Postoperative mortality associated with pancreaticoduodenectomy (PD) in high-volume hospitals is below 5%, yet morbidity rates range between 45% and 60%. Recent studies show a lower incidence of complications and postoperative pancreatic fistula (POPF) in pancreaticogastrostomy (PG). The primary objective was to assess the incidence and predictive factors for complications: POPF, post-pancreatectomy hemorrhage (PPH) and delayed gastric emptying (DGE) following the criteria of the ISGPS and Clavien-Dindo classifications.
MethodsA prospective observational study that included all patients who underwent PD between 2008 and 2016. PG was the surgical procedure of choice for PD reconstruction.
ResultsTwo hundred forty-nine (249) patients underwent surgery with intention of performing a PD. The feasibility of PG was 90.5%. One hundred and six (53%) patients had complications, 36 (18%) were severe (Clavien-Dindo grade ≥ III). Death within 90 postoperative days was 4%. DGE was the most frequent complication (22.5%), followed by PPH (21%). The clinical POPF rate was 15% (6% Clavien-Dindo grade ≥ III). The primary risk factors associated with complications were age >70 years (1.9 [1–3.55]), being male (1.89 (1; 3.6]) and soft pancreatic texture (3.38 [1.5; 7.37]).
ConclusionsIn this paper, we report a feasibility study for PG (90.5%). The primary risk factors associated with complications are age >70 years, being male and soft pancreatic texture. Soft pancreatic texture is also associated with the development and severity of POPF.
La mortalidad postoperatoria asociada a la duodenopancreatectomía (DP) en centros de alto volumen es inferior al 5%, sin embargo, las tasas de morbilidad oscilan entre el 45% y el 60%. Estudios recientes muestran una menor incidencia de complicaciones y fístula pancreática postoperatoria (POPF) con el uso de la pancreaticogastrostomía (PG). El objetivo de nuestro estudio es evaluar la incidencia y los factores predictivos de las complicaciones: POPF, hemorragia post-pancreatectomía (HPP) y retraso del vaciamiento gástrico (RVG) según los criterios de las clasificaciones ISGPS y Clavien-Dindo.
Material y métodosEstudio prospectivo observacional en el que se incluyeron todos los pacientes sometidos a DP entre 2008 y 2016. La PG fue la técnica de elección en la reconstrucción de la DP.
ResultadosDoscientos cuarenta y nueve (249) pacientes se sometieron a cirugía con la intención de realizar una DP. La viabilidad de PG fue del 90,5%. Ciento seis (53%) pacientes tuvieron complicaciones, 36 (18%) fueron graves (grado Clavien-Dindo ≥ III). La mortalidad a 90 días fue del 4%. El RVG fue la complicación más frecuente (22,5%), seguida de la HPP (21%). La tasa clínica de POPF fue del 15% (6% grado Clavien-Dindo ≥ III). Los principales factores de riesgo asociados a las complicaciones son la edad> 70 años (1,9 (1–3,55)), el sexo masculino (1.89 (1; 3.6)) y la textura blanda del páncreas (3.38 (1.5; 7.37).
ConclusionesEn nuestra experiencia la factibilidad de la PG es del 90.5%. Los principales factores de riesgo asociados a las complicaciones son la edad> 70 años, el sexo masculino y la textura blanda del páncreas. La textura blanda del páncreas también está asociada al desarrollo y la gravedad de la POPF.
Pancreaticoduodenectomy (PD) is a common procedure in tertiary hospitals. Over the past two decades, several studies have focused on technical changes in pancreatic reconstruction aimed at reducing the incidence of postoperative pancreatic fistula (POPF),1–3 the main factor associated with postoperative morbidity and mortality.
The two most commonly used techniques for pancreatic reconstruction are pancreaticojejunostomy (PJ) and pancreaticogastrostomy (PG). Currently, the advantages or disadvantages of these two techniques are being debated to determine which should be the technique of choice for PD. This is due in part to the use of different variants of the same two techniques as well as differences in defining and registering complications by different groups.
The use of the classifications proposed by the International Study Group of Pancreatic Surgery (ISGPS) regarding anastomoses, postoperative complications, delayed gastric emptying (DGE), post-pancreatectomy hemorrhage (PPH) and POPF, provides a more objective evaluation of the results.4–7
The aim of our study is to analyze the incidence and risk factors for these complications (POPF, PPH and DGE) in a group of 200 consecutive patients with PG, using the criteria of the ISGPS and Clavien-Dindo8 international classifications. As a secondary objective, we will analyze the technical feasibility of PG in a prospective series of patients treated surgically at a single hospital with a standardized technique and postoperative care.
MethodsStudy DesignIn April 2008, the first PG was selected and performed as a standard reconstruction technique for all patients undergoing PD. We conducted a prospective observational study including all patients who had undergone PD between April 2008 and April 2016 at our hospital. The technique of choice in the study involved an initial approach of the superior mesenteric artery9–11 to facilitate resectability assessment and the removal of the mesopancreas in patients with malignant tumors. Pancreatic reconstruction was performed using PG (type I-B S0)7 according to the technique described by Delcore.12 Reconstruction with PG was performed by the same surgical team of 3 surgeons. The surgical indication was determined by a multidisciplinary committee.
Patients signed the informed consent form before surgery in accordance with the hospital protocol, as approved by the Ethics Committee.
Postoperative CareA standardized clinical protocol for postoperative care was implemented.13 Following the ISGPS criteria,4 we analyzed the concentration of amylase in the abdominal wound drain fluid on the third day of the postoperative period (PDO3); drain tubes were removed when amylase levels reached ≤400 U/L. For higher values, the procedure was repeated every 48 h.
