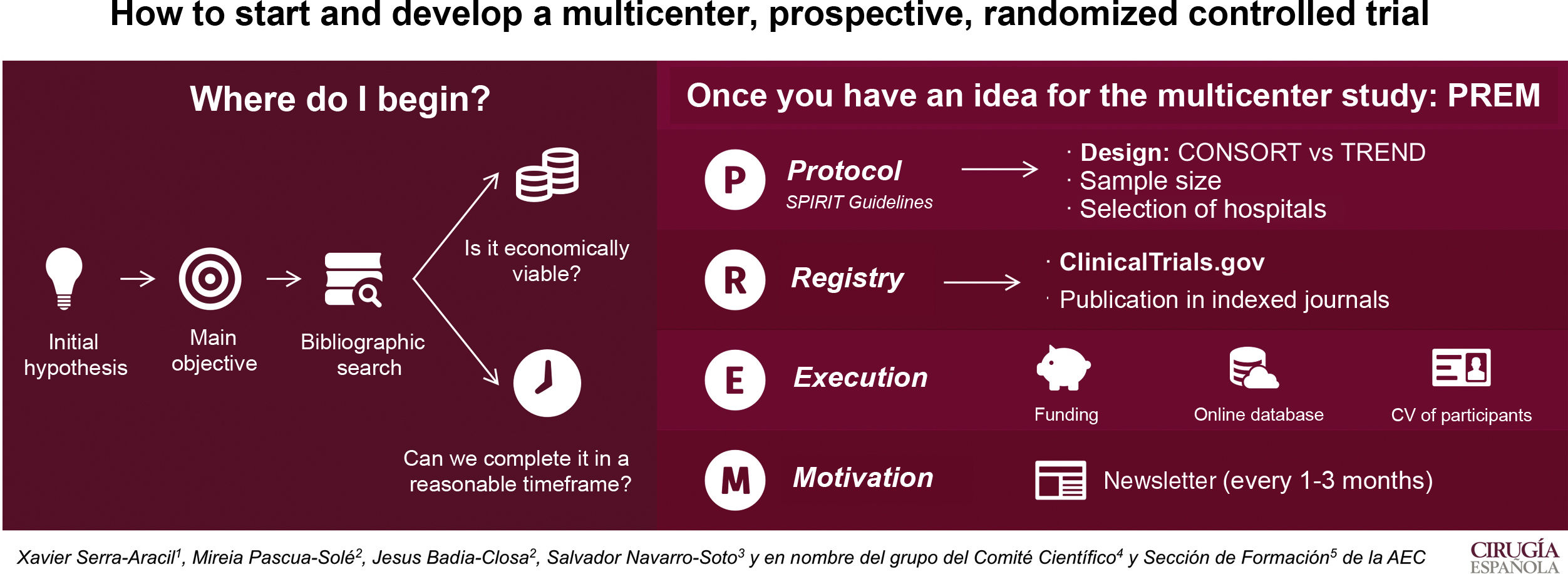
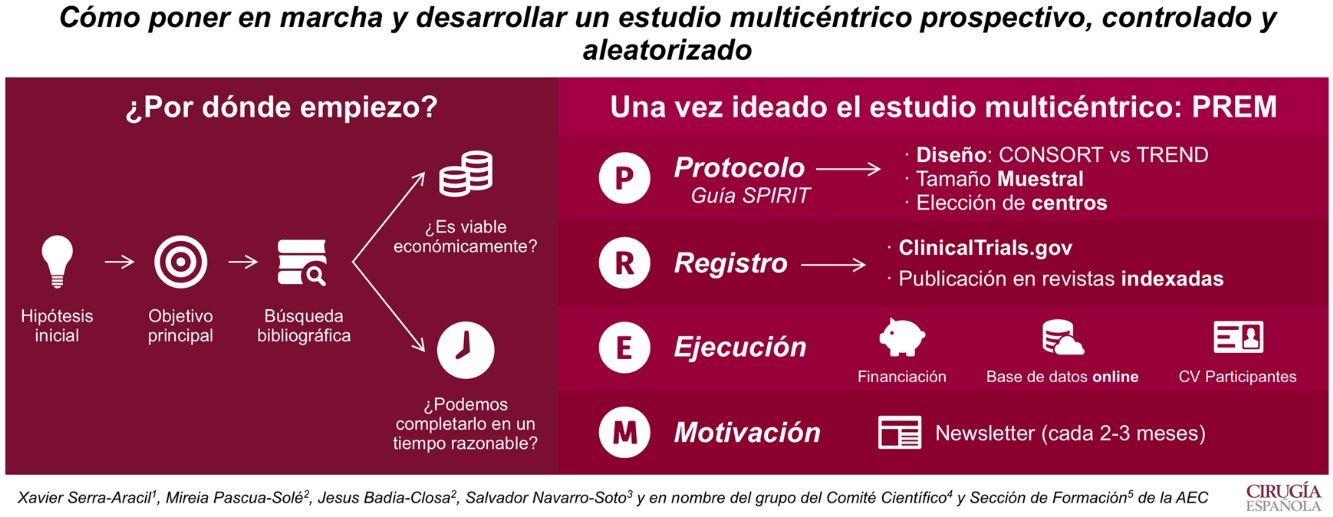
Our main goal is to describe how to start and develop a multicenter, prospective, randomized, controlled trial.
