The development of the lymphatic system begins in the sixth week of gestation, and the lymphatic vessels are formed by the thirtieth week.1 Lymphangiomas are residual embryonic lymphatic tissue, whose pathogenesis is not fully known. They are thought to represent isolated segments, sequestered from the lymphatic system and resulting from a failure of the lymphatic system to communicate with the venous system during embryonic development. These segments maintain the ability to produce lymph and, as the volume increases, the tumor grows.1–4 Morphologically, they are classified as single (unilocular), cavernous and intermediate cysts.5
We present the case of a 2-month-old infant with a family history of maternal multiple osteochondromatosis and hepatoblastoma in a maternal aunt during childhood. The patient was in multidisciplinary follow-up due to the existence of a right parotid congenital hemangioma. He was brought to the Emergency Department because of very limited oral intake and weight stagnation over the previous 2 weeks. Given the clinical suspicion of pyloric stenosis, a thoracoabdominal ultrasound was performed, which revealed a cystic-looking lesion with poor vascularization that was large in size (5.3cm×4.4cm×6.7cm). The mass occupied a large part of the left hemithorax, causing inversion of the left hemidiaphragm with anterior displacement of the ipsilateral lung, esophagus and gastroesophageal junction; this raised suspicion of its possible origin in the posterior mediastinum. Given the young age and the risk of complications derived from the size of the tumor mass, the patient was admitted for study and to rule out malignancy.
Upon examination, the patient presented an acceptable general condition, with a weight of 3.890kg. Cardiopulmonary auscultation showed no abnormal respiratory sounds. The patient was afebrile. A right submaxillary tumor of approximately 3cm in diameter and soft consistency was palpated, corresponding with the previously known parotid hemangioma.
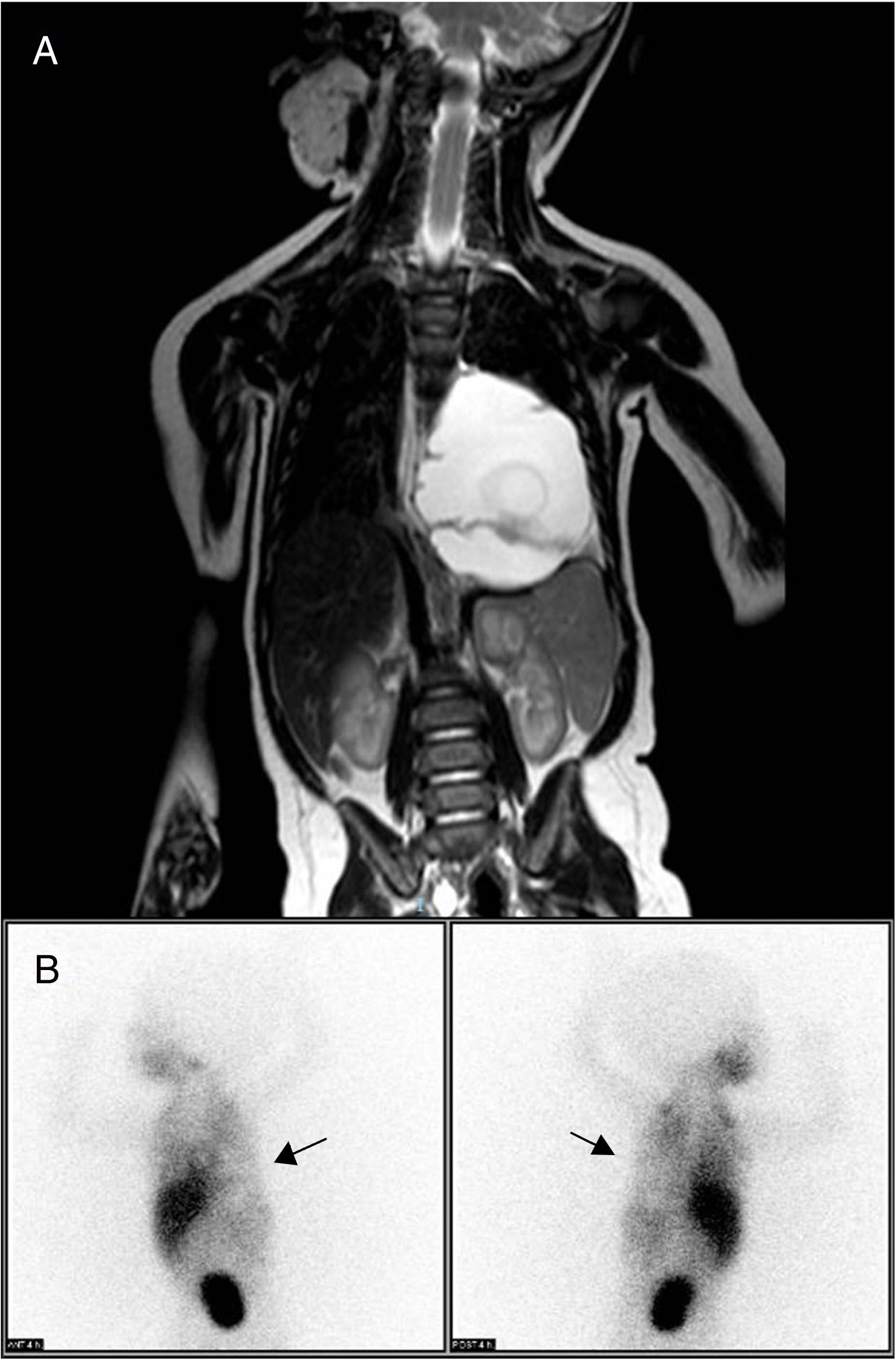
Thoracoabdominal magnetic resonance imaging study identified a mass in the posterior mediastinum (6.9cm×5.1cm×4.5cm), extraparenchymal and left paravertebral, that was macrocystic in appearance and caused deviation of the esophagus and gastroesophageal junction. There was no evidence of bone or medullary canal involvement, or compression of the airway (Fig. 1A), leading to a differential diagnosis with neuroblastoma, extralobar pulmonary sequestration and lymphatic malformation. The isotopic study with 123I-metaiodobenzylguanidine (MIBG), with acquisition of planar images 4h after the administration of the radiotracer, provided no findings indicative of chromaffin tissue, thereby ruling out neuroblastoma as a cause of the symptoms (Fig. 1B). Laboratory results did not reveal specific findings, and tumor markers and urine catecholamines presented normal values.