Data Collection and DefinitionsWe designed a database with FileMaker® and entered the data prospectively. The database included demographic data, American Society of Anesthesiologist (ASA) classification grade,14 body mass index (BMI), diagnosis, surgical procedures, date, surgeon and surgical complications according to Clavien-Dindo and ISGPS classifications for POPF, DGE and PPH within 30 days after surgery. Mortality included all events within the first 90 postoperative days.
The diameter of the pancreatic duct was measured using a 10-French (3.3 mm) silicone tube during the procedure. The duct was considered to be >3 mm when the tube was easily inserted and ≤3 mm when dilation was required or it was not possible to introduce the measuring device. The texture of the pancreas was classified as hard or soft, depending on the resistance of the tissue to the suture.
Statistical AnalysisWe used the epiR library and R package (version 2.13.1) tools to calculate the sample size in order to determine the risk factors for POPF, based on our previous results13 of percentage of pancreatic fistula and soft pancreas. For our study, a series of 200 patients was able to obtain a statistically significant relative risk difference (RR) of 1.5, with a power of 80% in two-tailed tests and a 95% confidence interval (CI).
Descriptive statistical tests were used to present sociodemographic variables and complications after PD. Binary logistic models were applied to analyze the correlation between variables and the appearance of POPF and DGE as complications; odds ratios (OR), measurement of risk and corresponding 95% CI are provided. In addition, we conducted a multivariate logistic regression analysis, including statistically significant variables according to the bivariate analysis. A P value <.05 was considered statistically significant.
ResultsPatient Selection and Feasibility of PGTwo hundred and forty-nine (249) patients underwent surgery at our hospital between April 2008 and April 2016 with the intention of performing a PD; 155 of these patients were included in a previous study.13 Out of the total, 181 patients had malignant tumors, which could not be resected in 26 cases due to local infiltration or metastasis; therefore, the resectability rate was 85.6%.
The surgical procedures conducted in the 223 resected patients included 221 PD and total pancreatectomy performed in 2 patients due to tumor involvement of the margin found on preoperative biopsy. PG was feasible in 200 (90.5%) of the 221 PD, and the remaining 21 (9.5%) underwent reconstruction with PJ. PG was not possible in these 21 patients due to extensive resection of the pancreas (42.8%), previous surgery (33.4%) or chronic pancreatitis (23.8%).
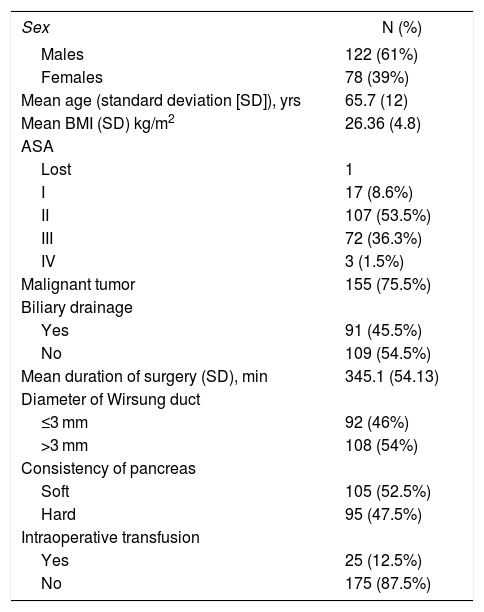
Demographic and Surgical DataTable 1 presents the main demographic variables of our series and summarizes the variables related with surgery.
Demographic Data.
| Sex | N (%) |
|---|---|
| Males | 122 (61%) |
| Females | 78 (39%) |
| Mean age (standard deviation [SD]), yrs | 65.7 (12) |
| Mean BMI (SD) kg/m2 | 26.36 (4.8) |
| ASA | |
| Lost | 1 |
| I | 17 (8.6%) |
| II | 107 (53.5%) |
| III | 72 (36.3%) |
| IV | 3 (1.5%) |
| Malignant tumor | 155 (75.5%) |
| Biliary drainage | |
| Yes | 91 (45.5%) |
| No | 109 (54.5%) |
| Mean duration of surgery (SD), min | 345.1 (54.13) |
| Diameter of Wirsung duct | |
| ≤3 mm | 92 (46%) |
| >3 mm | 108 (54%) |
| Consistency of pancreas | |
| Soft | 105 (52.5%) |
| Hard | 95 (47.5%) |
| Intraoperative transfusion | |
| Yes | 25 (12.5%) |
| No | 175 (87.5%) |
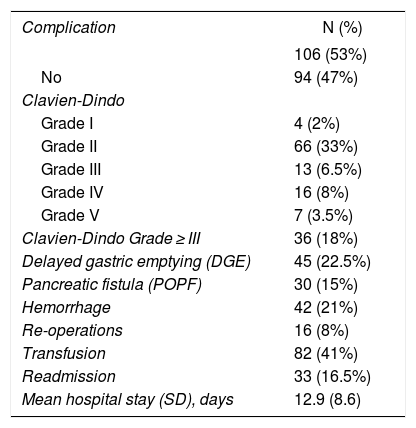
Table 2 shows the complications. There were 106 (53%) complications within 30 postoperative days, including hospital readmissions. The average number of complications per patient was 1.5.
