, there is little difference when comparing the use of soap and water with that of antiseptic solutions.
Nevertheless, in nasal methicillin-resistant Staphylococcus aureus (MRSA) carriers, nasal decontamination with chlorhexidine or mupirocin ointment combined with a chlorhexidine shower is recommended. This reduces the amount of bacteria and lowers the risk of infection.13,14
Hair RemovalHair has traditionally been removed from peri-incisional skin in diverse ways (depilation, shaving). The lowest rate of infection is achieved when the hair is not cut. When removal is considered necessary, electric shavers with disposable heads lead to lower rates of infection than manual devices.15 This procedure should be carried out a few hours prior to surgery with care taken not to cut the skin.14
Decontamination of the Skin at the Surgical SiteAlcohol is the most microbiologically active agent for disinfection, but its anti-microbial effect disappears after a few minutes; it is inflammable, and contraindicated on mucose membranes. Its use for surgical preparation has therefore been practically discontinued. Antiseptics such as chlorhexidine gluconate (CG) and povidone iodine (PI) are less active than alcohol, but they have a greater residual effect. Both come in aqueous or alcohol solutions, the latter being more effective than CG and (PI) alone. Despite recent studies,16,17 the debate continues on the most effective antiseptic. Systematic reviews confirm the significant role played by alcohol associated with antiseptics and highlight that the majority of studies compare 2 agents (alcohol and CG) against one (PI),18 and the efficacy of alcoholic chlorhexidine is put down to chlorhexidine alone. Disinfection with alcohol on its own is recommended for taking blood cultures19 and with CG–alcohol for the insertion of venous catheters.20 However, for abdominal surgery the question of whether CG–alcohol is better than PI–alcohol has not been resolved.
Any antiseptic should work for 2–3min and must be left to dry before placing surgical drapes. Alcohol-based antiseptics should be of low concentration and be left to evaporate to reduce the risk of burns when using the electric scalpel.14,17,21
Wound ProtectorsProtectors placed around the edges of the surgical wound protect the abdominal wall from drying out, trauma and contamination. Their use has been demonstrated to reduce the wound inoculum at the end of surgery although, depending on the type used, this does not always correlate with a lower incidence of SSI. Wet cotton compresses and buds are permeable to bacteria after a short time. However, one meta-analysis concludes that plastic protectors reduce SSI in abdominal surgery. The effect of the plastic barrier increases with the level of surgical contamination.22
Plastic adhesives on the surgical site are an attempt to minimise wound contamination with cutaneous germs. A Cochrane review found no evidence to show a reduction in SSI with their use; on the contrary, there is some evidence to demonstrate that it increases.23
Skin IncisionSkin incision with an electric scalpel significantly increases the risk of infection; therefore a cold scalpel should be used for dermal incision. When bleeding occurs around the edges, electrocautery should be selectively used on bleeding points.24
Aponeurotic SutureMonofilaments are less likely to become contaminated than braided ones. Bacteria can adhere better on the latter and the phagocytic capacity of the host cells decreases. Continuous sutures are associated with a lower risk of infection than interrupted ones, possibly due to more homogeneous distribution of pressure on the tissues and the smaller quantity of foreign bodies in the wound.25 Suture materials which are impregnated with antibacterial substances obtain a reduction of bacterial concentration in the wound.26,27 Several studies suggest that sutures impregnated with triclosan reduce incisional and organ-lining SSI,27,28 although independent, more statistically powered analysis is required in order to recommend their routine use.
Delayed Primary Suture of the WoundDelayed primary suture of the wound is valid for a selected group of highly contaminated surgical interventions.14,29 The wound is left open with compresses impregnated in serum and placed next to the edges when tissue appearance is correct.30 A randomised trial in dirty abdominal wounds showed an SSI rate of 42.5% with primary closure, compared with 2.7% with delayed primary suture.30
Intra-abdominal DrainsIn elective surgery, the objective of drains is to eliminate excess fluids from a cavity and control anastomosis. Both aspects have been questioned and there is extensive evidence that casts doubt on their use and demonstrates a negative effect of drains in abdominal surgery (surgery of the colon, cholecystecomy, hepatectomy, gastrectomy) and extra abdominal surgery (thyroid, hernioplasty). Drains should be avoided, but if they are used, they should be closed, one directional, and suction.14,31,32
Topical ProphylaxisAlthough inconclusive, there is evidence in favour of irrigating the surgical site with antibiotic solutions combined with systemic prophylaxis, which suggests that there are added benefits.33–35 The majority of these studies are in experimental peritonitis, showing reductions in SSI and mortality of up to 65%.36 However, clinical trials do not confirm these benefits,37 due to differences in the time of development and size of inoculum between clinical and experimental conditions. The effectiveness of prophylactic irrigation of the abdominal cavity with gentamycin and clindamycin in elective colorrectal surgery has been demonstrated, reducing SSI rates.38 Further trials to confirm these results and the most appropriate agent are needed.
