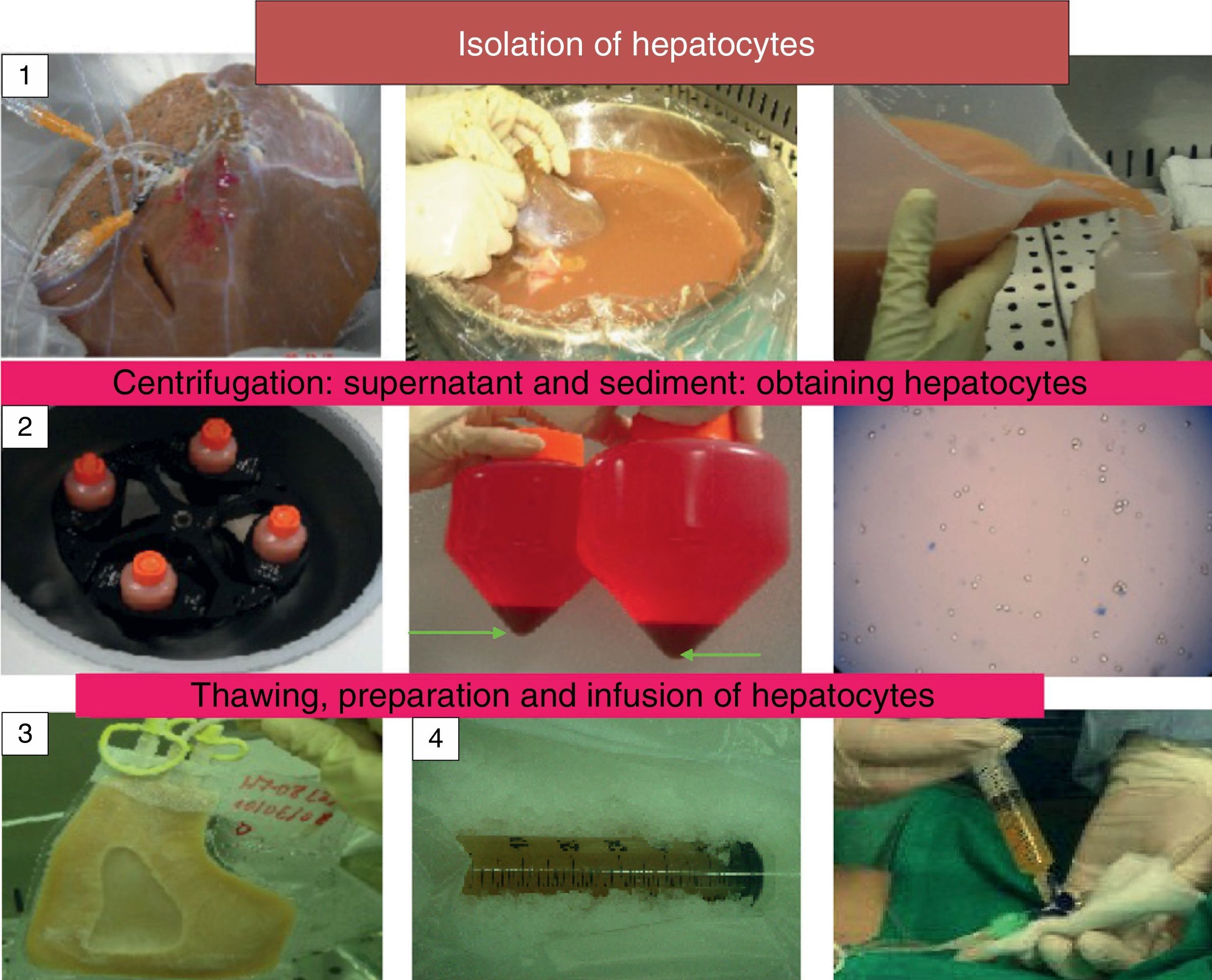
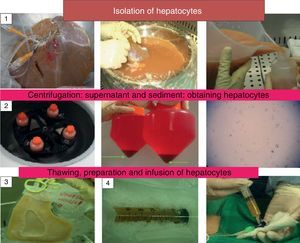
The imbalance between the number of potential beneficiaries and available organs, originates the search for new therapeutic alternatives, such as hepatocyte transplantation (HT). Even though this is a treatment option for these patients, the lack of unanimity of criteria regarding indications and technique, different cryopreservation protocols, as well as the different methodology to assess the response to this therapy, highlights the need of a consensus conference to standardise criteria and consider future strategies to improve the technique and optimise the results. Our aim is to review and update the current state of hepatocyte transplantation, emphasising the future research attempting to solve the problems and improve the results of this treatment.
