Enteroatmospheric fistulae (EAF) are a subgroup of enterocutaneous fistulae (ECF) in patients with laparostomy or open abdomen. They are characterized by being superficial, with a high volume of discharge and surrounded by viscera or granulation tissue.1–4 These factors can lead to a situation of metabolic and water–electrolyte imbalance, sepsis and severe malnutrition.
The conservative approach in functional short bowel syndrome associated with a complete jejunal fistula, using the reinfusion of the proximal fistula discharge through the distal jejunostomy, provides good results. In the physiological approach that we propose, we combine the reintroduction of the discharge with artificial enteral nutrition, which achieves improved nutritional state, a reduction in comorbidities and greater recovery of the intestinal mucosa, thereby facilitating the surgical closure of the EAF.
We present the case of a 19-year-old male with penetrating/stenosing Crohn's disease (CD), diagnosed 11 years earlier, with perianal involvement and ileocecal disease, which had had a terminal colostomy due to severe stenosis of the sigma 3 years earlier. He had presented with pancolitis and steroid-dependent extensive ileitis, refractory to various lines of biological treatment and currently treated with ustekinumab, with favorable clinical, radiological and endoscopic response, but stenotic changes persisted at the ileal level. The patient was hospitalized due to abdominal pain secondary to ileal stenosis and reactivation of his CD. During hospitalization, he presented symptoms of bowel obstruction that required emergency surgery, involving ileocecal resection after meticulous adhesiolysis. On the 10th day post-op, the patient was re-operated due to bowel leak, observing a catastrophic abdomen with involvement of multiple loops, so we opted for damage-control surgery and laparostomy assisted with negative pressure therapy for later surgical revision or a “second look”.
In the postoperative period, a complete fistula persistence was observed with high discharge with the afferent and efferent loops in the proximal jejunum, which resulted in a situation of functional short bowel syndrome. Initially, parenteral nutritional support was initiated, but worsened hepatic function was observed, with cytolysis of multifactorial etiology (fasting, infection, total PN, medication).