(A) Thoracoabdominal MRI showing a large mass in the posterior mediastinum and left paravertebral area. It is causing deviation of the esophagus and gastroesophageal junction, with no evidence of distant; (B) 123I-metaiodobenzylguanidine scintigraphy, performed 4h post-injection of the radiotracer, showing a cold area with zero tracer uptake in the left hemithorax, corresponding with the tumor mass (arrows) and ruling out neuroblastoma.
Percutaneous surgical drainage (40cc) was done with sclerosis using a fibrin sealant. The cytology of the cyst fluid presented mesothelial plaques and reactive lymphocytes, with no evidence of neoplastic cellularity, which supported the diagnosis of cystic lymphangioma-type lymphatic malformation. The postoperative period was uneventful. The patient was discharged on the seventh day after the procedure and was followed up in the outpatient surgery consultation.
The follow-up thoracoabdominal magnetic resonance study performed 2 weeks after the procedure showed the persistence of the posterior thoracic mass, with an increase in size compared to the diagnostic study (5.5cm×6cm×7.4cm). In addition, 2 hypodense images were observed in T2 sequences inside the lesion, which indicated a possible hemorrhagic complication or residual sclerosing material. Because the initial procedure was not successful, we decided to operate under general anesthesia for total excision of the tumor by thoracotomy in the fifth left intercostal space. A pleural lesion was observed under the lower lobe of the left lung that was compressing upwards. Subsequently, the lesion was opened and a sample taken for cytological study of the mass and dissection of the tumor from the upper part to the diaphragm adhered to the aorta, post-excision cytology and placement of a corrugated chest drain. The macroscopic pathology results of the intrathoracic mass showed multiple irregular fragments of whitish elastic tissue and other cystic-looking fragments. Pleural and tumor fluid cytology detected typical mesothelial plaques and a reactive lymphocyte component, with no evidence of neoplastic cellularity.
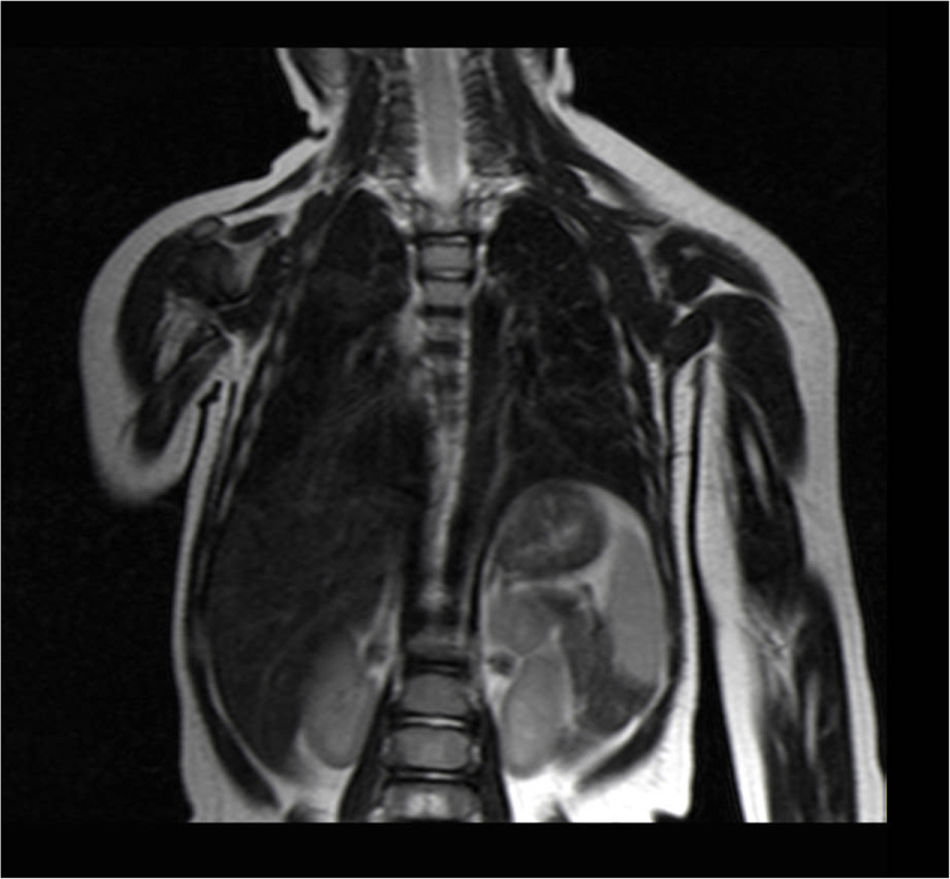
Currently, the patient is under scheduled outpatient follow-up, and the latest thoracoabdominal magnetic resonance imaging studies, performed 6 and 9 months after surgery, showed no remains or recurrence of the cystic lymphangioma (Fig. 2).