Existe un gran número de enfermedades hepáticas para las cuales el único tratamiento efectivo es el trasplante hepático. La disparidad entre el número de potenciales beneficiarios y de órganos disponibles motiva la búsqueda de nuevas alternativas de tratamiento, entre las que se encuentra el trasplante celular hepático (TCH). Esta terapia representa una alternativa de tratamiento en estos pacientes, sin embargo, la falta de unanimidad de criterios respecto a las indicaciones y técnica, los diferentes protocolos de criopreservación así como la distinta metodología para valorar la respuesta a esta terapia pone de manifiesto la necesidad de una conferencia de consenso que unifique criterios, planteando posibles estrategias futuras que mejoren la técnica y optimicen los resultados. Nuestro objetivo es realizar una revisión y puesta al día del estado actual del TCH, enfatizando las futuras líneas de investigación que tratan de solucionar los problemas y mejorar los resultados de esta terapia.
At the moment, advances in the field of regenerative medicine are providing ways of approaching certain diseases which involve the cellular deterioration of an organ and where performing a solid transplant of the organ does not always constitute the first therapeutic option or treatment of choice.
At present the only effective treatment for end-stage liver disease is orthotopic liver transplant (OLT). Although donor selection criteria have been broadened in an attempt to increase their number, the results obtained have not been satisfactory. The imbalance between offer and demand of organs is the main limitation of OLT, and alternatives to this treatment are needed. Liver cell transplantation (LCT) or human hepatocyte transplantation is the most promising alternative in terms of results obtained and is considered a cutting-edge therapeutic strategy complementary to solid organ transplant. Despite the fact that at present LCT is not a definitive therapeutic option, it does apply to patients with acute liver failure, where the intention is to replace or serve as a bridge for OLT and for paediatric patients with congenital metabolic defects where the defective enzyme is expressed in the hepatocyte.
Clinical observations have demonstrated the safety of the procedure and of patients who have undergone LCT. However, most publications refer to clinical cases with no unanimity of criteria as to indications, methodology, cellular cyropreservation or assessment of the response to the LCT.6–12
The objective of this manuscript is to review and update the current status of LCT and to highlight new lines of research which are attempting to find a solution to the scarcity of hepatocyte sources, their cryopreservation and their implantation in the recipient (Fig. 1).
Evolution of Liver Cell TransplantationLiver cell therapy is currently considered a cutting-edge therapeutic strategy complementary to solid organ liver transplantation, where the fundamental principle is to regain the lost function of the organ by transplanting its essential components: the cells.
Since Howard et al.1 first isolated hepatocytes in rats in 1967, laying the bases for current protocols, up until 1992 and 1998 when the first LCT was performed on adults with cirrhosis of the liver2 and a 10-year-old girl with type 1 Crigler–Najjar disease respectively,3 a great deal of research has been undertaken in this field.
The first LCT was performed on animals with metabolic disease and a sustained decrease in plasma bilirubin concentrations was described in recessive homozygous Gunn rats after portal infusion of hepatocytes obtained from heterozygous Gunn rats. It was considered that transplanting cells capable of providing the defective enzyme might be an effective form of treatment.4 Subsequently, Sutherland performed LCT on rats with acute liver failure induced by intravenous administration of dimethylnitrosamine, demonstrating how hepatocyte infusion can provide metabolic support enabling recovery from dimethylnitrosamine-induced liver necrosis.5
Clinical observations have demonstrated the safety of the procedure and of patients who have undergone LCT. However, most publications refer to clinical cases with no unanimity of criteria in indications, methodology, cellular cyropreservation or assessment of the response to the LCT.6–12 This highlighted the need to agree criteria at a consensus conference. Delegates at a consensus conference made an effort to identify and discuss possible strategies to overcome the technique's shortcomings and add criteria. They also made suggestions for the future towards finding a solution to problems and improving the results of this therapy.
