The increasing difference between the number of patients in waiting lists for liver transplantation and the number of available donors has generated a great interest in the use of non-ideal organs, like grafts obtained from cardiac death donors (DCD). However, the extreme sensibility to ischemia of these livers results in a low utilisation rate and a high percentage of post-transplant complications and re-transplantation. Normothermic perfusion machines (NMP) emerged as an alternative that tries to maintain the viability of the organ and even to improve its function. This review focuses on current results of DCD liver transplantation and on the role that NMP may have in this field.
La diferencia cada vez mayor entre el número de pacientes en espera para un trasplante hepático y el número de donantes disponibles ha generado un gran interés en la utilización de órganos «no ideales» como es el caso de los provenientes de donantes en asistolia. Sin embargo, la sensibilidad de estos hígados a la isquemia hace que su tasa de utilización sea baja y las tasas de complicaciones y retrasplante mayores que en el trasplante convencional. Las máquinas de perfusión normotérmica exvivo (MPN) surgen como una opción para intentar mantener la viabilidad de estos órganos e incluso mejorar su función. Esta revisión se centra en los resultados actuales obtenidos en el trasplante hepático con órganos provenientes de donantes en asistolia y el papel que puede tener la MPN en este campo.
The success of liver transplants (LT) has resulted, as a secondary effect, in a major difference between the number of patients on waiting lists (WL) for LT and the number of organs available. According to the latest data from UNOS, 15290 patients in the US were on WL for LT at the end of 2012, whereas only 6256 transplants were conducted in total that year.1 The scenario is similar in Spain, with 1093 LT conducted in 2013, but with 1997 patients on WL.2
One alternative available to increase the number of grafts is the use of organs from non-heart-beating donors (NHBD). However, due to the warm ischemia times (WIT) commonly associated with NHBD, the use of this type of organ is related to higher rates of graft loss and other complications. The use of ex vivo normothermic perfusion machines (NMP) would be interesting in LT, since the extreme responsiveness of these livers to ischemia results in a very low usage rate of organs from NHBD. This review focuses on the current state of NHBD in LT and on the role of NMP in its future development.
Historical Perspective and Current Legal Status of Non-heart-beating Donation in SpainEven though the donors used in the early stages of transplant programs were NHBD, their use has not become widespread after Harvard's definition of brain death in 1967, and due to poor results.3 However, the scarce availability of suitable donors and the success of NHBD liver transplants resulted in a review of this trend during the 1990s.
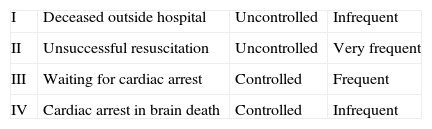
In 1995, the Maastricht consensus conference defined the classification of NHBD (Table 1).4
The division of NHBD into “controlled” and “uncontrolled” was added later, and it reflects the importance of WIT in the prognosis for the corresponding organ, once transplanted. In “controlled” donors, death is expected and, as a consequence, WIT is known (and lower, in most cases). Conversely, the “uncontrolled” NHBD implies an unexpected cardiopulmonary arrest. In these cases, WIT is usually more difficult to calculate (and usually longer).5
Both in the US and Europe, clinical guidelines have been developed to determine the key points of NHBD (definition of cardiac death, WIT and cold ischemia times [CIT], maximum transaminase levels, etc.).6,7 Although the application of these strict criteria for selecting donors has helped to lower post-transplant complication rates, the ratio of LT with grafts from NHBD has not increased over the last decade.6,8 Unlike NHBD kidney transplants, a significant number of livers are discarded.
In Spain, the current legal status makes NHBD donation even more interesting. Until recently, the only type of NHBD allowed in Spain was uncontrolled.9 As a consequence, our group has conducted an intense experimental study focused on NHBD type 2, to show the usefulness of normothermic oxygenation through the extracorporeal membrane,10–15 and it has later conducted the NHBD type 2 LT protocol with a systematic application of normothermic regional perfusion (NMRP), the results of which are not statistically lower than those for LT from brain-dead donors (BDD).7,16–18 The publication of RD 1723/2012,19 which includes type 3 donation, introduces a major shift in donation prospects, particularly for centres experienced in NHBD.