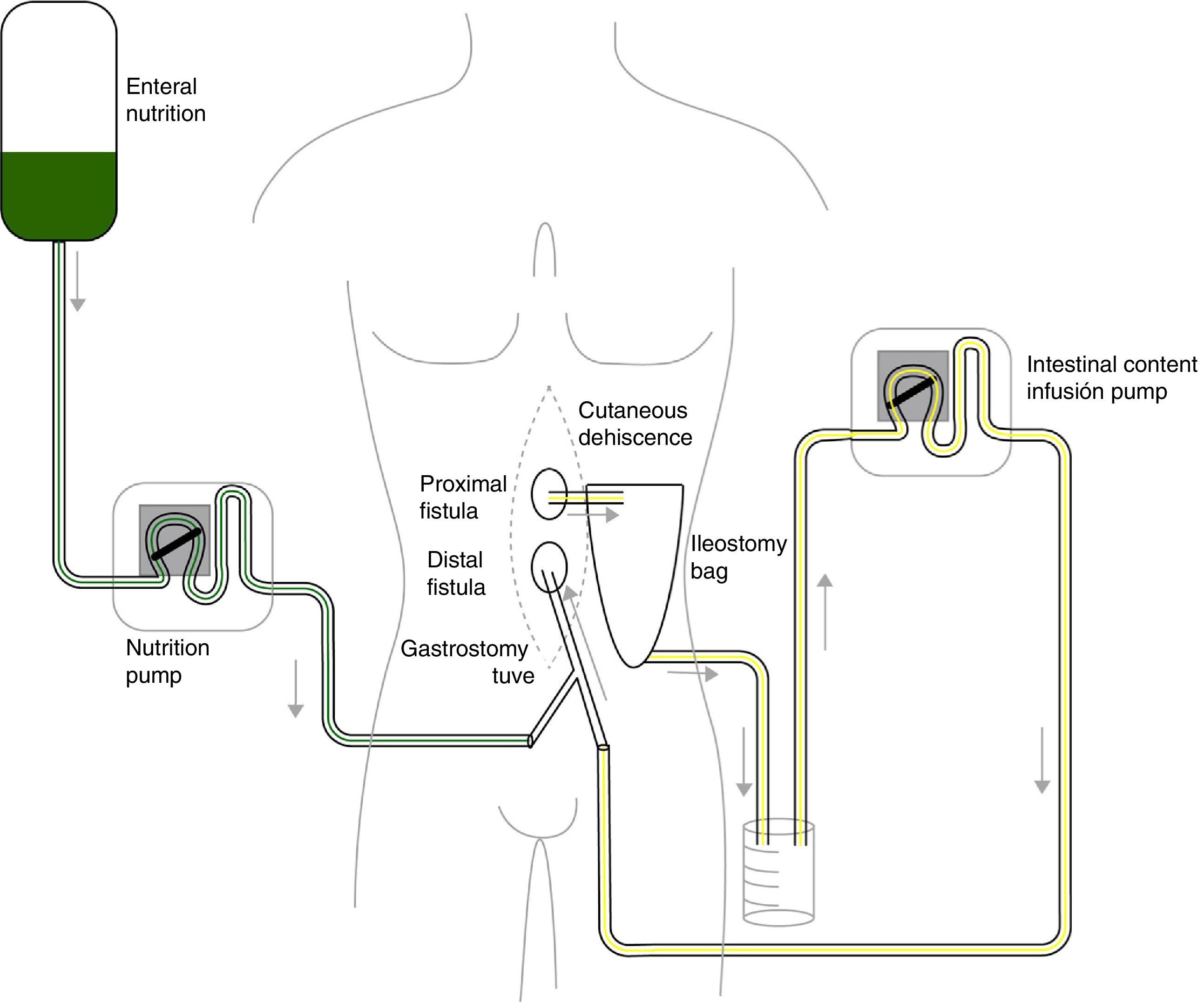
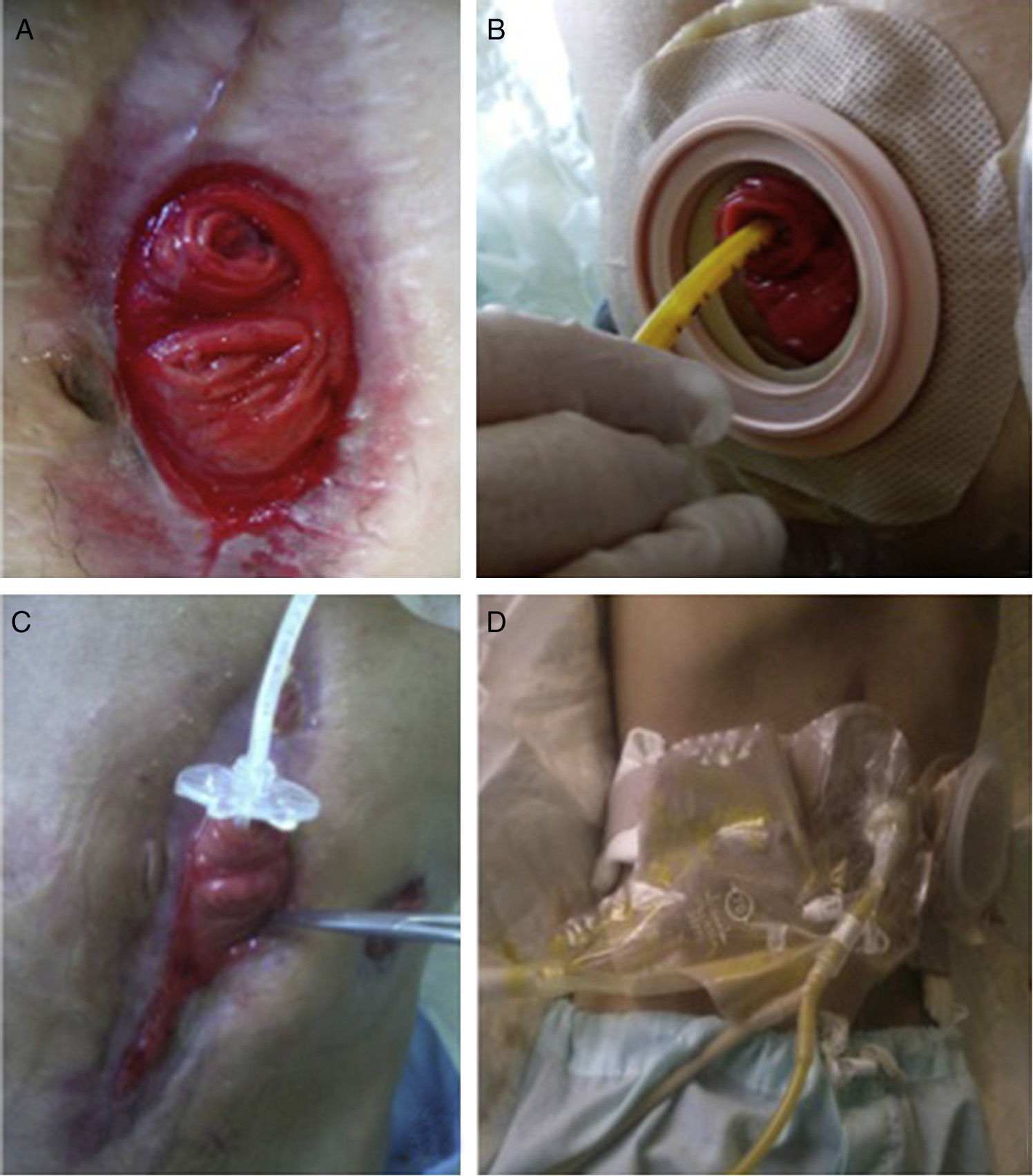
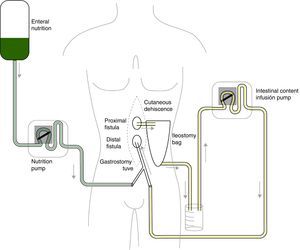
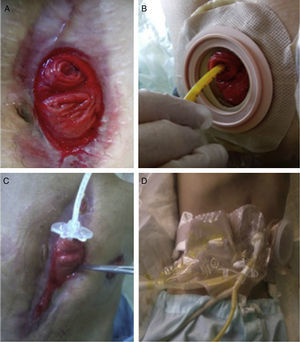
Given the situation of important malnutrition (41kg, BMI: 15.74kg/m2) and comorbidity associated with the use of parenteral nutrition, we considered optimizing the enteral nutritional support by means of a physiological model, using continuous infusion of the nutritional product and the discharge collected from the fistulous orifice through 2 simultaneous pumps connected in a Y (Fig. 1). First of all, in order to collect the proximal discharge in a laparostomy with vacuum-assisted closure, the fistulous orifices were isolated with moldable discs, paste and an ileostomy bag connected to a closed drainage system. For the administration of enteral nutrition (EN) and the discharge from the proximal fistulous orifice, we utilized a 24 F gastrostomy catheter in the distal fistulous orifice, through the ileostomy bag. In order to minimize leaks, we used an adapted cone with adhesive. We consider it recommendable to fill the balloon of the gastrostomy catheter above the indicated value, which in our case was 2cc, in order to guarantee a tight seal (requiring adaptation to the condition and diameter of the jejunum) (Fig. 2A–D). Six weeks after treatment, we observed normalization of the hepatobiliary function and improved nutritional parameters (52kg, BMI: 19.42kg/m2). Four months later, an adequate nutritional state was reached to schedule surgery, which involved resection of the fistulized jejunal loop, manual end-to-end primary anastomosis and reconstruction of the abdominal wall with biological mesh and animal collagen. Currently, the patient is being followed up in the outpatient clinic and has shown positive clinical and nutritional evolution with an ordinary diet.