Results regarding slow-release topical antibiotics are controversial. Animal models demonstrate SSI reduction with additive effects to systemic antibiotherapy.39 In colorectal surgery, subcutaneous collagen sponges with gentamycin obtain a significant reduction in SSI.40 However, other authors have not been able to reproduce these benefits and have even found that SSI increases, explained by the short half-life of gentamycin and the foreign body effect of the sponge.41
Irrigation of the abdominal cavity with antiseptic solutions such as PI or chlorhexidine has not demonstrated efficacy and is associated with the appearance of adverse effects such as sclerosing mesenteritis.42,43
Mupirocin ointment reduces complications associated with central venous routes in haemodialysis patients who are non-carriers of MRSA and could be valid in prophylaxis of abdominal surgery infection, but this hypothesis is yet to be confirmed.44
In colorectal surgery, carboxymethyl cellulose dressings which contain silver may have an effect on superficial incisional SSI,45 but not on deep incisional or organ space SSI.
Mechanical Bowel Preparation in Colorectal SurgeryMechanical bowel preparation (MBP) with evacuating solutions is used with the intention to reduce complications of infection and post-operative anastomotic dehiscence.46 Current evidence suggests that MBP does not reduce the rate of complications, including anastomotic failure.47–50 Several authors refer to an increase in complications associated with MBP, such as hydroelectrolytic conditions, convulsions or spontaneous oesophageal rupture.51 Due to this evidence, many colorectal surgeons have stopped using MBP52 and, unfortunately, administrating oral antibiotic prophylaxis as well.
Oral Antibiotic Prophylaxis in Colorectal SurgerySeveral meta-analyses have demonstrated that the combination of intravenous and oral antibiotic prophylaxis reduces up to 26% of SSI risk when compared with isolated intravenous prophylaxis.53,54 This practice may be of little use in the absence of MBP due to the high bacterial inoculum in a faeces-filled colon. However, no publications describe the effect of oral antibiotics in the absence of MBP. The most frequently administered oral antibiotics are: neomycin with erythromycin or metronidazole or kanamycin with erithomycin or metronidazole.55
Systemic Antibiotic ProphylaxisAntibiotic prophylaxis should achieve antibiotic levels in the tissues above the minimum inhibitory concentration of germs before they contaminate the surgical site. They should therefore be administered 30min prior to commencement of surgery.56 In general, prophylaxis is not indicated in clean surgery, as risk of SSI is lower than 2%.14,56 However, the latest meta-analyses on prophylaxis in herniorrhaphy demonstrate the antibiotic's protective effect, particularly in hernioplasty.57,58 Antibiotic prophylaxis is also recommended59 in breast surgery. In colorectal surgery, the combined administration of antibiotics compared with aerobic and anaerobic pathogens reduces this by 59%.14
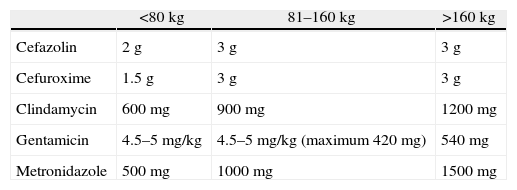
Obesity and renal failure require an adjustment of the prophylactic antibiotic dosage. Alexander et al.14 established an 80kg cut-off point to adjust the dosage (Table 1). The majority of antibiotics are eliminated via the kidney, and the dosage must therefore be adjusted in renal failure, depending on creatinine clearance.60
Preoperative Recommended Dose of Prophylactic Antibiotics.