The first step is to have an idea that will become the hypothesis and a main objective. A bibliographic search should be done to check for clinical interest and originality. Moreover, the study must be feasible and should be finished within 4 years.
In order to start the multicenter study, a protocol should be written (in accordance with the SPIRIT guidelines Standard Protocol items: Recommendations for Interventional Trials), including the design type, sample size and participating hospitals. Randomization is key to the design and, therefore, the CONSORT (Consolidated Standards of Reporting Trials) guidelines must be followed. However, if the study cannot be randomized, the TREND (Transparent Reporting of Evaluations with Non-Randomized Designs) guidelines are recommended.
When the protocol is approved by the Ethics Committee for Clinical Investigation of the hospital, we ought to create visibility. It is suggested to register the trial on ClincalTrials.gov and submit its publication to indexed magazines.
Financial resources are necessary to execute the study and maintain an online database. This allows the registry to be updated and accessible to all the participants in the study. What is more, randomization can be done immediately.
And last, but not least, is motivation. Multicentricity equals to participation of all the chosen medical centers. Updating and motivating them by sending a newsletter every 1–3 months keeps participants engaged in the study.
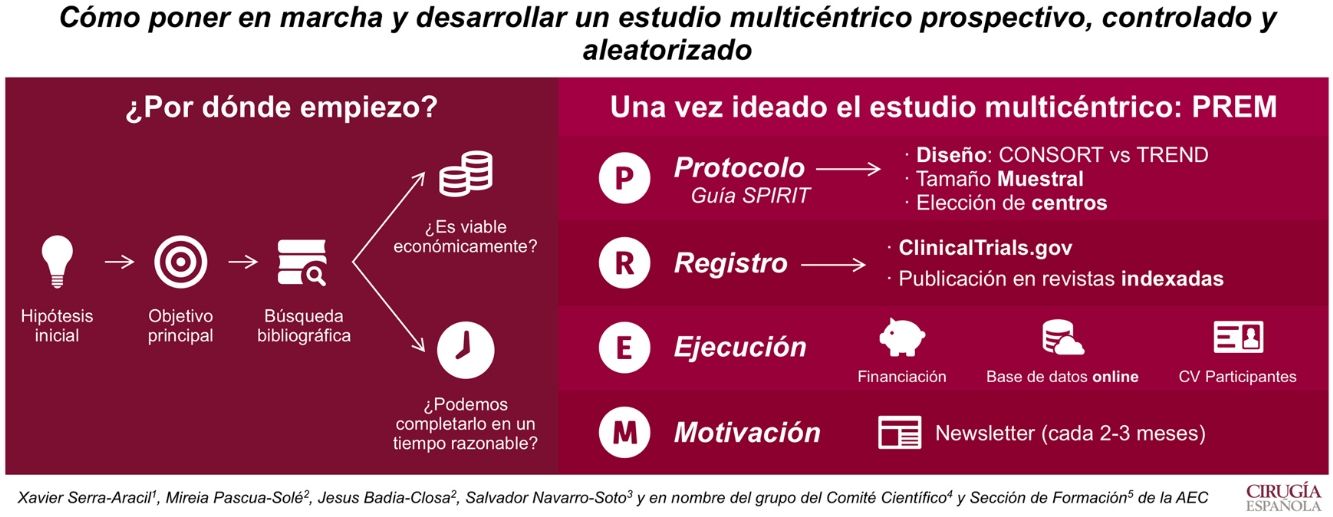
El objetivo de este artículo es ilustrar cómo poner en marcha y desarrollar un estudio multicéntrico prospectivo, controlado y aleatorizado.