Morbidity and Mortality of the Pancreaticogastrostomy Series.
| Complication | N (%) |
|---|---|
| 106 (53%) | |
| No | 94 (47%) |
| Clavien-Dindo | |
| Grade I | 4 (2%) |
| Grade II | 66 (33%) |
| Grade III | 13 (6.5%) |
| Grade IV | 16 (8%) |
| Grade V | 7 (3.5%) |
| Clavien-Dindo Grade ≥ III | 36 (18%) |
| Delayed gastric emptying (DGE) | 45 (22.5%) |
| Pancreatic fistula (POPF) | 30 (15%) |
| Hemorrhage | 42 (21%) |
| Re-operations | 16 (8%) |
| Transfusion | 82 (41%) |
| Readmission | 33 (16.5%) |
| Mean hospital stay (SD), days | 12.9 (8.6) |
| Clavien-Dindo | I | II | III | IV | V | TOTAL |
|---|---|---|---|---|---|---|
| DGE | 35 (17.5%) | 5 (2.5%) | 4 (2%) | 1 (0.5%) | 45 (22.5%) | |
| Hemorrhage | 15 (7.5%) | 11 (5.5%) | 11 (5.5%) | 5 (2.5%) | 42 (21%) | |
| POPF | 1 (0.5%) | 17 (8.5%) | 3 (1.5%) | 6 (3%) | 3 (1.5%) | 30 (15%) |
| ISGPS | A | B | C | |
|---|---|---|---|---|
| DGE | 17 (8.5%) | 19 (9.5%) | 9 (4.5%) | 45 (22.5%) |
| Hemorrhage | 7 (3.5%) | 17 (8.5%) | 18 (9%) | 42 (21%) |
| POPF | 5 (2.5%) | 16 (8%) | 9 (4.5%) | 30 (15%) |
Complications were mild (Clavien-Dindo grades I–II) in 70 patients (35%) and severe (Clavien-Dindo grade ≥ III) in 36 (18%). Seven (3.5%) patients died within 30 days and eight (4%) within 90 days.
Re-operationsSixteen (8%) patients were reoperated, mainly due to bleeding (56%): 7 pancreatic stump hemorrhage, one hemoperitoneum and one hematoma associated with a POPF. Sepsis was the second cause (3%): one due to POPF, one biliary fistula (BF), one POPF with associated BF, one dehiscence of the gastrojejunal suture and two cases with peritonitis with no observed origin during re-operation.
Hospital ReadmissionsThirty-three (16.5%) patients were re-admitted within the first 30 postoperative days, mainly due to infection (6.5%), hemorrhage (4%) and DGE (2.5%). There were three unjustified readmissions (1.5%). Readmissions increased the percentage of complications at discharge (43.5%) by 9.5% (53% total).
Hospital StayThe average length of hospital stay was 12.9 days (8.6). The average in patients with complications was 17.3 days versus 8 days in patients without complications.
Delayed Gastric EmptyingDGE was the most frequent complication; 45 patients (22.5%) met the ISGPS criteria. In 35 (77.8%) patients, DGE was classified as Clavien-Dindo grade II; in nine patients, it was classified as Clavien-Dindo grades III and IV. One patient died due to pulmonary aspiration secondary to DGE. The average hospital stay of patients with DGE was 19.67 days.
Out of the 45 patients who developed DGE, 21 (46.6%) had no other associated intra-abdominal complications and were classified as primary DGE. The remaining 24 patients (53.3%) were classified as secondary DGE.
Hemorrhagic complicationsForty-two (42; 21%) patients had hemorrhagic complications (Table 2), 27 (13.5%) were classified as severe (Clavien ≥ III).
Intraluminal hemorrhage (ISGPS) was observed in 30 (15%) cases, extraluminal in 10 (5%) and intra/extraluminal in two (1%). Regarding the time of onset of bleeding, in 32 (16%) cases it was late (>24 h) and in 10 (5%) early (<24 h).
Pancreatic Fistula (POPF)Thirty (15%) patients developed clinical POPF: eighteen (9%) of the POPF were classified as Clavien-Dindo grades I–II (Table 2) and the other 12 (6%) were classified as severe (Clavien-Dindo grade ≥ III).
We observed a higher incidence of POPF in patients with soft pancreas compared to those with hard pancreas: 25% versus 4.2% (P < .001).
Other OomplicationsForty (20%) patients had other complications that were generally mild: seven (3.5%) developed primary bacteremia and were treated with antibiotics; six (3%) patients developed BF; six (3%) developed surgical site infection, and three (1.5%) intra-abdominal abscess.
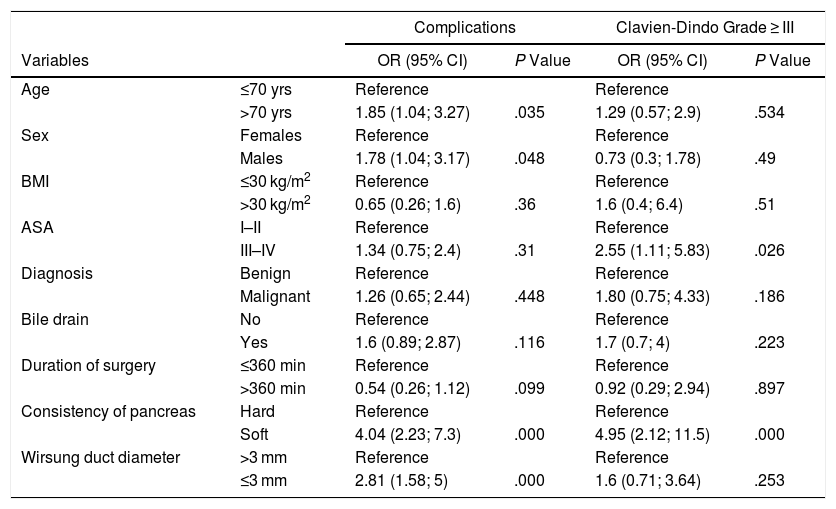
Risk Factors for Morbidity and Mortality in PGGeneral Complications and SeverityThe risk factors associated with the development of complications (Tables 3a, 3b and 4a, 4b) were analyzed in the 200 PG. First, we evaluated their correlation with the incidence of complications and subsequently with their severity, considering Clavien-Dindo complications ≥ III severe.