Sources of Hepatocytes for Liver Cell Transplantation and Its IndicationsThe main problem with LCT was tackled at this conference, i.e., the scarcity of sources for isolating hepatocytes for cell transplantation. As this is one of the main limitations, future strategies are geared at finding a solution for this issue. At the moment hepatocytes are obtained from organs rejected for OLT, and the use of tissue was considered from liver reductions, Split, IV segment after split for 2 recipients14 and grafts from cardiac death donors (DCD) with under 40min of warm ischaemia.13 With the need to find new hepatocyte sources, non-beating donors15 and neonatal donors have been suggested and recently it has even been proposed that hepatocytes from patients who have undergone OLT for metabolic diseases be used, considering the option of a domino transplant with the cells obtained.16
The current basis for LCT lies in transplanting adult hepatocytes, cells which have already been differentiated. However, new lines of research are focussing on obtaining hepatocytes from progenitors originating in the liver or derived from pluripotent progenitor stem cells.17–19 Administering hepatocytes with progenitor cells would combine the short-term metabolic effects of the hepatocyte with the long-term cell survival of the progenitor.
The liver progenitor cells proposed are: foetal liver progenitors, hepatoblasts and progenitor liver stem cells, oval cells, bipotential cells, as they differentiate from cholangiocytes and hepatocytes. They constitute a reserve of cells that proliferate after massive liver damage and colonise the liver parenchyma, raising the question as to whether it is possible to isolate them, grow and expand/differentiate them for therapeutic use.17,18 However, to date it they have been difficult to obtain.
Another option which is being developed is obtaining hepatocytes derived from pluripotent progenitors which come from embryonic stem cells (hES), mesenchymal stem cells (MSC), adipose/bone marrow and induced pluripotent stem cells (Ips).17–19
HES are cells which originate in the embryo at the blastocyst stage. They are able to grow indefinitely in vitro and sustain their pluripotentiality, differentiating from all the other cell types. However, they pose ethical and legal problems which prevent their use at present.19 Obtaining MSC from adipose tissue or bone marrow is simple and does not engender ethical or immunoreactive reservations, as this is autogenous tissue. Adipose tissue is rich in this type of cell and is easier to obtain than bone marrow; however, because they require manipulation to obtain them they enter the category of medicine as their use is complicated.19 Finally, induced Ips are somatic cells with the capacity to be reprogrammed as pluripotent cells and subsequently direct their differentiation to hepatocytes. These constitute a very promising therapeutical option and in combination with gene therapy could be of great use. However, problems with safety give rise to doubts about their use, particularly with regard to risks of neoplasia and immunogenicity. Nevertheless they could be promising for the future.17
With regard to the indications for LCT, the diseases where LCT has been used were summarised at the consensus conference. On a global level, more than 30 children with congenital errors of metabolism treated with LCT have been reported.6–12 Urea cycle alterations were highlighted as the metabolic disorder for which this therapy has been most frequently indicated13 (Table 1).
Pathologies for Which Liver Cell Transplantation has been Performed.