Current Results of Non-heart-beating DonationThe results for NHBD LT worldwide are still lower than those obtained in LT from BDD. Although there is no statistically significant difference between most studies with regard to patient survival (survival after three years 66.9%–77% in NHBD vs 77%–80% in BDD),20–23 graft survival is clearly lower in NHBD (48.8–65 vs 72%–80% in BDD, with a graft failure OR of 1.59–1.87 for NHBD).20,24–28
These differences recorded for graft survival are the result of higher rates of primary graft failure,20,24,28 but with reference to the ischemic cholangiopathy (IC) index, are much higher for NHBD grafts. IC occurs as a result of the responsiveness of the biliary epithelium to ischemic lesions and is evidenced by 15%–37% of NHBD recipients.24 It is evidenced by the appearance of bile duct stricture areas at the intrahepatic level. As a clinical consequence, IC patients present symptoms of cholangitis and recurring liver abscesses. When the causes of retransplant in NHBD liver recipients are analysed, IC is the most frequent cause.28
With regard to factors related to a higher graft failure rate, WIT and CIT are two of the most important factors related to poor post-transplant progress. Most centres call for a WIT of less than 20min.22 A CIT over 8h. is associated with 30.4% of graft failure, whereas for a CIT over 10h, there is graft failure in 58.3% of cases.20
These results have had the secondary effect of encouraging caution when deciding whether a NHBD liver is valid for LT. After analysing data from UNOS, Orman notes that the proportion of liver grafts discarded for LT based on their NHBD origin increased from 9% in 2004 to 28% in 2010.29
All this data points to the extreme responsiveness of NHBD livers to ischemia (both cold and warm). In this context, the use of NMP, particularly combined with NMRP, would keep graft exposure to ischemia at a minimum.
Normothermic Perfusion Machine in Experimental StudiesThe use of NMP is not a new technology. During the first half of the 20th century perfusion of several organs had been attempted with normothermic oxygenated serum, proving their viability for several days.30 In the first human LT, conducted by Starzl, the graft was prepared in a perfusion machine with diluted, oxygenated blood prior to transplantation.31 After the appearance of preservation solutions and the success of cold graft preservation, the use of NMP was deemed a far too complex process, especially in the context of little pressure on the WL, as was the case in the beginnings of LT. However, based on the need to use organs from donors with expanded criteria, the alternative of normothermic perfusion as an attempt to improve the quality of these grafts was raised again at the end of the 20th century.
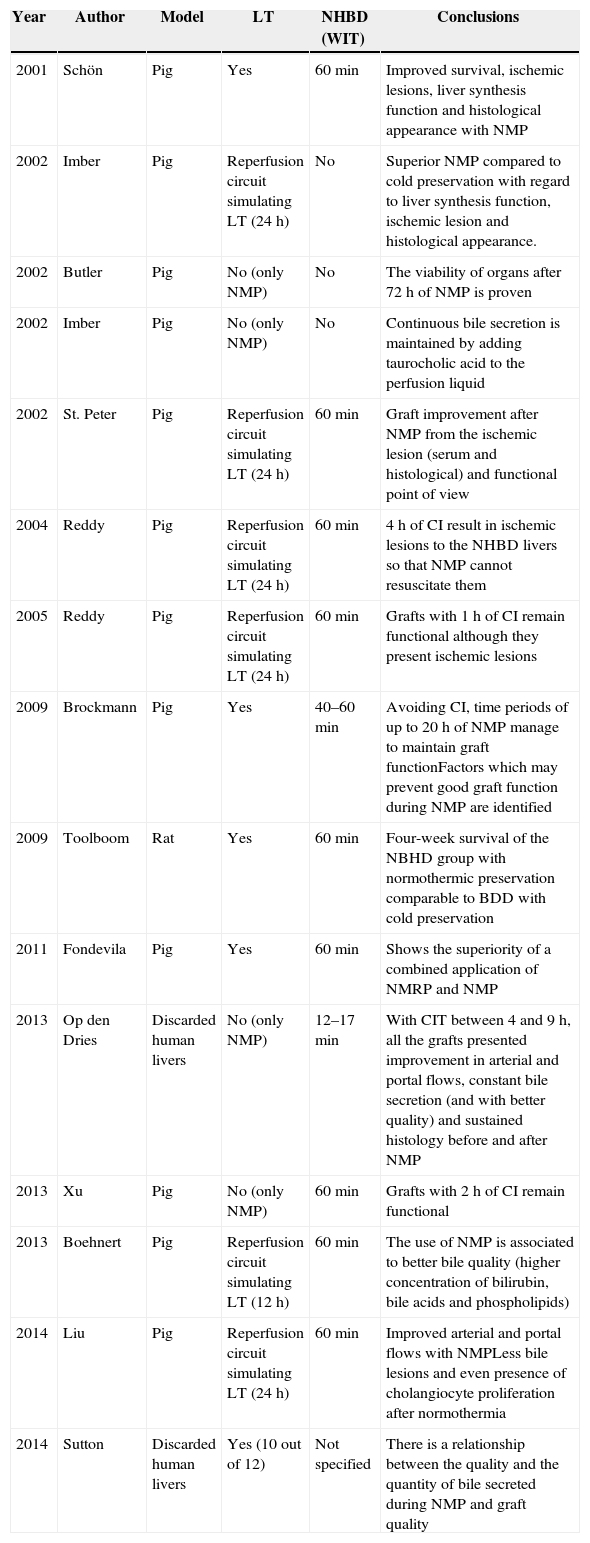
Following the first study, it was noted that the use of NMP might bring advantages to LT in general, and particularly in the field of NHBD. Apart from the obvious benefit of being able to analyse liver viability during ex vivo normothermic perfusion,32 NMP in the experimental field improves graft quality prior to LT (Table 2 summarises these experimental studies).
