There is no clear agreement on the type of gastrectomy to be used (either total [TG] or distal [DG]) in middle or distal gastric cancer, especially when it is undifferentiated or Lauren diffuse type. In this meta-analysis, we intend to define which of the two techniques should be recommended, based on survival, morbidity and mortality rates. Prospective and retrospective studies comparing both techniques have been included for a total of 6303 patients (3,641 DG and 2,662 TG). DG was significantly associated with fewer complications, fewer anastomotic fistulae, and less perioperative mortality. The number of lymph nodes in DG was significantly lower, but always above 15. Finally, even the 5-year survival of DG was also higher. Therefore, distal gastrectomy, as long as a safety margin is obtained and regardless of the histological type, should be performed in surgery for distal stomach cancer.
El tipo de gastrectomía, total (GT) o distal (GD), en el cáncer gástrico medio o distal no está claramente consensuada, sobre todo cuando es indiferenciado o difuso de Lauren. Pretendemos en este metaanálisis definir en términos de supervivencia y morbimortalidad cuál de las dos técnicas debiera ser recomendada. Se han incluido trabajos prospectivos y retrospectivos que comparen ambas técnicas hasta un total de 6303 pacientes (3.641 GD y 2.662 GT). La GD se asoció de forma significativa con menos complicaciones, menos fístulas anastomóticas y menos mortalidad peroperatoria. El número de ganglios en la GD fue significativamente menor, pero siempre por encima de 15. Finalmente, la supervivencia a cinco años de la GD fue también superior. Por tanto, la gastrectomía distal, siempre que se obtenga un margen de seguridad e independientemente del tipo histológico, debe ser realizada en la cirugía de cáncer distal de estómago.
Gastric cancer is the fifth most common cancer. In 2018, more than one million cases were diagnosed worldwide. Its prognosis is uncertain — in fact, out of the almost 10 million cancer-related deaths in the world that year, 782,685 (8.2%) were secondary to stomach cancer.1,2
Surgery is an essential pillar in the multidisciplinary treatment of this disease. Although more than a century has passed since Billroth and Schlatter performed, respectively, the first subtotal gastrectomy (subtotal distal gastrectomy [DG]) and the first total gastrectomy (TG) in stomach cancer,3,4 there is still no widespread agreement about which option is the best surgical treatment for distal and middle-third stomach cancer.
The best surgery for gastric adenocarcinoma should contemplate complete locoregional excision of the disease with negative resection margins, but without forgetting key issues such as the morbidity and mortality of the surgery and postoperative patient quality of life.
The extension of lymph node dissection has been the subject of debate in the past. Currently, most surgeons favor a D2, lymphadenectomy because it guarantees a lower rate of local recurrence, better survival results, D1, is reserved for elderly patients or those with comorbidities due to the higher morbidity, mortality of D2. However, when it comes to a cancer located in the middle or distal thirds of the stomach, there is not much consensus regarding the extent of resection of the stomach itself. Some authors argue that the resection must be, a TG, regardless of the location of the tumor, especially when dealing with poorly differentiated adenocarcinomas or adenocarcinomas of the diffuse type, according to Lauren’s classification. This is due to the possibility of metachronous or synchronous preneoplastic or neoplastic lesions in other parts of the gastric mucosa. In contrast, other authors advocate the use of DG due to its lower morbidity, mortality, provided that a minimum safety margin of 3- cm can be5, guaranteed, regardless of its differentiation or Lauren’s classification.
This lack of single criterion is evident in the scientific literature. According to a review of 62 hospitals in Europe.5 44% percent of surgeons opted for TG in cancer located in the antrum that is histologically defined as diffuse following Lauren’s classification. In the United States, 20% of surgeons would perform TG or near-total gastrectomy in patients with distal stomach cancer.6 More recently, 2 studies using the National Cancer Data Base as a reference 7,8 show lower figures, close to 12%, although the percentage approaches 40% if organs other than the stomach are included in the resection.