| - Congenital errors of metabolism |
| - Crigler–Najjar Type I (CNI) syndrome |
| - Familial hypercholesterolaemia |
| - Factor VII deficiency |
| - Type I Glycogenosis |
| - Refsum's disease |
| - Familial intrahepatic cholestasis type 2 |
| - Urea cycle defects |
| - Ornithine transcarbamylase deficiency |
| - Argininosuccinate lyase deficiency |
| - Carbamoyl phosphate synthetase deficiency type 1 |
| - Citrullinaemia |
| - Acute liver failure |
| - Drugs |
| - Viral |
| - Idiopathic |
| - Mushroom poisoning |
| - Liver failure after liver resection |
| - Acute liver failure in pregnancy |
| - Alpha-1 antitrypsin deficiency |
| - Alcohol |
A total of more than 40 patients with acute liver failure have been treated, of whom 18 presented fulminant liver failure. The objective with these patients was to sustain liver function as a bridge before OLT or until its regeneration. Although a reduction in bilirubin levels and ammonium, and an improvement in liver encephalopathy have been described, LCT does not have a significant influence on the clinical evolution of the condition.20
We started the LCT programme in the La Fe Hospital in Valencia in May 2008, and have performed 8 LCT, 4 on adults and 4 on children.11,12
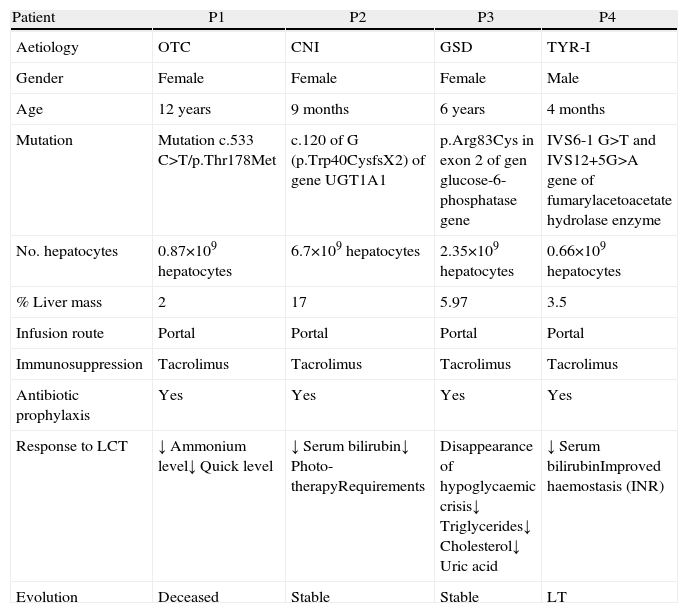
The indication for the paediatric patients was metabolic disease: one patient had ornithine transcarbamylase deficiency, one girl had Crigler–Najjar (CNI) syndrome, one girl had glycogenosis GSD and one boy had a tyrosinaemia type I (TYR-I) (Table 2). The diagnosis was made by determining the enzymatic activity of the liver and/or specific metabolites in serum, confirmed by genetic study. In all cases, therapeutic measures did not manage to stabilise the disease, and clinical and analytical deterioration was observed. Our indication for these children was based on the severity of the disease and the deterioration of their quality of life; the aim was to improve their clinical situation. These were children for whom LCT was indicated as a bridge before OLT or for whom, for various reasons, OLT was not indicated.12
Experience at the La Fe Hospital in Valencia With Liver Cell Transplantation in Children.
| Patient | P1 | P2 | P3 | P4 |
| Aetiology | OTC | CNI | GSD | TYR-I |
| Gender | Female | Female | Female | Male |
| Age | 12 years | 9 months | 6 years | 4 months |
| Mutation | Mutation c.533 C>T/p.Thr178Met | c.120 of G (p.Trp40CysfsX2) of gene UGT1A1 | p.Arg83Cys in exon 2 of gen glucose-6-phosphatase gene | IVS6-1 G>T and IVS12+5G>A gene of fumarylacetoacetate hydrolase enzyme |
| No. hepatocytes | 0.87×109hepatocytes | 6.7×109hepatocytes | 2.35×109hepatocytes | 0.66×109hepatocytes |
| % Liver mass | 2 | 17 | 5.97 | 3.5 |
| Infusion route | Portal | Portal | Portal | Portal |
| Immunosuppression | Tacrolimus | Tacrolimus | Tacrolimus | Tacrolimus |
| Antibiotic prophylaxis | Yes | Yes | Yes | Yes |
| Response to LCT | ↓ Ammonium level↓ Quick level | ↓ Serum bilirubin↓ Photo-therapyRequirements | Disappearance of hypoglycaemic crisis↓ Triglycerides↓ Cholesterol↓ Uric acid | ↓ Serum bilirubinImproved haemostasis (INR) |
| Evolution | Deceased | Stable | Stable | LT |
CNI, Crigler–Najjar disease; GSD, glycogen storage disease; INR, international normalised ratio; LCT, liver cell transplant; LT, liver transplant; TYR-I, type I tyrosinaemia; OTC, ornithine transcarbamylase deficiency.