Experimental Works on NMP.
| Year | Author | Model | LT | NHBD (WIT) | Conclusions |
|---|---|---|---|---|---|
| 2001 | Schön | Pig | Yes | 60min | Improved survival, ischemic lesions, liver synthesis function and histological appearance with NMP |
| 2002 | Imber | Pig | Reperfusion circuit simulating LT (24h) | No | Superior NMP compared to cold preservation with regard to liver synthesis function, ischemic lesion and histological appearance. |
| 2002 | Butler | Pig | No (only NMP) | No | The viability of organs after 72h of NMP is proven |
| 2002 | Imber | Pig | No (only NMP) | No | Continuous bile secretion is maintained by adding taurocholic acid to the perfusion liquid |
| 2002 | St. Peter | Pig | Reperfusion circuit simulating LT (24h) | 60min | Graft improvement after NMP from the ischemic lesion (serum and histological) and functional point of view |
| 2004 | Reddy | Pig | Reperfusion circuit simulating LT (24h) | 60min | 4h of CI result in ischemic lesions to the NHBD livers so that NMP cannot resuscitate them |
| 2005 | Reddy | Pig | Reperfusion circuit simulating LT (24h) | 60min | Grafts with 1h of CI remain functional although they present ischemic lesions |
| 2009 | Brockmann | Pig | Yes | 40–60min | Avoiding CI, time periods of up to 20h of NMP manage to maintain graft functionFactors which may prevent good graft function during NMP are identified |
| 2009 | Toolboom | Rat | Yes | 60min | Four-week survival of the NBHD group with normothermic preservation comparable to BDD with cold preservation |
| 2011 | Fondevila | Pig | Yes | 60min | Shows the superiority of a combined application of NMRP and NMP |
| 2013 | Op den Dries | Discarded human livers | No (only NMP) | 12–17min | With CIT between 4 and 9h, all the grafts presented improvement in arterial and portal flows, constant bile secretion (and with better quality) and sustained histology before and after NMP |
| 2013 | Xu | Pig | No (only NMP) | 60min | Grafts with 2h of CI remain functional |
| 2013 | Boehnert | Pig | Reperfusion circuit simulating LT (12h) | 60min | The use of NMP is associated to better bile quality (higher concentration of bilirubin, bile acids and phospholipids) |
| 2014 | Liu | Pig | Reperfusion circuit simulating LT (24h) | 60min | Improved arterial and portal flows with NMPLess bile lesions and even presence of cholangiocyte proliferation after normothermia |
| 2014 | Sutton | Discarded human livers | Yes (10 out of 12) | Not specified | There is a relationship between the quality and the quantity of bile secreted during NMP and graft quality |
IC: ischemic cholangiopathy; NHBD: non-heart-beating donors; BDD: brain dead donors; WI: warm ischemia (see WIT); CI: cold ischemia (see CIT); WL: waiting list; HPM: hypothermic perfusion machine; NMP: normothermic perfusion machine; NMRP: normothermic regional perfusion, preservation method for NHBD organs prior to their removal, by means of normothermic recirculation; LT: liver transplant; WIT: warm ischemia times, the time elapsed from the interruption of circulation in the donated organ until it is perfused with hypothermic preservation solution; CIT: cold ischemia times, the time elapsed from the preservation of an organ in hypothermic state until transplant to the recipient; UNOS: United Network for Organ Sharing, the transplant administrative network in the US.