Por ello, lo primero que se necesita es crear una idea que genere una hipótesis y un objetivo principal. La búsqueda bibliográfica nos permite ver su relevancia clínica y las evidencias publicadas. Además, hay que plantearse si el estudio es viable económicamente y si puede ser completado en un período menor a 4 años.
Una vez ideado el estudio multicéntrico, para ejecutarlo se debe redactar un protocolo (según la guía Standard Protocol items: Recommendations for Interventional Trials [SPIRIT 2013]). En él se recogerán el tipo de diseño, el tamaño muestral y los centros que participarán. La aleatorización es clave en el diseño. Si puede ser aleatorizado, se recomienda utilizar la guía Consolidated Standards of Reporting Trials (CONSORT), si no, la Transparent Reporting of Evaluations with Non-Randomized Designs (TREND).
Cuando el protocolo es aprobado por el Comité Ético de Investigación Clínica del hospital, hay que darle visibilidad. Es por eso que se recomienda su registro en ClincalTrials.gov y su publicación en revistas indexadas.
Para el inicio del estudio, se requiere buscar fuentes de financiación. Estas permiten tener una base de datos on line, que permiten aleatorizar al momento y mantener el registro al día desde cualquier centro.
Por último, hay que destacar que es imprescindible la motivación. La multicentricidad solo se entiende si todos los centros participan. Así que informar de resultados y dar ánimos cada 1–3 meses (en forma de newsletter) es una manera de conseguir un buen funcionamiento del estudio.
Prospective, controlled and randomized studies (PCA) are the studies with the highest scientific evidence and internal validity. These studies are identified with level of evidence 1A–B according to the Oxford Center for Evidence-Based Medicine.1
Multicenter studies have the added value of associating reproducibility at other hospitals. For this reason, multicenter PCA studies (EMPCA) have a maximum level of validity, both internal and external.
How to initiate a multicenter, prospective, randomized controlled trialEverything should start with a question: what surgical problem am I addressing? This question generates an idea that translates into a hypothesis and the main objective of the study. The next step is an exhaustive search of the medical literature. To do this, the question is translated into different keywords that are entered in the bibliographic bases (like PUBMED, SCOPUS, COCHRANE) to conduct the search using the advanced search form through the use of logical or ‘Boolean’ operators (AND, OR, NOT). Subsequently, the search results are evaluated, and the articles of greatest interest are selected.
After the bibliographic update, we must ask ourselves the following questions: does the medical literature we have consulted sufficiently answer our question? If not, is our idea clinically relevant?
How to express an idea that leads to a multicenter, prospective, randomized controlled trial: description of the study protocolAn EMPCA protocol provides the grounds for planning, executing, publishing and evaluating the study. However, the protocols and guidelines that exist vary greatly in terms of quality and content. To respond to this problem, the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT 2013)2 statement was published, which are guidelines or an instruction manual that establish the minimum content of a clinical trial or PCA study protocol. This statement document also provides a checklist of 33 elements.
A well-written protocol facilitates an appropriate evaluation of scientific, ethical, and safety aspects, as well as the complete assessment of its execution and results once finalized. This homogenization facilitates the development of the study and avoids gaps at the end of the study that are difficult to solve.
Design of a superior multicenter, prospective, randomized controlled study of superiorityPCA studies with an adequate design are the reference method for the evaluation of new treatments (medical or surgical). However, if they lack methodological rigor, they can give biased results.