It is evident that, in distal stomach cancers, TG continues to be an approach used for many patients, despite the fact that DG is simpler from a technical perspective, has less morbidity and mortality and, more importantly, does not seem to have worse oncological results.9–14
This meta-analysis aims to analyze the results in terms of efficacy in oncological safety, morbidity and mortality of DG versus TG in middle-third and distal stomach cancer. The lack of consensus on the two techniques justifies the need for this study.
MethodsSearch strategyThe databases included for the article search were PubMed, Cochrane and EMBASE, using the search terms ‘total gastrectomy’, ‘subtotal gastrectomy’, ‘distal gastrectomy’, ‘gastric cancer’ and ‘partial gastrectomy’.
All articles were read by 2 independent reviewers. In the absence of agreement between both reviewers, a third person was consulted before rejecting or considering an article for the database.
Inclusion criteria and study objectivesThe selection criteria were: 1) article written in English, French or Spanish; 2) studies comparing TG and DG in middle-third and distal stomach cancer performed for curative, not palliative, purposes; and 3) retrospective and prospective studies.
The primary objectives of our meta-analysis were: 5-year survival and perioperative mortality. The secondary endpoints were: lymph nodes obtained, postoperative complications (intra-abdominal abscess, paralytic ileus, postoperative hemorrhage), and anastomotic fistula.
Data collectionData collection and subsequent assessment were carried out by 2 independent researchers. The following variables were included for each study: name of the authors, year of publication, type of study. The following variables were extracted from each of the study groups: number of patients, postoperative mortality, anastomotic fistula, postoperative complications (paralytic ileus, postoperative hemorrhage, intra-abdominal abscess), number of lymph nodes removed, and 5-year survival.
Statistical analysisThe comparative data of the studies were expressed as odds ratio (OR) with a 95% confidence interval (CI). We assessed the heterogeneity of the studies with the I-squared index (I2) and the Cochrane Q test (P). When heterogeneity was significant, we used the random-effects model. Statistically significant differences in heterogeneity were considered when P<.1 or I2> 35%. To assess the existence of publication bias, a funnel plot was created.
ResultsArticle search and selectionThe initial search with the keywords identified a total of 4500 articles.