Schön's group proved in 2001 that the use of normothermia for only 4h prior to LT caused improved synthesis functions of the liver, when compared to 4h of cold preservation.33 The results were confirmed by the Oxford group, which concluded that the use of NMP is superior to cold preservation with regard to bile secretion, protein synthesis, ischemic lesions and histological aspects.32
Regarding the duration that NMP can maintain organs in working condition, Butler managed to maintain viability for 72h.34 The only parameter with unfavorable progress in the last part of the study was a decrease in bile secretion, but Imber proved that this effect is related to the challenge of filling the liver deposit with bile salts. The liver has the capacity to absorb and use the exogenous bile acids. Once added to the perfusion solution, a normal bile secretion can be re-established.35
Effects of Normothermia in NHBD ModelsIf in BDD models, normothermia manages to maintain organ viability for a long time, the case of NHBD is different. These organs have already undergone major ischemic lesion, with ATP reserves significantly depleted. For this reason, adding cold ischemia (CI) at the time of preservation means adding even more ischemic lesions which many organs cannot tolerate well.36,37
In his 2001 study, Schön also studied LT in a NBHD model with one hour of warm ischemia.33 Perfusion by means of NMP was accompanied by less ischemic lesions, better histological appearance, and a major survival improvement (100% after seven days), when in the control group (WIT for one hour+cold preservation) all the transplanted animals died less than 18h afterwards, due to primary graft failure. When the experiment was repeated in a reperfusion circuit simulating LT, it was further noted that organs preserved by NMP and “transplanted” presented continuous and good quality bile secretion, unlike cold-preserved organs.38
A bile secretion improvement following normothermic perfusion appears to be related to changes at the level of the biliary epithelium. Liu shows that the use of NMP is associated with an improved appearance of the biliary epithelium and he even notes indirect signs of cholangiocyte proliferation.39 These changes at the histological level are accompanied by an improvement in the quality of bile secreted by the graft, with higher concentrations of bilirubin, bile acids and phospholipids.40
However, all these preliminary studies used animal models without CI. From the logistic point of view, most LT cannot occur without cold preservation, simply because it is the method of graft transportation between the donor centre and the recipient hospital. In the context of using NMP in NHBD, there are only two logical alternatives: transportation with cold preservation for later placement of the organ in normothermia, or immediate application of normothermic perfusion (conditioned by the transportation of NMP to the donor hospital).
Oxford's group studied the first alternative in depth.41,42 When a 4h CIT is added to a one hour WIT prior to the beginning of normothermic perfusion, organs cannot maintain their synthesis function. However, if CIT is reduced to 1h, bile secretion and the levels of factor V are similar to patients without CI, although the ischemic lesion is still noteworthy, both at the serum and histological levels. The viability of organs may be maintained even with 2h of CIT, achieving a regeneration of ATP reserves of up to 80% of the levels prior to death.43 It is obvious that strictly maintaining time periods of one hour of CI, or even 2h, is impossible in many cases, therefore the most reasonable alternative is a mobile NMP to reperfuse the graft directly after extraction.
As Brockmann shows, if CI is kept at a minimum, even prolonged normothermic perfusions are associated with high graft survival rates.44 An algorithmic model is shown in the same study, detailing the perfusion parameters which may indicate good graft progress. This model includes bile flow, base excess, AST, ALT, hyaluronic acid, pressure and portal resistance within the first 4h of perfusion.
With regard to mid-term results after using NMP, Tolboom reports survival of 92% after four weeks in a model of NHBD in rats, with postoperative results comparable to the BDD group with cold preservation.45 However, these results must be analysed with caution, since the rat experimental model is quite different from conventional human transplants.
Our group published a study in 2011 combining the prior experience acquired in NMRP using NMP in a model of NBHD LT with 90min of WIT.46 The combined use of NMRP and NMP is related to improved survival after five days, in addition to less ischemic lesions and reduced development of inflammatory parameters and of endothelial lesions. This study confirms the importance of NMRP to start the process of cellular repair and of replenishment of energy reserves in models of NBHD transplant, but it also shows that NMP offers the physiological conditions and substrate to continue with quality improvement in this type of graft.