Univariate Logistic Regression Analysis of Total Complications and Clavien-Dindo ≥ III.
| Complications | Clavien-Dindo Grade ≥ III | ||||
|---|---|---|---|---|---|
| Variables | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | ≤70 yrs | Reference | Reference | ||
| >70 yrs | 1.85 (1.04; 3.27) | .035 | 1.29 (0.57; 2.9) | .534 | |
| Sex | Females | Reference | Reference | ||
| Males | 1.78 (1.04; 3.17) | .048 | 0.73 (0.3; 1.78) | .49 | |
| BMI | ≤30 kg/m2 | Reference | Reference | ||
| >30 kg/m2 | 0.65 (0.26; 1.6) | .36 | 1.6 (0.4; 6.4) | .51 | |
| ASA | I–II | Reference | Reference | ||
| III–IV | 1.34 (0.75; 2.4) | .31 | 2.55 (1.11; 5.83) | .026 | |
| Diagnosis | Benign | Reference | Reference | ||
| Malignant | 1.26 (0.65; 2.44) | .448 | 1.80 (0.75; 4.33) | .186 | |
| Bile drain | No | Reference | Reference | ||
| Yes | 1.6 (0.89; 2.87) | .116 | 1.7 (0.7; 4) | .223 | |
| Duration of surgery | ≤360 min | Reference | Reference | ||
| >360 min | 0.54 (0.26; 1.12) | .099 | 0.92 (0.29; 2.94) | .897 | |
| Consistency of pancreas | Hard | Reference | Reference | ||
| Soft | 4.04 (2.23; 7.3) | .000 | 4.95 (2.12; 11.5) | .000 | |
| Wirsung duct diameter | >3 mm | Reference | Reference | ||
| ≤3 mm | 2.81 (1.58; 5) | .000 | 1.6 (0.71; 3.64) | .253 | |
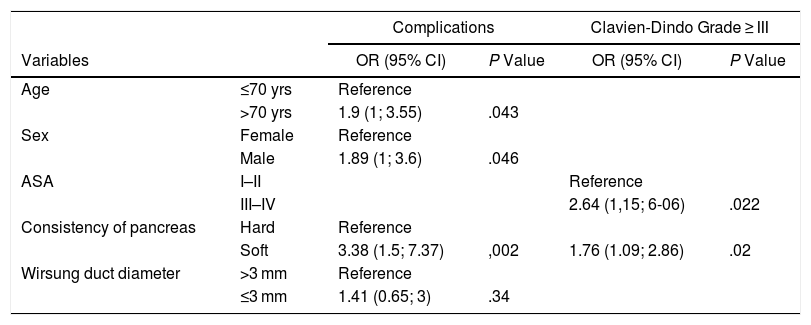
Multivariate Logistic Regression for Total Complications and Clavien-Dindo ≥ III.
| Complications | Clavien-Dindo Grade ≥ III | ||||
|---|---|---|---|---|---|
| Variables | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | ≤70 yrs | Reference | |||
| >70 yrs | 1.9 (1; 3.55) | .043 | |||
| Sex | Female | Reference | |||
| Male | 1.89 (1; 3.6) | .046 | |||
| ASA | I–II | Reference | |||
| III–IV | 2.64 (1,15; 6-06) | .022 | |||
| Consistency of pancreas | Hard | Reference | |||
| Soft | 3.38 (1.5; 7.37) | ,002 | 1.76 (1.09; 2.86) | .02 | |
| Wirsung duct diameter | >3 mm | Reference | |||
| ≤3 mm | 1.41 (0.65; 3) | .34 | |||
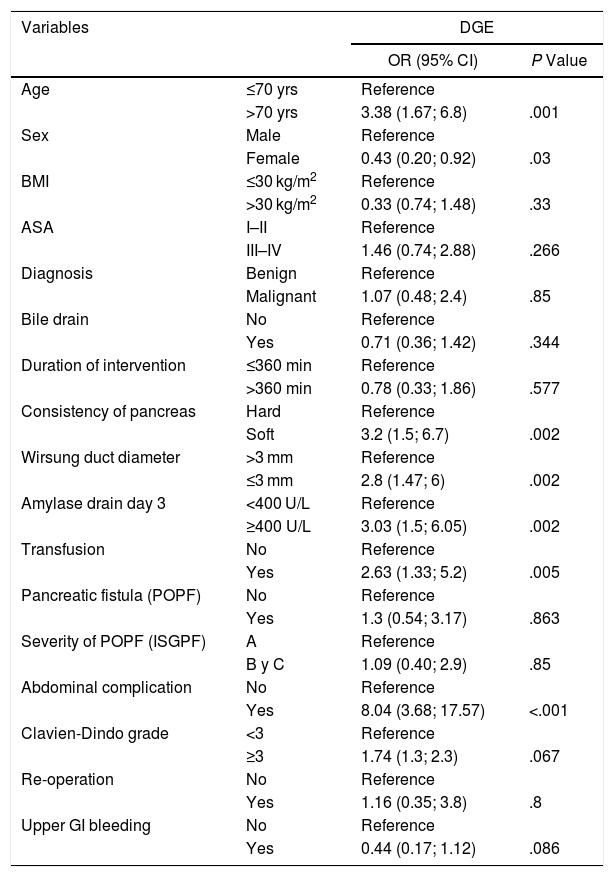
Univariate Logistic Regression for Delayed Gastric Emptying (DGE).