The flowchart (Fig. 1) illustrates the reasons for discarding articles and reaching the 15 selected. They were discarded for the following: not having any relationship or not dealing with surgery in gastric cancer; not selectively studying middle or distal gastric cancer; not comparing both techniques; lacking adequate statistical methodology; dealing with quality of life after both techniques without including complications after the interventions; because they were written in languages other than those designated in the inclusion criteria; because they included patients with different surgical techniques or non-curative surgeries; and, lastly, because they did not include certain data on postoperative mortality or mid-term survival.
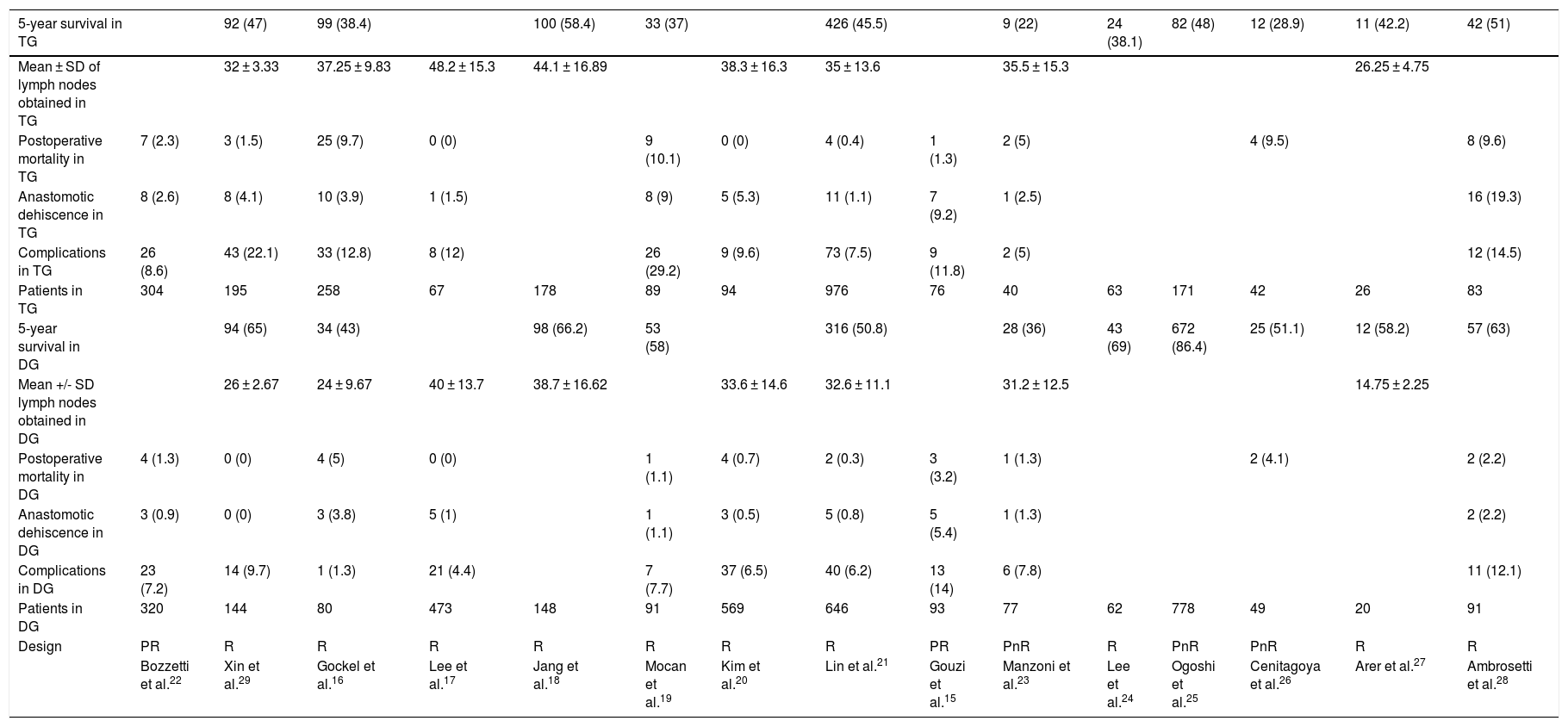
The characteristics and variables of the 15 studies included15–29 are shown in Table 1.
Characteristics of the articles included in the meta-analysis.
| 5-year survival in TG | 92 (47) | 99 (38.4) | 100 (58.4) | 33 (37) | 426 (45.5) | 9 (22) | 24 (38.1) | 82 (48) | 12 (28.9) | 11 (42.2) | 42 (51) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD of lymph nodes obtained in TG | 32 ± 3.33 | 37.25 ± 9.83 | 48.2 ± 15.3 | 44.1 ± 16.89 | 38.3 ± 16.3 | 35 ± 13.6 | 35.5 ± 15.3 | 26.25 ± 4.75 | |||||||