Nutritional therapy is an essential part of the management of ECF due to the rapid and inevitable protein catabolism.5,6 Unfortunately, there are no randomized controlled clinical trials about their overall management, which limits the ability to establish specific nutritional support strategies. However, it is clear that the treatment of these patients requires specialized surgeons backed by a multidisciplinary team (MDT) with experience in the treatment of complex cases.7 In Spain, the implementation of these multidisciplinary units has been slow, and ours is the first in our region.
In patients with EAF, we are faced with the anatomical difficulties secondary to an open abdomen.3 This continuous infusion system of the proximal fistula discharge by means of a gastrostomy catheter resolves the problems derived from the poor fixation of other catheters to the efferent loop. Our experience indicates that small variations may be necessary in the placement of these devices, depending on the anatomical situation of the fistula. Furthermore, periodical revisions may be needed to avoid complications secondary to decubitus caused by the balloon.
This continuous reinfusion leads to maximal optimization of EN support and provides the ability to decrease or discontinue parenteral nutrition (PN), which has classically been more widely used.8 This results in fewer complications secondary to prolonged PN, such as improved hepatobiliary function and the lower risk of sepsis associated with central venous catheters. It also facilitates the recovery of trophism, achieving the main objective of providing high levels of calories and nutrients.9,10 As a whole, these factors optimize the nutritional state and ensure better results in the definitive surgical treatment for closing the jejunal fistula.
FundingNo funding was received for the completion of this study.
Conflict of InterestsThe authors have no conflicts of interests to declare.
We would like to thank the members of the Grupo de Trabajo de Enfermedad Inflamatoria Intestinal (Inestinal Inflammatory Disease Work Group) at the Complexo Hospitalario Universitario of Ferrol (Spain) for their assistance in this case.
Please cite this article as: Sánchez-Guillén L, López de los Reyes R, Vives-Rodríguez E, Mato Iglesias A, Cantón-Blanco A. Nutrición enteral en enfermedad de Crohn con fístula enteroatmosférica de alto débito. Cir Esp. 2016;94:547–550.
This article was presented as an oral presentation at the XX Reunión Nacional de Cirugía, Granada, 21-23 October 2015.