| <80kg | 81–160kg | >160kg | |
| Cefazolin | 2g | 3g | 3g |
| Cefuroxime | 1.5g | 3g | 3g |
| Clindamycin | 600mg | 900mg | 1200mg |
| Gentamicin | 4.5–5mg/kg | 4.5–5mg/kg (maximum 420mg) | 540mg |
| Metronidazole | 500mg | 1000mg | 1500mg |
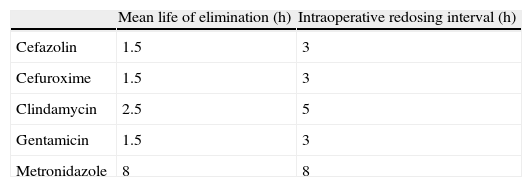
A single prophylactic antibiotic dose is as effective as multiple doses.61,62 Prolonged use of antibiotics provides no benefits and actually increases the risk of developing resistance.63 Intraoperative redosing is more important than prolongation of prophylaxis after surgery. Indications for redosing are blood loss over 1500ml and prolongation of the operation over twice the half-life of the antibiotic14,55 (Table 2). When creatinine clearance is below 50ml/min, the redosing interval should be twice that established for a patient with normal renal function, but when it is under 20ml/min, the time interval should be quadrupled.14
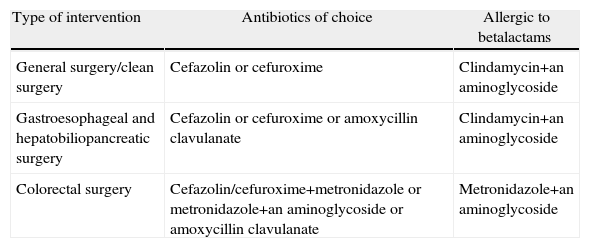
It is important to evaluate the local patterns of bacterial sensitivity when selecting the appropriate antibiotic. We know the cut-off points of minimal inhibitory concentration (MIC) from which a bacteria is considered resistant, but there is no consensus on the cut-off percentage of resistant strains from which we consider an antibiotic ineffective for prophylaxis.55 The antibiotics used in prophylaxis have to be different from those used in treatment (Table 3). 1st and 2nd generation cephalosporins are thus regarded as ideal drugs.14,64,65 Broad spectrum drugs in high risk surgery prophylaxis have been proposed. Itani et al.4 analysed ertapenem in colorectal surgery, demonstrating a lower incidence of SSI compared with cefotetan. This recommendation has been disputed, arguing that the use of broad spectrum drugs may increase resistance and colonisation by Clostridium difficile.66 Despite this, several clinical guidelines include it as an alternative in elective colorectal surgery.14,67
Antibiotics Recommended for Systemic Prophylaxis.
| Type of intervention | Antibiotics of choice | Allergic to betalactams |
| General surgery/clean surgery | Cefazolin or cefuroxime | Clindamycin+an aminoglycoside |
| Gastroesophageal and hepatobiliopancreatic surgery | Cefazolin or cefuroxime or amoxycillin clavulanate | Clindamycin+an aminoglycoside |
| Colorectal surgery | Cefazolin/cefuroxime+metronidazole or metronidazole+an aminoglycoside or amoxycillin clavulanate | Metronidazole+an aminoglycoside |
The most recommended antibiotics in gastroesophageal and hepatobiliary surgery are 1st and 2nd generation cephalosporins and amoxycillin clavulanate. In some Spanish regions prophylaxis with amoxycillin clavulanate is not recommended, due to non urological high resistance rates of Escherichia coli which may reach 25%.68 The 3rd and 4th generation cephalosporins are also effective, but not recommended due to their cost and their relationship with the appearance of MRSA and ESBL enterobacteria. In patients who are allergic to beta-lactams, the combination of vancomycin or clindamycin with an aminoglycoside may be used.14,55,64
Combinations of metronidazole with 1st and 2nd generation cephalosporins are recommended in colorectal surgery (cephuroxim/cefotetan) or with gentamycin. Amoxycillin clavulanate can be used depending on the local resistance pattern. In allergic patients, clindamycin is not recommended due to the rate of resistant Bacteroides fragilis and metronidazole must be opted for. The use of aminoglycosides may be controversial, although they are very effective agents compared with gram-negative bacilli and particularly with Pseudomonas aeruginosa.69 Due to the short half-life of gentamycin (1.5h), single doses of 4.5–5mg/kg are recommended, with a very low toxicity risk. Another disadvantage is its antimicrobial spectrum, since the combination of metronidazole with an aminoglycoside does not cover gram positive microrganisms. This may explain why Streptococcus spp.36 presents in 27% of SSI after elective colorectal surgery.