The adult patients, all males, had acute liver failure. Of the 4 patients, 2 had previously undergone transplantation for hepatitis C viral cirrhosis, and were on the waiting list for liver retransplantation due to a recurrence of the virus; the objective of the LCT was to serve as a bridge until retransplantation. In the other 2, acute liver failure presented after a major liver resection for hepatic metastases and after significant alcohol ingestion in the context of one case of undiagnosed alcoholic liver cirrhosis (Table 3). Both patients underwent the LCT as a bridge towards re-establishing liver function. Our indication for LCT was when all therapeutic measures had failed and the patients presented clinical and analytical deterioration with grade II or IV liver encephalopathy despite having started aggressive medical treatment, requiring them to be admitted to the Intensive Care Unit.11
Experience With Cellular Transplantation in Adults With Acute Liver Failure.
| P1 | P2 | P3 | P4 | |
| Aetiology | VHC | VHC | Liver metastases | Alcoholic |
| Gender | Male | Male | Male | Male |
| Age | 42 | 49 | 66 | 50 |
| Child–Pugh | 15 | 15 | – | 13 |
| MELD | 39 | 32 | – | 35 |
| ABO | 0 | 0 | A | A |
| Response to antiviral treatment | No | Yes | – | – |
| MARS response | No | No | – | – |
| Predisposing factor | Haemorrhage due to oesophageal varices | Spontaneous bacterial peritonitis | No | Alcohol abuse |
| Encephalopathy | IV | III | III–IV | III–IV |
| Encephalopathy treatment response | No | No | No | No |
| Renal failure | Renal | No | No | Renal |
| Fresh plasma | Yes | Yes | Yes | Yes |
| Tacrolimus | Yes | Yes | Yes | Yes |
| Antibiotic prophylaxis | Yes | Yes | Yes | Yes |
| Prior LT | Yes (LT 10 years before) | Yes (LT 2 years before LT) | No | No |
| No. hepatocytes | 2.209×109 hepatocytes | 3.610×109 hepatocytes | 2.300×109 hepatocytes | 1.200×109 hepatocytes |
| % Liver mass | 1.57 | 2.4 | 1.44 | 0.76 |
| Infusion route | Splenic artery | Splenic artery | Splenic artery | Arteria esplénica |
| Hepatocytes | Cryopreserved | Cryopreserved | Cryopreserved | Cryopreserved |
| Retransplant | No | Yes | No | No |
| Evolution | Deceased | Living | Deceased | Deceased |
ABO, blood group; MARS, molecular adsorbent recirculating system; MELD, model for end-stage liver disease; LT, liver transplantation; VHC, virus hepatitis C.
There is a consensus on the procedure for isolating hepatocytes by perfusion of the liver in situ with collagenase. Cell viability is determined using the trypan blue exclusion test, which measures the integrity of the cell membrane. The dead cells are permeable to the colourant and are dyed blue; however, their correlation with metabolic function and the possibility of their being grafted into the recipient has not been demonstrated. New lines of research propose adding an antioxidant such as N-acetylcysteine to the perfusion solution when the cells are isolated in order to improve the viability and metabolic function of hepatocytes. An improvement in these parameters has been described in hepatocytes isolated from fatty livers and their routine use is being proposed.21
It is considered appropriate to determine viability prior to cryopreservation and after thawing the cells as well as prior to and after their infusion. This should exceed 80% in order to cryopreserve the hepatocytes and exceed 60% after thawing, prior to their infusion into the recipient.13
Cryopreservation of HepatocytesCryopreservation presents one of the great difficulties with liver cell therapy. The hepatocytes for use can be fresh or cryopreserved. Cryopreservation causes mitochondrial damage, increases the permeability of the mitochondrion, causing a drop in ATP synthesis capacity and alterations at the level of cellular respiration as it causes changes in the activity of the respiratory chain enzymes.13 Some European centres only transplant cryopreserved hepatocytes. This allows thorough microbiological controls to be undertaken to prevent the risk of transmitting infection, enabling the creation of a cell bank so that they are available in emergency situations and it provides the flexibility to perform this therapy as a planned treatment.13,22 Nevertheless, cryopreservation has harmful effects on the viability and metabolic function of the cells and this implies that they may not be suitable for use after thawing.