| Postoperative mortality in TG | 7 (2.3) | 3 (1.5) | 25 (9.7) | 0 (0) | 9 (10.1) | 0 (0) | 4 (0.4) | 1 (1.3) | 2 (5) | 4 (9.5) | 8 (9.6) | ||||
| Anastomotic dehiscence in TG | 8 (2.6) | 8 (4.1) | 10 (3.9) | 1 (1.5) | 8 (9) | 5 (5.3) | 11 (1.1) | 7 (9.2) | 1 (2.5) | 16 (19.3) | |||||
| Complications in TG | 26 (8.6) | 43 (22.1) | 33 (12.8) | 8 (12) | 26 (29.2) | 9 (9.6) | 73 (7.5) | 9 (11.8) | 2 (5) | 12 (14.5) | |||||
| Patients in TG | 304 | 195 | 258 | 67 | 178 | 89 | 94 | 976 | 76 | 40 | 63 | 171 | 42 | 26 | 83 |
| 5-year survival in DG | 94 (65) | 34 (43) | 98 (66.2) | 53 (58) | 316 (50.8) | 28 (36) | 43 (69) | 672 (86.4) | 25 (51.1) | 12 (58.2) | 57 (63) | ||||
| Mean +/- SD lymph nodes obtained in DG | 26 ± 2.67 | 24 ± 9.67 | 40 ± 13.7 | 38.7 ± 16.62 | 33.6 ± 14.6 | 32.6 ± 11.1 | 31.2 ± 12.5 | 14.75 ± 2.25 | |||||||
| Postoperative mortality in DG | 4 (1.3) | 0 (0) | 4 (5) | 0 (0) | 1 (1.1) | 4 (0.7) | 2 (0.3) | 3 (3.2) | 1 (1.3) | 2 (4.1) | 2 (2.2) | ||||
| Anastomotic dehiscence in DG | 3 (0.9) | 0 (0) | 3 (3.8) | 5 (1) | 1 (1.1) | 3 (0.5) | 5 (0.8) | 5 (5.4) | 1 (1.3) | 2 (2.2) | |||||
| Complications in DG | 23 (7.2) | 14 (9.7) | 1 (1.3) | 21 (4.4) | 7 (7.7) | 37 (6.5) | 40 (6.2) | 13 (14) | 6 (7.8) | 11 (12.1) | |||||