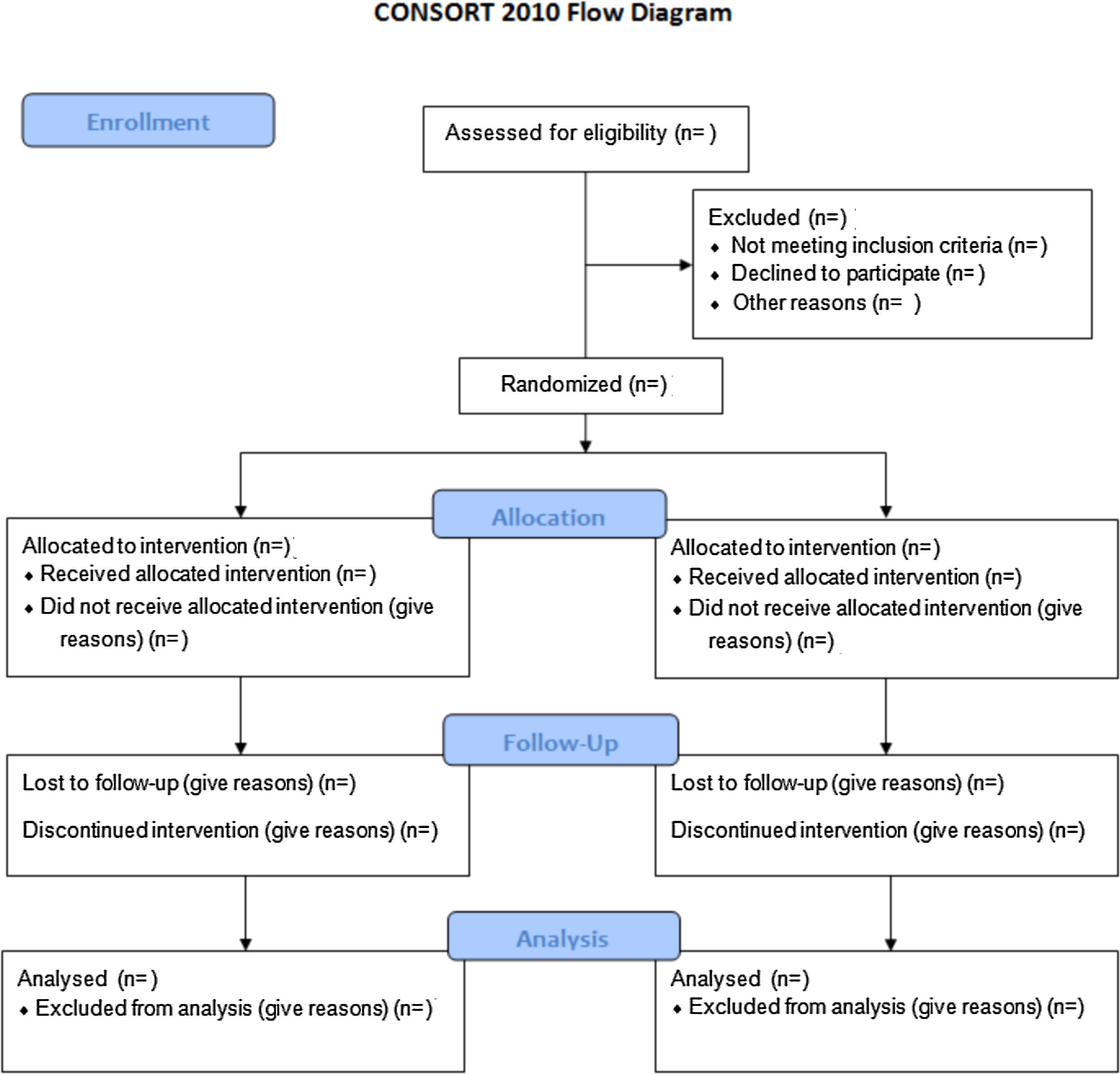
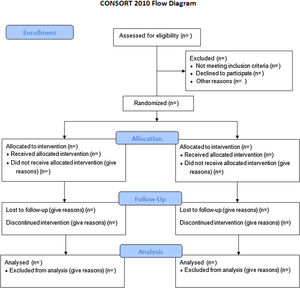
In order to evaluate a PCA study accurately, a complete and clear description is required of its methodology and the findings obtained. In 1996, the lack of rigor resulted in the publication of the Consolidated Standards of Reporting Trials (CONSORT) guidelines. This manifesto caused an increased quality of studies, even though many of them continued to be incorrect.3 As a consequence, the CONSORT Group made an update that they called the CONSORT 2010 Statement.4 This document included a list of 25 elements in checklist format and a flow chart (Fig. 1) to aid in the development of the study.
This manuscript provides guidance for designing the most common PCA studies: parallel, randomized, individual and 2-group trials. Other designs of PCA studies, such as randomized group trials, require other types of variables. The CONSORT extensions for these designs can be found through the CONSORT5 website.
Design of a multicenter, prospective, randomized controlled and non-inferiority multicenter studyGenerally, PCA studies are designed with the aim of trying to demonstrate that a medical or surgical treatment is superior to the standard or conventional one. However, sometimes the purpose is not to show if a treatment is better, but that it has certain advantages (such as menores postoperative complications, better quality of life, etc.), which is not inferior to the standard treatment.
Unlike PCA studies of equivalence, in those of non-inferiority, we are not worried if the new treatment is similar, provided that it “is not much worse”.6 How much worse (how much less effective) will depend on the research team, which will consider to what extent the importance of the effectiveness result is acceptable. This acceptable percentage of worse treatment is what is called the “margin of non-inferiority”, described with the letter delta (δ).