The differences in cryopreservation procedures between the different groups create differences in the quality of the cells after they have been performed, making it difficult to compare results. This highlights the need to combine and improve these protocols. Some authors propose that cryopreservation injuries are Fe+ dependent and therefore suggest the addition of iron chelators such as desferoxamine and LK 614 in order to reduce the damage caused by the cold. Studies show that the use of preservation solutions rich in ions (Cl−, Na+, K+, and Ph 7,0) contribute towards reducing cryopreservation injuries.23 Other authors propose adding cryprotectors in order to improve the DMSO-UW cryopreservation protocol. The team at King’ College proposes new measures such as reducing cellular density (107cells/mL) in cryobags and improving the cryopreservation solution. With this protocol they achieve cryopreservation of the hepatocyte without losing function for up to 3 years with improved viability and performance.24 After the consensus conference, the authors conclude that fresh cells are more viable than cryopreserved cells.
Quality Control of HepatocytesThe result of LCT is closely related to the quality of the transplanted cells. Quality control of the cells is an attempt to gather information in order to select the best cells according to the recipient's underlying pathology in the shortest time possible.13,25 To that end, it is proposed that ATP production be determined, which provides an indirect estimate of the viability and functional activity of the hepatocytes, and that the hepatocyte's metabolic functions be determined, such as ureogenesis. It is proposed that the enzyme activity levels in the cell and the metabolisation of drugs should be determined by measuring the activity of cytochrome P 450.22
New lines of research propose establishing the metabolic profile of the cells by quantifying cellular respiration at the mitochondrial level and obtaining fluorescent and luminescent metabolites by using fluorescence and luminescence equipment. The possibility is proposed of performing a liver biopsy to perform a histological study and analysis of oxidative stress and apoptosis at the same time as undertaking a metabonomic analysis with enzymatic determination by nuclear magnetic resonance (Hydrogen-1 NMR) or by mass spectometry, which would enable selection of the cells according to their enzyme activity.26 This raises the possibility of filling hepatocytes with greater enzyme activity. However, despite everything that has been outlined above, the cell quality parameters which best correlate with cell integration and a favourable cell transplant result are not yet known.
Performing Liver Cell TransplantationABO compatibility must be respected when hepatocyte transplantation is performed. The following are key aspects: the route and site of infusion of the hepatocytes and the number of infusions and cells administered by infusion. We transplant between 5% and 10% of the recipient's estimated theoretical liver cell mass, the maximum number of cells per infusion must be taken into account in order to prevent side effects, especially an increase in portal pressure, which is monitored by performing an eco-doppler.13
Hepatocyte transplantation is performed by manual infusion of fresh or cryopreserved cells via the portal route (having first placed a port-a-cath in the portal vein) for metabolic disorders, or into the splenic artery (for adults with liver cirrhosis). The number of infusions required is variable.2,6–12,15 One of the alternative infusion sites proposed by lines of research for the future is the intraperitoneal infusion of hepatocytes encapsulated in alginate microspheres, which would offer the advantage of avoiding immunosuppression. Studies have been undertaken which point to an improvement in the survival of hepatocytes due more to metabolic support than to stimulating liver generation.27,28
An immunosuppression pattern is necessary, based on the administration of a bolus of corticosteroids prior to the cellular infusion followed by double therapy with corticosteroids and calcineurin inhibitors.
Engraftment of Transplanted Cells Into the RecipientLCT results are limited by the low efficiency of integration of transplanted cells in the recipient and by the difficulty in monitoring these cells after LCT. Their identification in the liver of the recipient can help to determine the mechanisms involved. However, it is not easy to demonstrate this cellular integration in humans. It is considered that the best method to monitor hepatocytes after LCT is to determine the deficient enzyme activity. This would provide indirect evidence of cellular function after transplant, although it does not give information on the location or number of functioning cells.