Body TemperatureModerate hypothermia (34–36°C) is associated with diverse adverse effects, such as coagulopathies, which result in greater blood loss and which may increase the need for blood transfusion.70 Hypothermia is also associated with prolonged anaesthetic recovery,71 increased hospital stay72 and SSI.73 In colorectal surgery hypothermia was confirmed to triple the risk of wound infection74 and in clean surgery to multiply it by 6.75 The most accepted explanation is the constriction of blood vessels, which reduces blood flow to subcutaneous cellular tissue and oxygen tension.72,73,76 Hypothermia affects cellular and humoral immunity, and its cytokine-mediated regulation.77 Some studies maintain that hyperthermia up to 40°C may be more beneficial, based on the mechanism of fever as an enhancer of the body's defence mechanisms.7,78 However, further studies are needed to confirm these results. The mechanisms for maintaining body temperature must be systemic and local, and include the administration of warming IV fluids, heat lamps and thermal blankets.14,79
Oxygen TherapyThere has been speculation regarding supranormal oxygen delivery increasing partial oxygen pressure in the surgical wound, and thereby increasing the oxidative destruction of bacteria by neutrophils. Studies have been designed comparing the perioperative administration of an 80% FiO2 compared with a 30% FiO2. Although no adverse effects with 80% FiO2 have been described, results are contradictory, with studies which show lower incidences of SSI with hyperoxia80,81 and others which show no significant differences.82,83
Control of Postoperative GlycaemiaDiabetics suffer from a greater incidence of postoperative complications and mortality, including delay in SSI healing.84 In hyperglycaemic situations, there is an increase in catecholamine, cortico-steroid and growth hormone levels, inhibiting the release of oxygen to the wound.85 In studies on hyperglycaemia and SSI in heart surgery with sternotomy, maintaining glycaemic levels between 120 and 160mg/dl for the first 2–3 days after surgery reduces the risk of SSI.86,87 A retrospective study on general and vascular surgery establishes that each increase of 40mg/dl in glycaemia above 110mg/dl represents a 30% increase in the risk of SSI.88 Monitoring of postoperative glycaemia in diabetics is recommended, maintaining levels below 180mg/dl.14
Perioperative Blood TransfusionsPerioperative blood transfusions are an independent predictive factor of SSI,14,89 with a link between the amount of blood transfused and the risk of infection. In surgery for gastrointestinal tumours, transfusion of over 1000ml of red blood cell concentrate is associated with a 6.5 times higher risk of SSI.90 Multivariate analyses with animals, confirm that blood component transfusions are a separate risk factor for the development of SSI.91
A recent review of perioperative care strategy confirms that the need for a blood transfusion is associated with a higher incidence of SSI, which results in poorer postoperative recovery.92 However, according to Hranjec et al., reducing leucocytes in transfused elements may be beneficial.93
Infusion of Intraoperative IV LiquidsIntraoperative hyperhydration leads to oedemas which hinder correct healing, and this leads to an increase in SSI. A multi-centre trial demonstrates that the restriction of intraoperative fluids is associated with a lower incidence of SSI.94 Furthermore, liquid restriction is associated with lower postoperative morbimortality, due to an improvement in respiratory function, postoperative ileus and the risk of deep vein thrombosis.95
Multimodal rehabilitation programmes in colorectal surgery include the administration of glycoside solutions up to 3h before surgery, aimed at reducing the catabolic response induced by surgical aggression. The ingestion of fluids ending with 400ml of glucose solution would help to prevent postoperative dehydration.95
Nutritional StatusPreoperative malnutrition is associated with changes in body composition and the dysfunction of cardiopulmonary, renal and digestive systems. Immunosuppression associated with malnutrition facilitates SSI and intraabdominal sepsis. The term immunonutrition refers to the potential usefulness of certain immuno-nutrients in clinical development. The 3 most important immunonutrients are omega 3 fatty acids, glutamine and arginine. Several studies have attempted to demonstrate that the perioperative ingestion of immunonutrients is associated with a lower incidence of infections. A meta-analysis by Cerantola et al.96 shows a decrease in complications, particularly postoperative infections, and a reduction in hospital stay. However, no significant differences arise in postoperative mortality. In 2012, a meta-analysis on digestive cancer showed that perioperative immunonutrition is effective, safe, and reduces hospital stay and postoperative infections and complications.97 However, Klek et al.98 found no significant differences in the rate of complications, postoperative infection, mortality and hospital stay in a randomised clinical trial. In light of the discrepancies between these results and the high price of formulas, there is a need for independent studies to be conducted before including or eliminating immunonutrition from the general recommendations on SSI reduction.