| Variables | DGE | ||
|---|---|---|---|
| OR (95% CI) | P Value | ||
| Age | ≤70 yrs | Reference | |
| >70 yrs | 3.38 (1.67; 6.8) | .001 | |
| Sex | Male | Reference | |
| Female | 0.43 (0.20; 0.92) | .03 | |
| BMI | ≤30 kg/m2 | Reference | |
| >30 kg/m2 | 0.33 (0.74; 1.48) | .33 | |
| ASA | I–II | Reference | |
| III–IV | 1.46 (0.74; 2.88) | .266 | |
| Diagnosis | Benign | Reference | |
| Malignant | 1.07 (0.48; 2.4) | .85 | |
| Bile drain | No | Reference | |
| Yes | 0.71 (0.36; 1.42) | .344 | |
| Duration of intervention | ≤360 min | Reference | |
| >360 min | 0.78 (0.33; 1.86) | .577 | |
| Consistency of pancreas | Hard | Reference | |
| Soft | 3.2 (1.5; 6.7) | .002 | |
| Wirsung duct diameter | >3 mm | Reference | |
| ≤3 mm | 2.8 (1.47; 6) | .002 | |
| Amylase drain day 3 | <400 U/L | Reference | |
| ≥400 U/L | 3.03 (1.5; 6.05) | .002 | |
| Transfusion | No | Reference | |
| Yes | 2.63 (1.33; 5.2) | .005 | |
| Pancreatic fistula (POPF) | No | Reference | |
| Yes | 1.3 (0.54; 3.17) | .863 | |
| Severity of POPF (ISGPF) | A | Reference | |
| B y C | 1.09 (0.40; 2.9) | .85 | |
| Abdominal complication | No | Reference | |
| Yes | 8.04 (3.68; 17.57) | <.001 | |
| Clavien-Dindo grade | <3 | Reference | |
| ≥3 | 1.74 (1.3; 2.3) | .067 | |
| Re-operation | No | Reference | |
| Yes | 1.16 (0.35; 3.8) | .8 | |
| Upper GI bleeding | No | Reference | |
| Yes | 0.44 (0.17; 1.12) | .086 | |
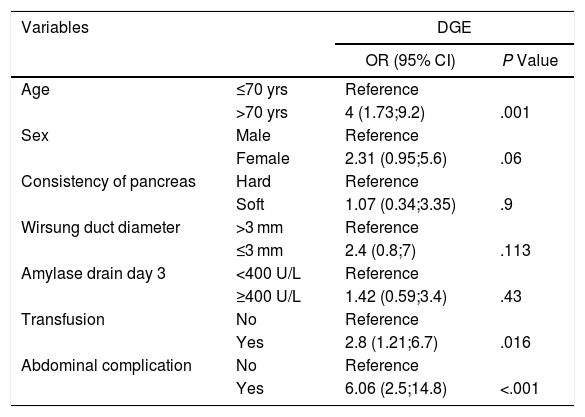
Multivariate Logistic Regression Analysis for Delayed Gastric Emptying.
| Variables | DGE | ||
|---|---|---|---|
| OR (95% CI) | P Value | ||
| Age | ≤70 yrs | Reference | |
| >70 yrs | 4 (1.73;9.2) | .001 | |
| Sex | Male | Reference | |
| Female | 2.31 (0.95;5.6) | .06 | |
| Consistency of pancreas | Hard | Reference | |
| Soft | 1.07 (0.34;3.35) | .9 | |
| Wirsung duct diameter | >3 mm | Reference | |
| ≤3 mm | 2.4 (0.8;7) | .113 | |
| Amylase drain day 3 | <400 U/L | Reference | |
| ≥400 U/L | 1.42 (0.59;3.4) | .43 | |
| Transfusion | No | Reference | |
| Yes | 2.8 (1.21;6.7) | .016 | |
| Abdominal complication | No | Reference | |
| Yes | 6.06 (2.5;14.8) | <.001 | |
As for preoperative clinical and demographic characteristics, age over 70 (OR = 1.85 [95% CI: 1.04–3.27]) and male gender (OR = 1.78 [95% CI: 1.04–3.17]) were identified as risk factors for complications.
The surgical risk factors associated with complications were the soft consistency of the pancreas (OR = 4.04 [95% CI: 2.23–7.3]) and the diameter of the pancreatic duct ≤3 mm (OR = 2.81 [95% CI: 1.5–8.5]). ASA ≥ III (OR = 2.55 [95% CI; 1.11–5.83]) and soft consistency (OR = 4.95 [95% CI; 2.12–11.5]) were related with the severity of complications.
Age >70 years, male gender and soft consistency of the pancreas (P = .002) continued to be predictive variables in the multivariate model. Patients with ASA ≥ III and soft consistency of the pancreas were also associated with the severity of complications (P = .02).
Delayed Gastric EmptyingThe univariate analysis (Tables 4a, 4b) revealed that age >70 years (P = .001) and male gender (P = .03) were risk factors for DGE. Other correlating factors included: soft consistency of the pancreas (OR = 3.2 [95% CI 1.5–6.7]), pancreatic duct diameter ≤3 mm (OR = 2.8 [95% CI 1.47–6]), amylase concentration in PDO3 ≥ 400 IU/L (OR = 1.83 [95% CI 0.8–4.13]), blood transfusion (OR = 2.63 [95% CI 1.33–5.2]), and POPF (OR = 8.04 [95% CI 3.68–17.57]). The presence of abdominal complications was the variable most associated with DGE.