In the same way that there is the manual or guidelines of PCA studies of superiority through CONSORT, Piaggio et al.7 have described their adaptation to studies of non-inferiority and equivalence with an appropriate checklist for these studies.
In prospective randomized controlled studies, is it always possible or ethical to randomize?A PCA study ensures that all included patients come from the same population and, therefore, are comparable. Subsequently, randomization masks possible unknown confounding variables. The results of this type of design are those that provide the greatest scientific evidence and allow them to be applied to future patients. However, this does not imply that any therapeutic decision should be based on a PCA8 design.
An example where the application of the results from a prospective study base on randomization is doubtful is the study by Barendse et al.9 It is an EMPCA comparing transanal endoscopic microsurgery (TEM) vs. endoscopic mucosal resection (EMR) in large adenomas of the rectum. They included medical centers with extensive experience in EMR but associated others with limited experience in TEM. They obtained TEM results worse than the usual results described in the literature, so they concluded that RME was the technique of choice due to a lower complication rate and a better cost-effectiveness ratio than TEM. Despite having a perfect EMPCA design, the study conclusions were questionable.10
Thus, non-randomized studies are accepted when:
- 1.
It is impossible or unethical to perform randomization (e.g., randomize the effect of the parachute when you jump out of a plane).
- 2.
The objective of the study is to analyze the effectiveness of 2 treatments in conditions of real clinical practice, having more experience in one of them.
- 3.
It is interesting to evaluate the cost-effectiveness of a therapeutic intervention.8
For the development of prospective, non-randomized controlled studies, Transparent Reporting of Evaluations with Non-Randomized Designs (TREND)11 studies have been designed. As in other guidelines, “checklist” was published, which allows instructions on how to develop this type of study.
Related to this circumstance, our group wanted to design an EMPCA comparing intracorporeal versus extracorporeal anastomoses in right hemicolectomies. We observed that there were hospitals that had no experience with intracorporeal anastomosis, so they had to go through a learning curve. Furthermore, those who performed intracorporeal anastomosis already had previous experience in extracorporeal anastomosis and believed that the former was better. Given this circumstance, our Clinical Research Ethics Committee (CEIC) did not allow us to randomize because it was unethical to randomize between two techniques when one of them is believed to be better than the other or there is less experience.12
Why conduct multicenter studies?The answer is twofold. Mainly, to gather a greater number of patients in less time. In addition, these studies have greater external validity. They lessen the effect that one hospital may have with better surgeons than another, so the results are more similar to reality.
What is the total number of patients I should include in the multicenter, prospective, randomized controlled study? How is sample size calculated?The calculation of the sample size is important because, if we do not include a sufficient number of patients, we can give inconclusive results, even with an impeccable study design. Including an excessive number of patients, however, implies an expense of resources.
There are rules that should not be infringido, otherwise we commit a bias that may invalidate our study:
- –
The calculation must be done in relation to the main study variable. Sometimes, we observe how the calculation is made on secondary variables, because with the main variable the number we obtain is excessive.
- –
The risk of the ‘level of significance’, ‘alpha risk (α)’ or type I risk, the value of 0.05–0.025 is given by default. It indicates the probability of rejecting a null hypothesis that is actually true. In other words, we have a probability of 0.05–0.025 of a ‘false positive’ and saying that the groups are different when in reality they are not.
- –
The risk called ‘test power’, ‘1–beta (1–β)’ or type II is the probability of rejecting a null hypothesis that is false. In other words, it represents the probability of accepting an alternative hypothesis as true when it is. A power of 90%–80% or a β risk of 0.1–0.2 is considered prudent.
- –
The calculation method will vary depending on whether the main variable is quantitative or categorical (they are the most frequent cases).
- –
The percentage of losses during recruitment. This is usually considered 10% of the total calculation.
- –
Finally, we calculate the sample based on the study design.
This is the most typical study with a categorical main variable. In these cases, as researchers we must have a series of clear data:
- –
The percentage of successes with the standard treatment and which is the percentage of successes that will improve the experimental (or study) treatment.
- –
The α and β risks that we will assume, adding a 10% loss.
There are several online calculators that calculate the sample size. One of the best known in our setting is the GRANMO13 sample size calculator.
As an example, the sample size of the study of intra- and extracorporeal anastomoses in right hemicolectomies12 was calculated by taking the anastomotic dehiscence (AD) as the main variable. DA is estimated at 8% in the extracorporeal group and 2% in the intracorporeal group, with an α risk of 0.05 and 1–β of 0.9, and a loss of 10%. The estimated number of cases to include is 208 patients per group, so the sample size is 416 patients.