ChecklistIn the last few years several checklists have been designed to ensure that correct perioperative parameters are applied. These facilitate the interrelation between members of the surgical team and an improvement in overall patient care. Haynes et al. carried out a prospective study of the fulfilment of the WHO checklist. They confirmed that perioperative mortality was lowered from 1.5% to 0.8% and the rate of complications fell from 11% to 7%.99
BundlesThe use of bundles or systemised packages for the prevention of postoperative complications has become popular during the last decade. These are either applied generally or in high risk situations such as colorectal surgery. In 2004, the CDC initiated the Surgical Infection Prevention Project (SIP) to ensure that a few basic evidence-based measures for the prevention of SSI are implemented (appropriate use of prophylactic antibiotics, administration during the hour prior to incision and removal before 24h, good protocol for body hair management and maintenance of normothermia). When these measure were applied, in 2007 Hedrick et al. reported higher compliance with the prophylaxis protocol and a reduction in SSI from 25.6% to 15.9% in colon surgery compared with a previous series.100 However, a similar study found no differences in SSI rate (19%) despite detecting an improvement in the process.101 In 2011, a randomised study of 211 colorectal operations compared its “traditional” bundle with another bundle which, among other modifications, had removed mechanical preparation of the colon and oral antibiotics. Despite managing to improve compliance with evidence-based measures, an increase in SSI from 24% to 45% was detected.102 In Spain, a multicentre observational study of SSI incidence in colorectal surgery with the application of a bundle similar to that of the SIP Project presented 23.2% of SSI in 611 colorectal interventions.103
To conclude, the adoption of a package of measures for the prevention of SSI with good scientific evidence (Table 4), its protocolisation and monitoring that it is adhered to lead to an improvement in the surgical process. Adherence to these bundles by preparing a checklist also helps to reduce the rate of SSI. However, in the struggle to reduce postoperative infection there remain several fairly unknown and poorly systematised factors, and therefore an impeccable individual surgical technique combined with good criteria in choosing the most appropriate prophylaxis measures is still irreplaceable.
Recommendations to Reduce SSI.
| 1. Administer a preoperative shower with soap and water |
| 2. If intranasal MRSA is detected: nasal decontamination with mupirocin+administer preoperative shower with chlorhexidine soap |
| 3. Do not eliminate hair during surgery or eliminate hair with an electric shaver with a disposable head |
| 4. Decontaminate the skin during surgery with a chlorhexidine alcohol solution or povidone. Do not dry. Leave the solution to take effect for 2–3min |
| 5. Make skin incision with a cold scalpel. Do not overuse electrocoagulation |
| 6. Perform fascial closure with monofilament sutures |
| 7. Use primary deferred suture of the wound in dirty surgery |
| 8. Avoid intra-abdominal drains. If they are used: closure is to be one directional and aspirative |
| 9. In colorectal surgery do not administer mechanical preparation of the colon. Administer a combination of oral and systemic antibiotics |
| 10. Systemic antibiotic prophylaxis:Commence 30–60min before incisionFlat dose, adjusted to the ideal weight and kidney functionRedosing if blood loss>1500ml or if surgery prolonged>twice the mean age of the antibioticPreoperative monodose. Do not prolong the prophylaxis with postoperative dose1st and 2nd generation cephalosporins usage preferred |
| 11. Avoid hypothermia |
| 12. Maintain postoperative glycaemia below 180mg/dl in diabetics |
| 13. Avoid perioperative blood transfusions |
| 14. Restrict IV introperative liquids |
The authors have no conflict of interest to declare related to this review.
Please cite this article as: Ruiz Tovar J, Badia JM. Medidas de prevención de la infección del sitio quirúrgico en cirugía abdominal. Revisión crítica de la evidencia. Cir Esp. 2014;92:223–231.