In the multivariate model, the factors associated with DGE were age > 70 years (P = .001), the presence of abdominal complications (P < .001) and transfusion (P = .016).
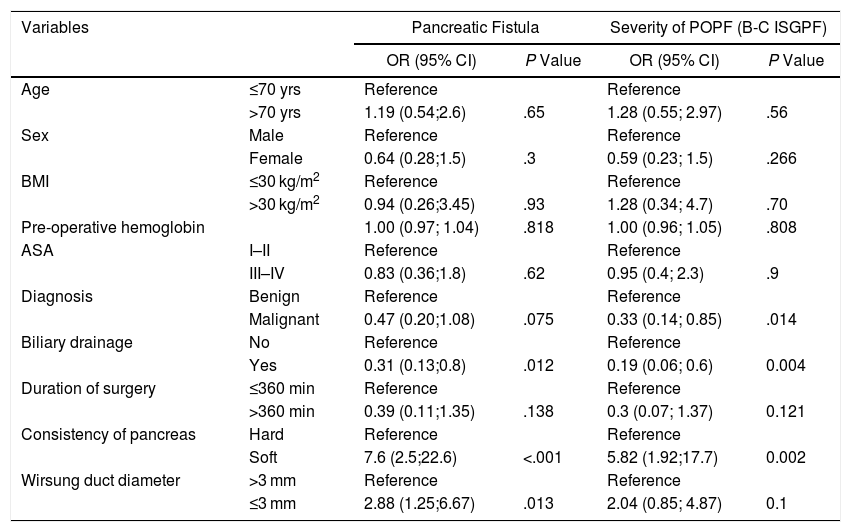
Pancreatic FistulaWe analyzed the risk factors associated with POPF (Table 5a and 5b) and their severity (grades B and C). The univariate model revealed that the risk factors for POPF were the soft vs hard consistency of the pancreas (OR = 7.6 [95% CI 2.5–22.6], P < .001) and duct diameter ≤ 3 mm (OR = 2.88 [95% CI 1.25–6.67]). In contrast, the analysis identified preoperative biliary drainage as a protective factor (OR = 0.31 [95% CI 0.13–0.8], P = .012).
Univariate Logistic Regression Analysis for Pancreatic Fistula (POPF) and Severity of POPF According to ISGPS.
| Variables | Pancreatic Fistula | Severity of POPF (B-C ISGPF) | |||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Age | ≤70 yrs | Reference | Reference | ||
| >70 yrs | 1.19 (0.54;2.6) | .65 | 1.28 (0.55; 2.97) | .56 | |
| Sex | Male | Reference | Reference | ||
| Female | 0.64 (0.28;1.5) | .3 | 0.59 (0.23; 1.5) | .266 | |
| BMI | ≤30 kg/m2 | Reference | Reference | ||
| >30 kg/m2 | 0.94 (0.26;3.45) | .93 | 1.28 (0.34; 4.7) | .70 | |
| Pre-operative hemoglobin | 1.00 (0.97; 1.04) | .818 | 1.00 (0.96; 1.05) | .808 | |
| ASA | I–II | Reference | Reference | ||
| III–IV | 0.83 (0.36;1.8) | .62 | 0.95 (0.4; 2.3) | .9 | |
| Diagnosis | Benign | Reference | Reference | ||
| Malignant | 0.47 (0.20;1.08) | .075 | 0.33 (0.14; 0.85) | .014 | |
| Biliary drainage | No | Reference | Reference | ||
| Yes | 0.31 (0.13;0.8) | .012 | 0.19 (0.06; 0.6) | 0.004 | |
| Duration of surgery | ≤360 min | Reference | Reference | ||
| >360 min | 0.39 (0.11;1.35) | .138 | 0.3 (0.07; 1.37) | 0.121 | |
| Consistency of pancreas | Hard | Reference | Reference | ||
| Soft | 7.6 (2.5;22.6) | <.001 | 5.82 (1.92;17.7) | 0.002 | |
| Wirsung duct diameter | >3 mm | Reference | Reference | ||
| ≤3 mm | 2.88 (1.25;6.67) | .013 | 2.04 (0.85; 4.87) | 0.1 | |
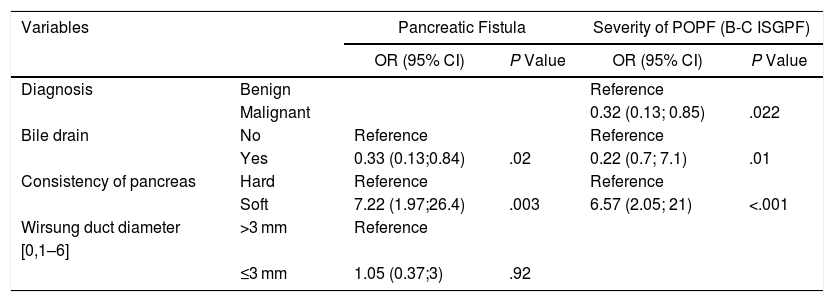
Multivariate Logistic Regression Analysis for Pancreatic Fistula (POPF) and Severity of POPF According to ISGPS.
| Variables | Pancreatic Fistula | Severity of POPF (B-C ISGPF) | |||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Diagnosis | Benign | Reference | |||
| Malignant | 0.32 (0.13; 0.85) | .022 | |||
| Bile drain | No | Reference | Reference | ||
| Yes | 0.33 (0.13;0.84) | .02 | 0.22 (0.7; 7.1) | .01 | |
| Consistency of pancreas | Hard | Reference | Reference | ||
| Soft | 7.22 (1.97;26.4) | .003 | 6.57 (2.05; 21) | <.001 | |
| Wirsung duct diameter | >3 mm | Reference | |||
| [0,1–6] | |||||
| ≤3 mm | 1.05 (0.37;3) | .92 | |||
Regarding the severity of the POPF, the only risk factor was the soft consistency of the pancreas, while the diagnosis of adenocarcinoma and preoperative biliary drainage were protective factors.