| Patients in DG | 320 | 144 | 80 | 473 | 148 | 91 | 569 | 646 | 93 | 77 | 62 | 778 | 49 | 20 | 91 |
| Design | PR | R | R | R | R | R | R | R | PR | PnR | R | PnR | PnR | R | R |
| Bozzetti et al.22 | Xin et al.29 | Gockel et al.16 | Lee et al.17 | Jang et al.18 | Mocan et al.19 | Kim et al.20 | Lin et al.21 | Gouzi et al.15 | Manzoni et al.23 | Lee et al.24 | Ogoshi et al.25 | Cenitagoya et al.26 | Arer et al.27 | Ambrosetti et al.28 |
The percentages of each of the variables of the total number of patients are in parentheses.
PnR: prospective not randomized; PR: prospective randomized; R: retrospective.
15 articles with a total of 6303 patients were studied (TG = 2662; DG = 3641).
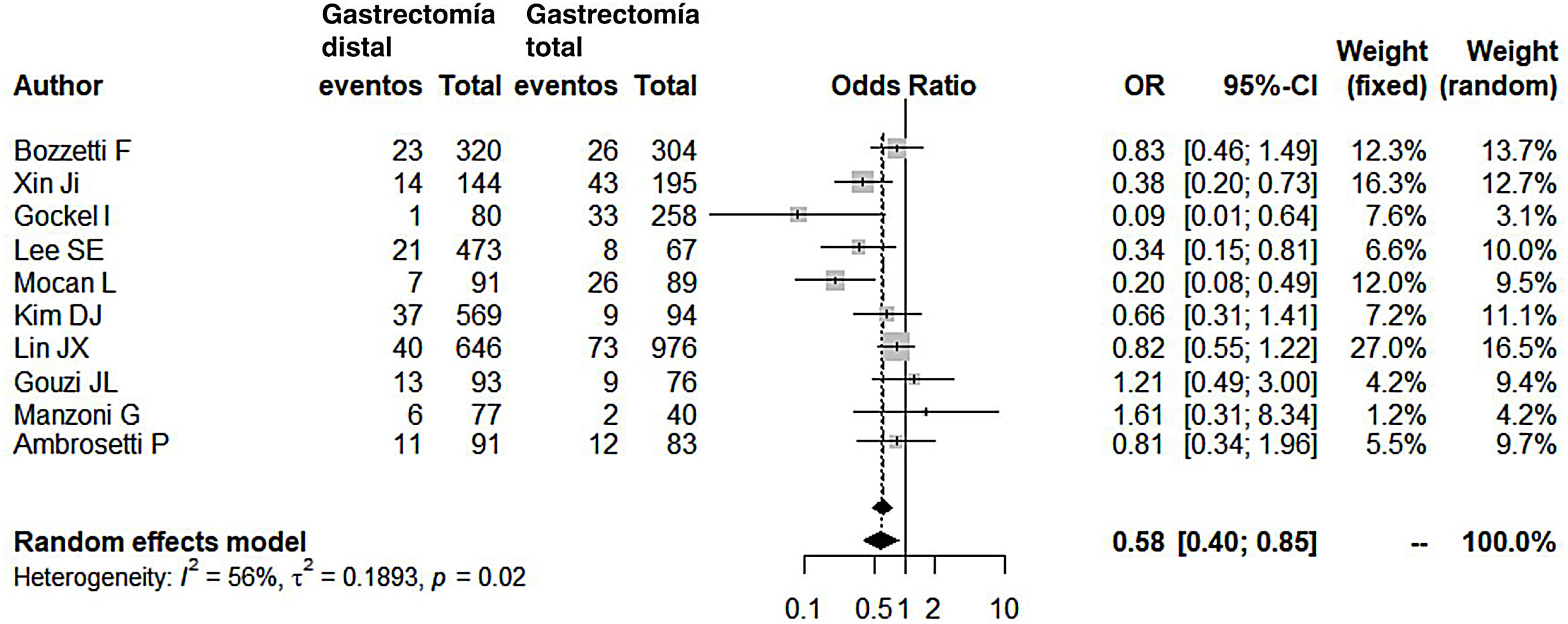
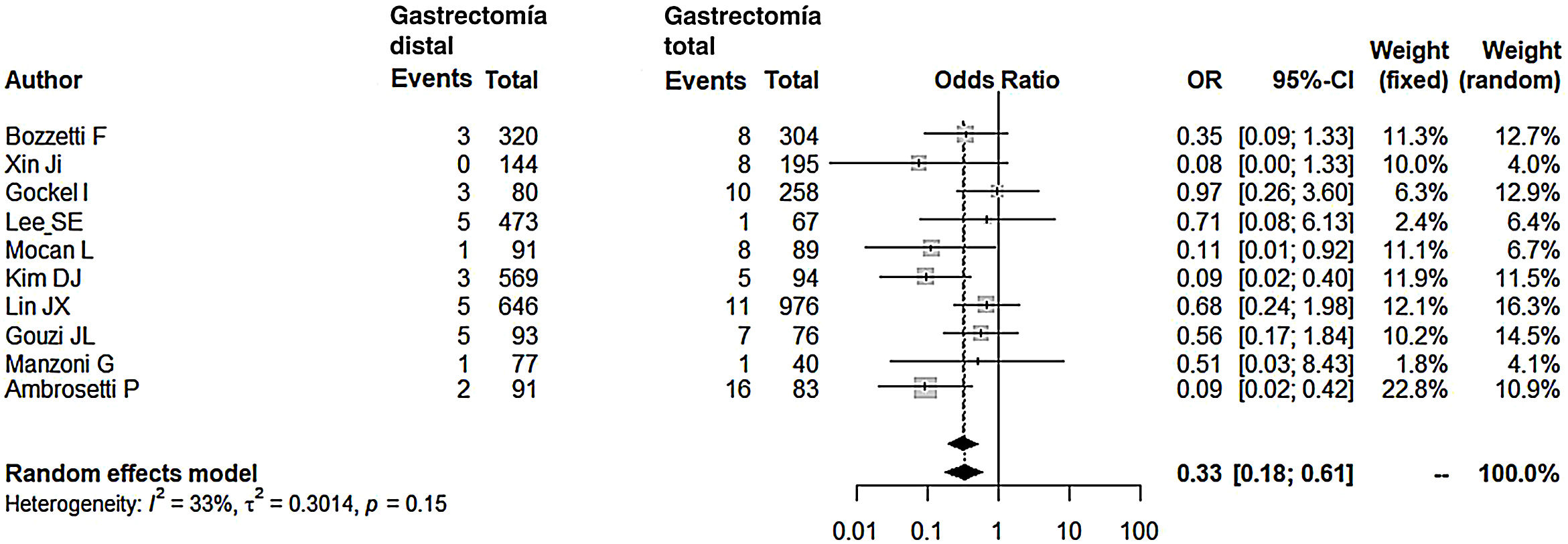
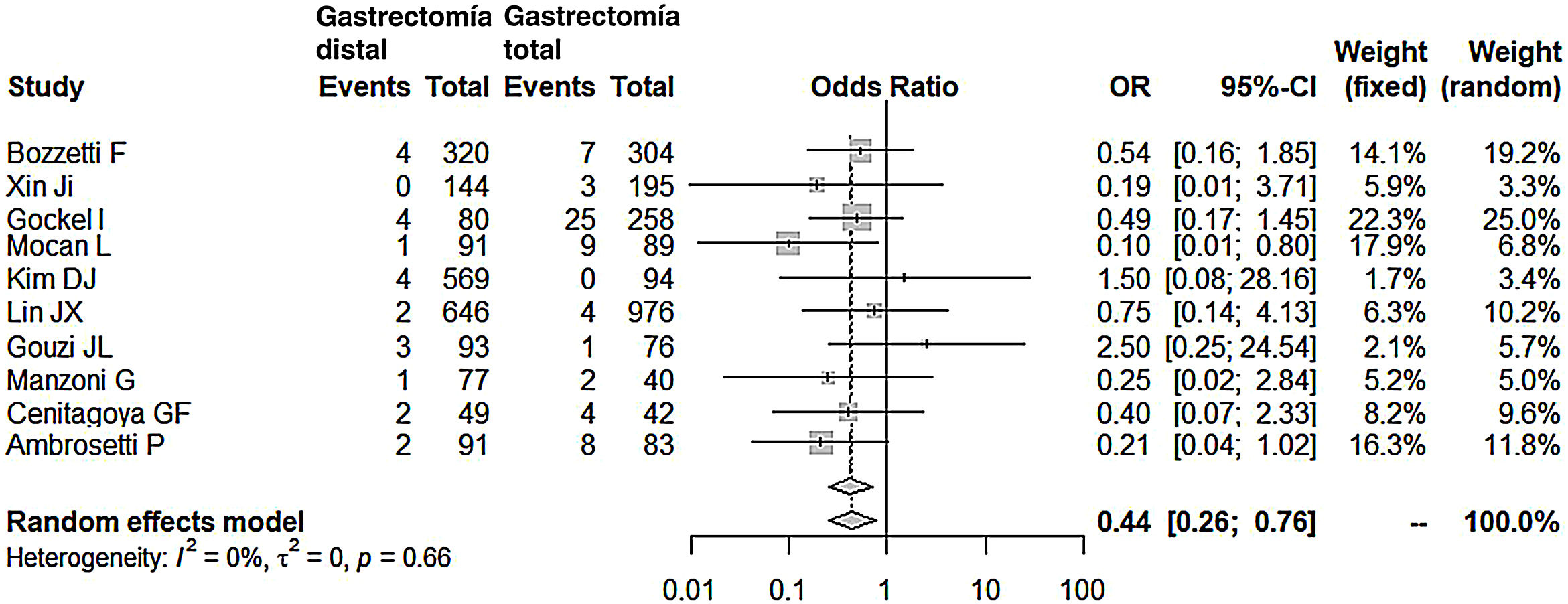
Compared with the patients in the TG group, the patients in the DG group presented fewer complications (OR: 0.58; 95% CI: 0.40-0.85; I2: 86%) (Fig. 2). Similarly, the appearance of an anastomotic fistula was significantly lower in the DG group (OR: 0.33; 95% CI: 0.18-0.61; I2: 33%) (Fig. 3). With these 2 data, the result for postoperative mortality was as expected, with lower mortality in the DG group (OR: 0.44; 95% CI: 0.26-0.76; I2: 0%) (Fig. 4).