Sample calculation in a multicenter, prospective, randomized controlled study of no inferiorityIn this type of studies, the data that we should know are:
- –
The percentage of success with standard treatment.
- –
The percentage of the ‘delta-δ non-inferiority margin’.
- –
The α and β risks that we will assume, adding a loss of 10%.
As an example, in a study by our group on treatment with and without antibiotics in uncomplicated acute diverticulitis,14 the conditions for estimating the percentage of success were 90%–91%, with a margin of non-inferiority delta-δ of 7%, an α risk of 0.025 and 1–β risk of 0.8, and a loss of 10%. The estimated number of cases to include is 230 patients per group; the sample size is 460 patients.
Next, we must ask ourselves: will the multicenter, prospective, randomized controlled study be viable?Now that we know the difficulties of the design and the number of patients to be included in the EMPCA, we must ask ourselves if it is viable from the point of view of infrastructure, economic and duration. If the study is prolonged excessively, it may become obsolete. In the same way, it can cause wear and tear of the entire research team that prevents it from being finished.
Choice of hospitals, authorship policy in multicenter, prospective, randomized controlled studiesMany EMPCAs are publicized and open to all centers that wish to participate, but our experience has not been favorable in this situation. There is usually an avalanche of requests from hospitals to participate that subsequently do not receive approval for the study from their CEIC or do not receive an answer or do not include patients. We believe that the selection must include those we know and have a commitment to the study, both in terms of people and means.
Another important point is to establish the authorship policy for the artices that arise from the study and in the presentation of communications to medical conferences. Therefore, the order and number of the main authors should be specified (most journals limit the number of authors) from the beginning. The name of the study must be generated with the group of all participating professionals by hospitals, being named in the acknowledgments section. Writing ‘on behalf of … group’ at the end of the authorships allows all participants to have their recognition reflected in PubMed.
Clinical Research Ethics Committees at the promoting hospital and collaborating hospitals; international registry of clinical trialsThe EMPCA must be approved by the Ethics and Clinical Research Committee (CEIC) of the promoter center.
In this type of studies, instead of sending the request for approval to the CEIC, it is recommended to hold meetings with them and have the ability to self-criticize in order to improve the protocol. Thanks to these meetings in the study of right hemicolectomies with intra- or extracorporeal anastomosis, they indicated the impossibility to randomize, but gave the solution of the TREND12 design. The more elaborate the protocol is of the study of the promoting hospital, the easier approval will be at the collaborating hospitals.
In case of using medication in the EMPCA, registration must be requested in the Spanish Medicines Agency (Agencia Española del Medicamento).
All indexed and high-impact journals request, prior to the start of the study, its registration in international clinical trial websites. One of the best known is the clinicaltrials.gov (https://register.clinicaltrials.gov).15 After registration, the study is given an identification number, and all interested parties will be able to follow its development.
Study insuranceThis is not necessary if the EMPCA is considered to have a low intervention level and makes a comparison of standard treatments. Complementary diagnostic or follow-up procedures must involve a very limited risk or additional burden for the safety of the subjects, which is minimal compared to that of common clinical practice.16 Otherwise, the CEIC itself will request that insurance be contracted for the approval of the study.
Visibility of multicenter, prospective, randomized controlled studiesIt is very important to give visibility to these types of studies. Not only so that the international community knows about the study that is being carried out, but to be able to contact different groups that are interested in the subject.
Visibility is not enough with registration on international clinical trials websites. We should try to publish in indexed PubMed journals. Many of them are in Open Access format (requiring payment of publication costs), but there are others with mixed format that do not cause additional costs.
An example of visibility is the case of a classic study by our group, known as TAUTEM. It was registered at ClinicalTrial.gov in 2009, but until its publication in 2018, we had not received information from other groups interested in our study.17
Creation of cuaderno de datos on lineFor proper data management and automatic randomization, the creation of an online database is essential. This can be created through specialized companies or with a contract research organization, which is a company that provides all clinical trial management services.
This online notebook can be accessed by all collaborating hospitals from any computer connected to the Internet, providing randomization and data entry at any time of the day. The promoters can access the status of the study and updated results at anytime.
Search for financingAll this work is not free. In addition to requiring leadership from the principal investigator, there is a lot of work that joins our daily lives. Therefore, it is advisable to hire a data manager, who will be responsible not only for the introduction of data, but also for the coordination of all patient tests and the problems that arise with the rest of the centers.