In the multivariate model, the soft consistency of the pancreas continued to be a significant risk factor (OR = 7.22 [95% CI: 1.97–26.4]) and preoperative biliary drainage a protective factor (OR = 0.33 [95% CI 0.13–0.84]). With regard to POPF severity, preoperative biliary drainage (OR = 0.33 [95% CI 0.13–0.84]) and the diagnosis of adenocarcinoma (OR = 0.32; [95% CI 0.13–0.85]) were protective factors, while the soft consistency of the pancreas was a risk factor (OR = 7.2 [95% CI 1.97–26.4]).
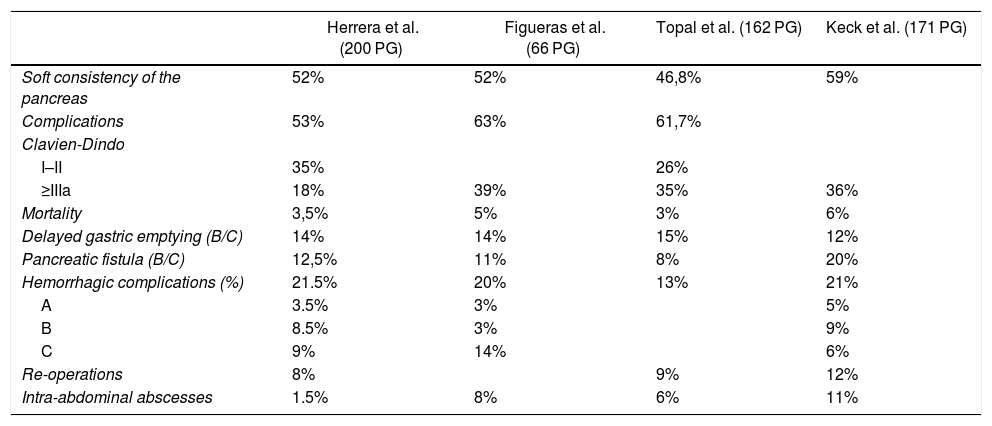
Finally, Table 6 compares our morbidity and mortality results with those from the main published series.
Comparison of Morbidity and Mortality With Other Pancreaticogastrostomy (PG) Series.
| Herrera et al. (200 PG) | Figueras et al. (66 PG) | Topal et al. (162 PG) | Keck et al. (171 PG) | |
|---|---|---|---|---|
| Soft consistency of the pancreas | 52% | 52% | 46,8% | 59% |
| Complications | 53% | 63% | 61,7% | |
| Clavien-Dindo | ||||
| I–II | 35% | 26% | ||
| ≥IIIa | 18% | 39% | 35% | 36% |
| Mortality | 3,5% | 5% | 3% | 6% |
| Delayed gastric emptying (B/C) | 14% | 14% | 15% | 12% |
| Pancreatic fistula (B/C) | 12,5% | 11% | 8% | 20% |
| Hemorrhagic complications (%) | 21.5% | 20% | 13% | 21% |
| A | 3.5% | 3% | 5% | |
| B | 8.5% | 3% | 9% | |
| C | 9% | 14% | 6% | |
| Re-operations | 8% | 9% | 12% | |
| Intra-abdominal abscesses | 1.5% | 8% | 6% | 11% |
A previous study by our group13 with 332 PD (177 PJ and 155 PG) indicated that reconstruction with PG had a lower morbidity and incidence of POPF. Although the surgical technique of choice continues to be a matter of debate, data from nine clinical trials (RCT)15–22 comparing PJ versus PG revealed heterogeneous results with similar morbidity and mortality rates. The previous RCT were not designed to evaluate the influence of the anastomosis type on the incidence of grade C POPF. Moreover, an intention-to-treat analysis was not performed to analyze the technical feasibility of PG.
In some high-volume hospitals, PG is the reconstruction technique of choice in patients at a higher risk for developing POPF.17 In the current debate on the best technique for reconstruction after PD, additional information will probably not be obtained to provide a Level 1 recommendation. However, we can focus on certain factors to improve results, such as standardization of the technique, postoperative care, and technical resources of the surgical team to adapt to the circumstances of each patient, while considering not only morbidity and mortality, but also patient quality of life or nutritional status and long-term glycemic control.
Next, we analyze three studies that have used the ISGPS classification and have assessed the incidence of POPF separately, while considering pancreas consistency and pancreatic duct diameter.17,21,22 For the analysis of the complications and their risk factors, we conducted a search of the literature, finding that only the most recent series followed ISGPS and Clavien-Dindo classifications to report complications.8 Pancreas consistency is a significant risk factor for complications; therefore, when this information is missing, comparisons between series are difficult. It is true that pancreas consistency is subjective and depends on the opinion of the surgeon, while the diameter of the pancreatic duct is more objective; however, in our experience the consistency has a greater predictive value for POPF.
Overall, in our series there were 105 soft (52.5%) and 95 hard (48.5%) pancreata. These data are in line with the series recently published by Keck,17 with 59% and 41%, respectively (58% with a diameter ≤ 3 mm). Figueras22 reported 52% of patients had a soft pancreas, while Topal21 reported 46.8% soft and 60% had a pancreatic duct diameter ≤ 3 hmm. In our series, 75% of cases with soft pancreata had pancreatic duct diameters ≤ 3 mm, and in the Topal series21 it was 78%.