Studies of NMP in Organs Discarded for LTTwo studies have been published in recent years on the subject of normothermic perfusion of human livers discarded for transplant. In the first, conducted by the Groningen group, four organs from NBHD with a WIT between 12 and 17min and a CIT of between 4 and 9h were analysed.47 All the grafts presented improvement of arterial and portal flows after 30min, with constant bile secretion and with improved biochemical characteristics over time. The second, published by Sutton, finds a relationship between quality and quantity of bile secreted during NMP and the quality of the organ.48 Furthermore, it suggests that a time of 2.5h of perfusion in NMP might be sufficient to assess the potential transplantability of the organ.
Although the results obtained in these studies are promising, the study on discarded organs represents a theoretical model of the behavior of a liver once transplanted. A more detailed analysis at the molecular level might offer more insight into the changes that appear in this type of graft and their reversibility by means of NMP.
NMP in Clinical StudiesIn contrast with the multiple experimental studies, clinical experience with NMP is still very limited. In 2014, the Oxford group presented the first clinical study in LT at the ILTS conference in London using NMP in a group of 20 patients.49 Of these, 10 patients received grafts with expanded criteria and three were organs from NBHD type 3. The grafts were maintained in normothermic perfusion for a mean time of approximately 12h before the transplant. During this time, the organs presented good macroscopic appearance, a constant secretion of bile of approximately 10ml/h, and the pH of the perfusion fluid was maintained constant. There were no differences in the clinical behavior of organs from BDD and NBHD. All patients presented a correct postoperative recovery, with 100% survival rate after 30 days, and with first graft failure and primary graft dysfunction rates of 0% and 15%, respectively. When the results of this 20-patient group were compared to a historic control group with a 1/2 ratio, the only data showing a statistical difference was the transaminase peak in the first seven days. Although follow-up was limited to three months, no mid-term complications were reported. However, these results require confirmation using a randomised prospective study with a greater number of patients.
As the next step in the use of normothermia in the clinical environment, the Trial COPE-WP2 is currently being developed. This is a prospective, randomised, multi-centre, international study comparing LT results using NMP vs cold preservation.50 Coordinated by the Oxford group, some of the most important LT centres in England, the Leuven and Essen centres, and the Hospital Clínic de Barcelona are participating in the study. The study expects to include 260 patients, randomised with a 1/1 ratio to receive a graft preserved with normothermic perfusion or in cold temperature. The main purpose is to assess the effect of NMP compared to cold preservation for the prevention of lesions related to preservation and of primary graft dysfunction. The project offers very interesting perspectives for the study of actual organ behavior during normothermic perfusion, since a large volume of data and of serum and histological samples are expected to be compiled.
Since this work also expects to include LT with grafts from NHBD type 3, the results in the long term will be very interesting with regard to complications that are characteristic to this type of donation (IC) and the effects of NMP in this context. Data such as the quality and quantity of bile secretion, a biopsy of the bile duct prior to implantation and the performance of a magnetic cholangioresonance 6 months after LT specifically aimed at assessing potential intra-hepatic stenosis, could offer new assurances about the use of normothermia in NHBD.
Although COPE-WP2 is not expected to use organs from type 2 NHBD, the application of normothermia in this type of donation could be interesting. The current success of NHBD in the field of LT is closely related to the use of very strict criteria, which renders the transplantability rate of these livers very low. The use of NMP could improve the quality of these organs and increase their usage. However, any shift from a highly restrictive use of these livers to the application of more liberal criteria, associated with the use of NMP, should be undertaken by means of a prospective clinical study and later validated in a large series of patients.
ConclusionsTo conclude, the use of NMP in LT offers new and interesting prospects. With particular regard to the field of NBHD, where currently the limiting factor is the quality of the graft, normothermic perfusion may increase the number of available grafts and also provide organs of better quality. It is the belief of our group that the combined use of NMRP and NMP is the ideal alternative for these grafts. However, clinical studies are needed to validate the good results obtained in the experimental field.
Conflict of InterestI hereby state that there are no conflicts of interest.
Please cite this article as: Pavel MC, Fondevila Campo C, Calatayud Mizrahi D, Ferrer Fabrega J, Sanchez Cabus S, Molina Santos V, et al. La máquina de perfusión normotérmica en el trasplante hepático con injertos provenientes de donación en asistolia. Cir Esp. 2015;93:485–491.