To these personnel are added the expenses generated by the management of some CEICs that request it, the English translation and the publication of the protocol, the contract with the CRO, the creation, management and maintenance of the online database, study insurance (if required), meetings with collaborating centers, statistical analysis of the final results, correct translation into English of the various manuscripts and communications derived from EMPCA, attendance at national and international conferences for presentation of the study, the costs of publishing in Open Access (if we consider it appropriate), etc.
Search for fundingAll of this work does not come free. In addition to requiring leadership from the principal researcher, there is a lot of work on top of our daily practice. Therefore, it is advisable to hire a data manager, who will be responsible not only for data processing, but also for the coordination of all patient tests and the problems that arise with the other hospitals.
Further expenses include: CEIC management costs (required by some); the English translation and publication of the protocol; the contract with the CRO; the creation, management and maintenance of the online database; study insurance (if required); meetings with collaborating hospitals; the statistical analysis of the final results; correct translation into English of the various manuscripts and communications derived from EMPCA; attendance at national and international conferences to present the study; the costs of publishing in Open Access (if appropriate); etc.
Therefore, funding must be sought. We can look for it in scientific societies (Spanish Association of Surgeons, Spanish Association of Coloproctology Foundation, Catalan Society of Surgery, etc.), the regional healthcare administrations of the autonomous communities (provinces), private foundations, grants from the Instituto de Salud Carlos III (FISSS ), grants from European research funding programs, etc.
In addition to the protocol, it is important to have the resume/CV of all the main researchers at each hospital (in standardized Spanish CVN format, if possible).18 This will avoid missing out on opportunities to apply for public grants requiring CVN.
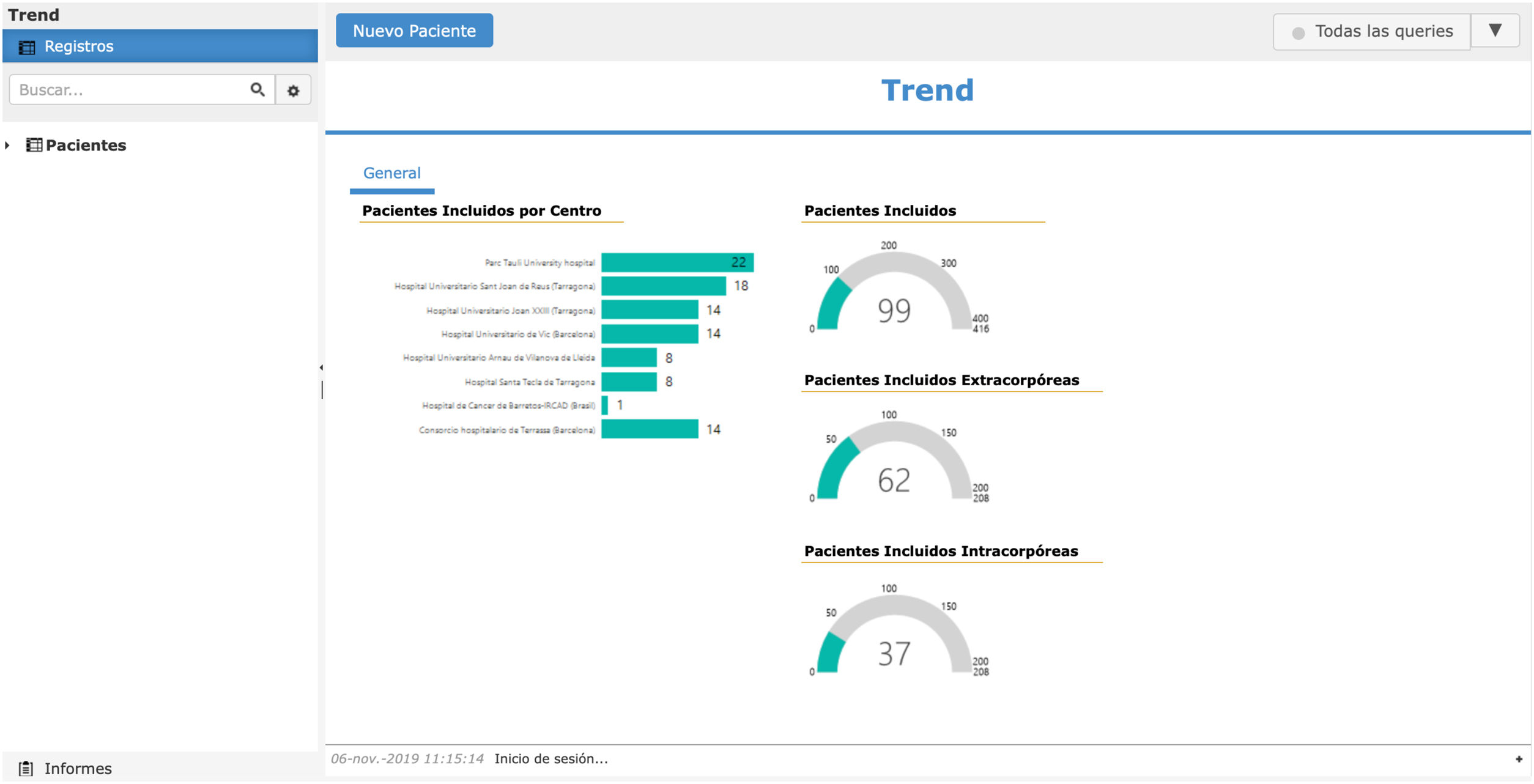
Last phase: starting and maintaining group motivationThe final challenge is finishing the study. Therefore, it is the obligation of the main researcher and the promoter group to maintain the motivation of the other collaborating hospitals. It is very important to have a motivating online database that provides updated information every time a patient is added (Fig. 2). It is essential to create a newsletter every 1–3 months, with charts and figures, objectives, compliance ideas and partial results, without compromising results bias.