In our series, 43.5% of hospitalized patients had complications at discharge, which increased 9.5% by including readmissions within 30 days (53% in total): 18% Clavien-Dindo ≥ IIIA, 8% IV and 3.5% mortality. Table 6 summarizes the complications of three RCT using the ISGPS1–3 and Clavien-Dindo8 classifications. In addition, the total incidence of complications in our series was around 53%, compared to 63% reported by Figueras22; Keck17 did not evaluate the total complications. It is not clear whether these two studies included the complications from readmissions during the 30 days after surgery, as was done in our series.
Eighteen percent (18%) of the complications reported in our study were severe (Clavien-Dindo ≥ III A), which is lower than the percentages presented by other series with PG (Table 6). Mortality in the first 30 days ranged between 3 and 6% in the different series. Mortality within 90 days in our series increased 0.5%–4%, a percentage lower than 10% reported by Keck.17 The rest of the studies did not contain such information. These morbidity and mortality results meet the quality standards of pancreatic cancer surgery.23,24
The percentages of POPF grade B/C were 8% in the Topal study,21 11.5% in Figueras,22 12.5% in the present study and 20% in Keck.17 It is relevant to note the association between the percentage of soft pancreas (46.8% Topal21 and 59% Keck17) and the degree of POPF B/C.
The incidence of PPH ranged between 13%22 and 21%.13,17,21 Except for the Topal series,21 early intraluminal hemorrhage of the pancreatic stump was significantly higher in the PG group compared to the PJ group.3,17,25,26 In our series, patients with visceral artery hemorrhage were treated by percutaneous embolization, and the seven (3.5%) patients with hemorrhage from the pancreas section edge were reoperated for this cause, performing a gastrotomy and hemostasis along the pancreatic excision edge. In view of the results, PPH can be considered the severest postoperative complication, more so than POPF, and we should have performed an analysis of the prognostic factors associated with PPH as well as that performed with DGE and POPF. We consider that the factors associated with PPH cannot be analyzed globally since the hemorrhage includes two different scenarios, visceral artery hemorrhage and hemorrhage of the resection edge of the pancreas; therefore, we are now carrying out a more extensive study on this specific complication.
The transfusion percentage of the series (41%) was higher than that of hemorrhagic complications. This is mainly due to the fact that, in the first part of the series, we treated patients in worse preoperative condition (malnutrition and anemia) who required transfusion either intraoperatively or within the first postoperative hours, with no hemorrhage or other complications. Currently, we are implementing a preoperative optimization protocol, starting at the anesthesia consultation, in order to reduce the percentage of transfusion and the need for postoperative TPN.
Although the overall incidence of DGE in the four series varied between 15 and 37% reported by Keck,17 the percentage of DGE grade B/C was similar between them (12%–15%). This difference in results may be due to the percentage of grade A DGE (which is not included in all) and to the variability in the nasogastric and feeding tube management protocols.
In our study, age > 70, male sex and soft consistency of the pancreas (OR 3.38) were risk factors associated with complications. Factors associated with the severity of the complications were the ASA grade ≥ III and the soft consistency of the pancreas (OR 1.76). In the Figueras series,22 associated factors included the diameter of the pancreatic duct and POPF. In the multivariate analysis of the incidence and severity of the POPF, Figueras22 reported a Hazard Ratio (HR) of 17.76 for the soft consistency of the pancreas, followed by BMI > 25 kg/m2 with an HR of 11.21. The HR of the soft consistency of the pancreas in the series presented by Keck17 was 2.09. In contrast, preoperative biliary drainage was found to be a protective factor, which does not concur with the results of previous studies. A study by Fujii27 with 122 patients undergoing PD (72 [59%] of whom had preoperative biliary drainage) concluded that there was an increase in the percentage of PF in patients with preoperative biliary drainage if the intervention was delayed more than one month after placement of the drain tube. A possible explanation of our result may be the association of obstructive jaundice with hard pancreas consistency, so that patients with biliary drainage would have a pancreas that is harder in consistency than patients without drainage.
As a secondary objective, we have evaluated the limitations of PG reconstruction in a consecutive, unselected series of patients from a single hospital where three surgeons perform 30 PD per year. The feasibility of PG at our hospital was 90.1%, while in the Keck series17 the feasibility was 93%, and 97% in the Figueras group.22 In our experience, these feasibility values allow us to use PG as a standard technique and reserve PJ, which is technically more complex, for patients with previous gastric surgeries or with a pancreas that cannot be mobilized due to fibrosis of chronic pancreatitis or extensive resections.
This is an observational study, with a single technique and a limited number of patients, so it is not possible to draw a definitive conclusion about which reconstruction type is better (PG or PJ), as is the case with the analysis of RCT and the series compared in this study. However, it seems that, regardless of the reconstruction technique, pancreas consistency and the diameter of the pancreatic duct are variables associated with complications, their severity and the development of POPF. In the Keck series,17 a volume of more than 10 PD per year reduced the incidence of POPF, although not significantly. The RECOPANC study conducted by Keck17 is methodologically the most solid RCT to date, although the reconstruction technique was not the same for all PJ or PG. In our study, we used the same reconstruction technique and the same protocol for postoperative care in all patients and presented similar results. In our experience, PG is a safe technique that meets the morbidity and mortality criteria required by PD and has a feasibility of 90.5%.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Herrera J, Zazpe C, Sánchez P, Tarifa A, Eguaras I, Lera JM. Factibilidad, morbimortalidad en doscientos casos consecutivos de pancreaticogastrostomía después de duodenopancreatectomía. Cir Esp. 2019;97:501–509.s