Lymphangiomas are rare and benign lymphatic malformations.6 In 90% of cases, they are congenital due to sporadic or inherited/hereditary mutations. Some are even related to endothelial growth factor C. Associated aneuploidy rates have been reported from 45% to 60% (Turner syndrome, Down syndrome, Edwards syndrome, Patau syndrome and others). An association has also been observed with other syndromes, such as Noonan, multiple pterygium, type I achondrogenesis, Fryns syndrome, Roberts syndrome, Cowchock syndrome, fetal alcoholic syndrome and teratogen.1–3,6,7 Acquired presentations are related to a history of surgery, chronic infections or radiation.2
The most frequent locations of lymphangiomas are the head and neck (80%), presenting some extension to the mediastinum (2%–3%) followed by the axillary region.1,2 Only 1% affect the thorax, representing 0.7%–4.5% of mediastinal tumors, with a higher prevalence in the anterior mediastinum.1–3 Lymphangiomas in children, adolescents and young adults are located in the anterior mediastinal or craniocervical regions, have vascular behavior and are often considered congenital. In adults, they are located in the middle or posterior mediastinum and have liquid cysts, with evidence of an acquired origin.3
The exceptional nature of this case lies in the low incidence of lymphangiomas originating in the posterior mediastinum with no craniocervical connection in the general population (which are even less frequent in children) as well as their possible confusion with other diseases with malignant behavior at this age, such as neuroblastomas.
Neuroblastomas are the most frequent extracranial solid tumors during infancy and the first year of life. 123I-MIBG has a sensitivity for the diagnosis of neuroblastoma between 90% and 95%, both for the detection of the primary tumor and for bone, bone marrow and lymph node involvement. In addition, MIBG scintigraphy can help predict the response to treatment as well as disease-free survival in these patients.8 Therefore, in the presence of a mass in the abdomen and/or in the posterior mediastinum in a patient of pediatric age (especially during the first year of life), it is mandatory to rule out the existence of neuroblastoma as an etiological cause, and scintigraphy with 123I-MIBG is the imaging test of choice.
Regarding the treatment of lymphangiomas, surgical excision is the treatment of first choice to avoid superinfection, rapid growth, risk of rupture or urgent laparotomy.5,9,10 Its disadvantage is the existence of complications associated with various factors (patient age, size and location of the lymphatic malformation, possible infiltration of vascular/nerve structures) and sequelae of major surgery.3,4,6 Given the age of the patient and size of the lesion, the decision was made to initially perform treatment with sclerosis versus surgery with the intention of avoiding possible postoperative complications. It is important that the excision of the lesion be complete to avoid recurrences, which appear in up to 50% of cases when the dissection is partial.2,3,6,10 Alternative therapeutic options to surgery range from the use of sclerosing agents, such as OK-432 (lyophilisate obtained from the incubation of Streptococcus pyogenes with benzathine penicillin G), bleomycin, triamcinolone, tetracycline or fibrin sealants and even the use of so-called ‘mixed therapies’ (intralesion injection of these substances prior to surgery to reduce tumor volume or recurrences).1,3,4,6,9,10 It is important to highlight that recently published series opt for the use of sclerosing agents like OK-432 or bleomycin, as described in the review on sclerotherapy in childhood lymphatic malformations by Torres Palomino et al. In this study, the percentage of response to these therapies was 60.8% with OK-432 and 47.4% with bleomycin, and the authors concluded that the most effective sclerosing therapy is OK-4326.9.
Please cite this article as: Espinosa Muñoz E, Ramírez Ocaña D, Martín García AM, Lumbreras Vega LJ, Puentes Zarzuela C. Linfangioma quístico mediastínico en un lactante: estudio isotópico con 123I-MIBG en el diagnóstico diferencial con neuroblastoma. Cir Esp. 2020;98:161–163.