Final comments (Fig. 3)The objective of this manuscript was to provide step-by-step information of all the phases of the development of an EMPCA, not as an obstacle course, but to show how it is possible to do, stage by stage, provided that there is a good team working together (Table 1).
Checklist of the stages for conducting a multicenter, prospective, randomized controlled trial.
| 1 Idea: hypothesis; main objective |
| 2 Bibliographic search |
| 3 It is clinically relevant. It is viable: economically and with available infrastructure. |
| 4 It is clinically relevant. It is viable: economically and with available infrastructure. |
| 5 Design type: |
| 5a Randomized: CONSORT: superiority, non-inferiority |
| 5b Non-randomized: TREND |
| 6 Sample size: superiority, non-inferiority |
| 7 Viable: recruitment no more than 4 yrs |
| 8 Selection of hospitals for the multicenter study |
| 9 9a Approval by CREC at promoting hospital |
| 9b Approval by CREC at collaborating hospitals |
| 9c Registration in ClinicalTrial.gov |
| 9d If medications are used, registration with the Spanish Medicines Agency |
| 9e Study insurance |
| 10 Visibility: publication of the protocol in an indexed journal (PubMed) |
| 11 Creation of an online data notebook. CRO contract |
| 12 Search for funding |
| 13 Request CVN from all co-authors |
| 14 Motivation: newsletter (every 1-3 months) |
CREC: clinical research ethics committee; CRO: contract research organization; CVN: curriculum vitae normalizado, a standardized Spanish format for resumes/CV.
Every research group aims to contribute knowledge to our daily clinical practice that is reflected in high-impact journals. One of the most important ways is through these EMPCA.
FundingNo funding was received for the creation of this article.
Conflict of interestNone.
Thanks to the Board of Directors of the AEC for the opportunity to develop this article. Also, thanks to all the members of the General and Gastrotintestinal Surgery Department of the Hospital Universitario Parc Taulí, the Fundación de Investigación Parc Taulí and all the collaborating hospitals of the multicentric studies for their immesurable collaboration.
Salvador Navarro Soto, Raquel Sánchez Santos, Luís Sabater Ortí, Manuel Pera Román, Victor Soria Aledo, Eduardo M. Targarona Soler and Xavier Serra Aracil.
José Luis Ramos Rodríguez, María Socas Macías, Sergio Moreno, Ignacio Rey Simó, Sandra García Botella, Helena Vallverdú, Inés Rubio, Laura Armananzas, Ivan Arteaga, J.M. Miguelena, Vicenç Artigas Raventos, Enrique Mercader, Dieter Morales García, Monica Millan, María Dolores Frutos, Gonzalo de Castro, Manuel López Cano, Baltasar Pérez Saborido, Itziar Larrañaga and Xavier Serra Aracil.
Please cite this article as: Serra-Aracil X, Pascua-Sol M, Badia-Closa J, Navarro-Soto S. Cómo poner en marcha y desarrollar un estudio multicéntrico prospectivo, controlado y aleatorizado. Cir Esp. 2020;98:119–126.