Experimental studies demonstrate that 70% of hepatocytes are eliminated from the portal system and liver sinusoid by the immune system, the Kupffer cells and neutrophils in 24–48h; therefore an issue pending is how to prevent the early loss of these transplanted cells. Research supports the expression of a hepatocyte tissue factor activating coagulation cascade. Under this premise, research is underway on inhibiting procoagulant activity with N-acetyl-cysteine and the use of inhibitors of complement and coagulant activation with dextran sulphate as possible courses of action to prevent the loss of hepatocytes.
Other authors consider that monitoring vascular changes and liver inflammation can improve the integration of hepatocytes in the recipient. Infusing cells through the inside of vessels causes an inflammatory response which gives rise to an increase in vascular permeability and disruption of the vascular endothelium, detecting over-regulation of cytokines associated with the activation of neutrophils, macrophages and regulatory cytokines (tumour necrosis factor and interleukin 6). This would raise the possibility of directly modulating the cell implantation process using drugs.29,30
Furthermore, it has been seen in animal studies that the integration of hepatocytes in the parenchyma takes between 3 and 7 days, confirming that immune response mediators such as the CD4 and CD8 T lymphocytes contribute towards the loss of graft function.31 Patients show clinical improvement and/or partial correction of the underlying metabolic disorder, although, in the majority of cases, no sustained benefits were observed.
Lines of research for the future are based on the use of preconditioning techniques, the aim of which is to find acceptable methods from a clinical viewpoint which stimulate the proliferation of implanted hepatocytes. These techniques include irradiation of the liver and partial portal embolisation in isolation or combined with surgery.32–34
Liver irradiation is a preconditioning method proposed to improve cellular integration in the liver parenchyma. Experimental models show that liver irradiation in doses of 15–50Gy about 7 days prior to LCT improves integration and cellular proliferation; cell implantation should be performed between 1 and 7 days afterwards. Irradiation should be avoided in patients with cirrhosis of the liver, as the liver parenchyma is more sensitive. There is no conclusive data with regard to liver irradiation in children under 2; above this age isolated fractions of 3–5Gy are safe, although it is not known whether this would be sufficient to stimulate repopulation with donor cells. Further studies are necessary to define the patients and pathologies for which this application would be indicated.33,34
Portal embolisation is another pre-conditioning technique, considered a less invasive procedure, which promotes liver regeneration. The use of particles of absorbable material (Gelofoam®) has been suggested, performing a very distal embolisation. This induces proliferation of the hepatocytes and hypertrophy of the non-embolised lobe; the complete recanalisation of the portal vein is observed after 13 days.35
If the initially planned studies with these techniques were to establish that pre-conditioning of the liver is safe and offers an improvement for the graft, new opportunities would certainly be opened up in the field of LCT.
At the moment, hepatocyte transplantation constitutes an advance in the treatment of liver failure. The infusion of hepatocytes into the liver or spleen is a safe procedure which patients tolerate well. It has the advantage of not being a major surgical procedure. It has lower morbidity, mortality and cost and is a far less invasive therapy than conventional transplant, offering the possibility of maximising resources because one single donor can be used for several recipients. Clinical experience shows metabolic improvement in patients when we transplant differentiated adult cells although the benefit obtained is not sustained indefinitely over time. The integration of hepatocytes in recipients requires faster-growing cells, therefore new cell lines are being researched such as liver or foetal progenitors, although these types of cells are difficult to obtain at the moment. Hepatocytes derived from pluripotent cells are a very promising option, in particular the use of induced pluripotent stem cells in combination with gene therapy. However, at present, safety issues are yet to be addressed –the risk of neoplasias and immunogenicity in particular –which would mean their use could be contemplated.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Pareja E, Cortés M, Gómez-Lechón MJ, Maupoey J, San Juan F, López R, et al. Estado actual y perspectivas futuras del trasplante de hepatocitos. Cir Esp. 2014;92:74–81.