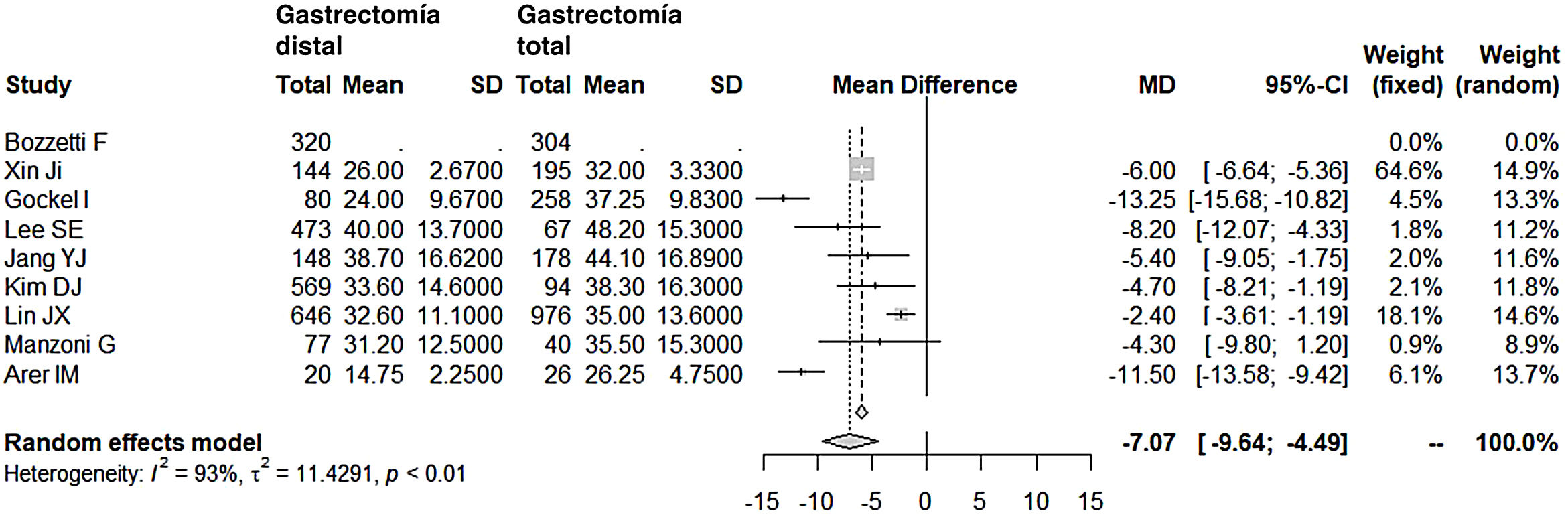
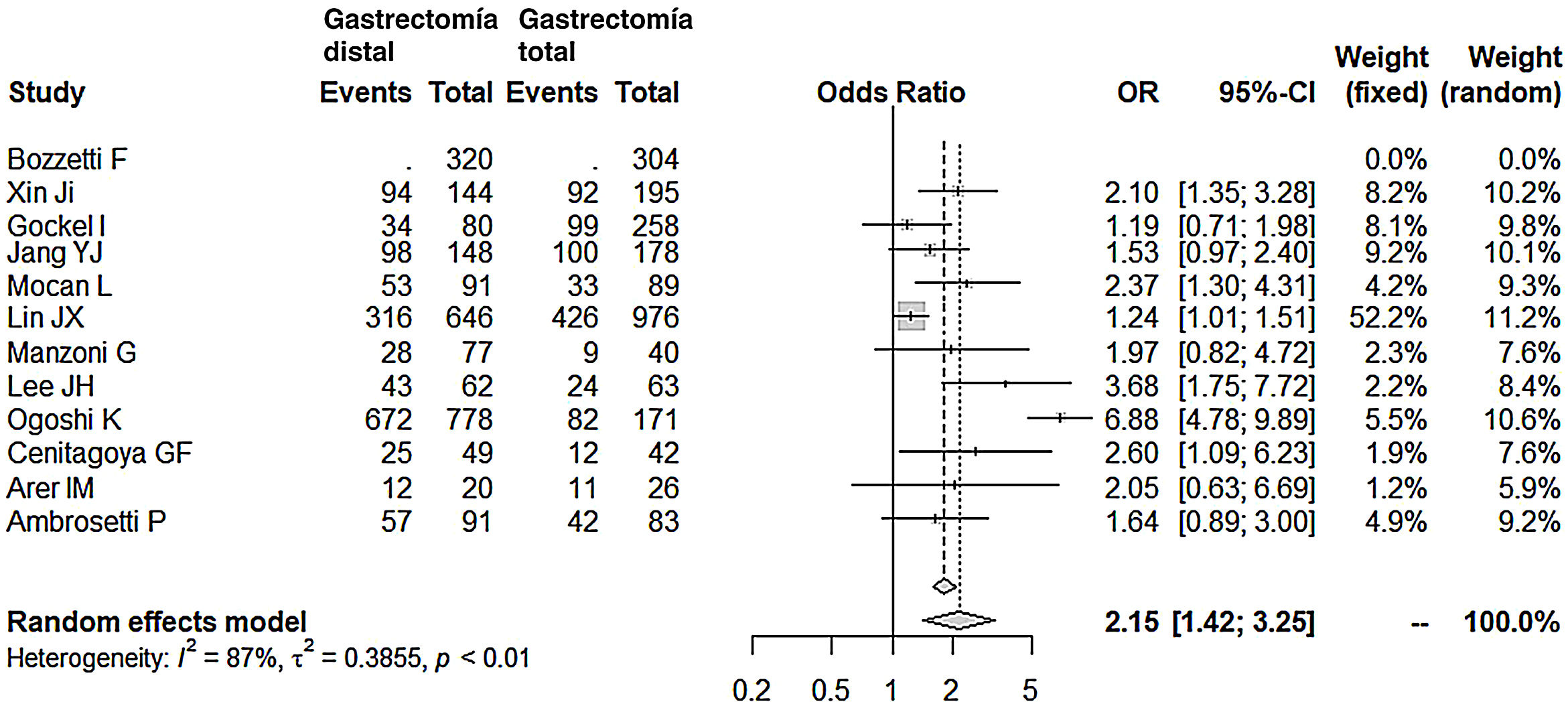
Lymphadenectomy in the DG group (range 15-40) obtained a lower number of nodes than the TG group (range 26-48), with 7 fewer nodes on average (OR: −7.07; 95% CI: [−9.54]-[−4.49]; I2: 93%) (Fig. 5). However, removal of a smaller number of nodes did not reduce the mean 5-year survival rate in this group. In fact, the patients who underwent DG had higher survival rates compared to the TG group (OR: 2.15; 95% CI: 1.42-3.25; I2: 87%) (Fig. 6).
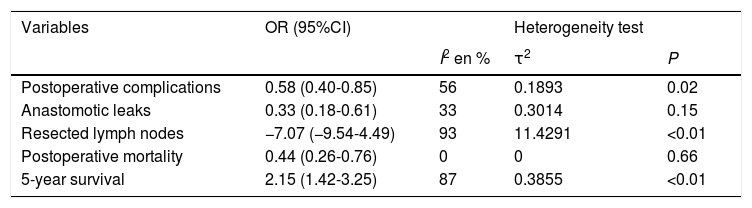
The summary of the comparative statistics between both groups is shown in Table 2.
Summary of the statistics obtained from the comparison between DG and TG.
| Variables | OR (95%CI) | Heterogeneity test | ||
|---|---|---|---|---|
| I2 en % | τ2 | P | ||
| Postoperative complications | 0.58 (0.40-0.85) | 56 | 0.1893 | 0.02 |
| Anastomotic leaks | 0.33 (0.18-0.61) | 33 | 0.3014 | 0.15 |
| Resected lymph nodes | −7.07 (−9.54-4.49) | 93 | 11.4291 | <0.01 |
| Postoperative mortality | 0.44 (0.26-0.76) | 0 | 0 | 0.66 |
| 5-year survival | 2.15 (1.42-3.25) | 87 | 0.3855 | <0.01 |
To date, and to our knowledge, no meta-analysis has been published by Western authors comparing which gastrectomy should be planned for middle-third and distal stomach cancer in terms of postoperative complications and mortality, without forgetting safety in oncological efficacy (5-year survival).
From a strictly surgical point of view, the type of gastrectomy for middle or distal stomach cancer is a reason for divergence of opinions, and our intention with this meta-analysis is to shine some light on this poorly defined panorama. There are surgeons who routinely perform TG because they understand that it does not lead to greater morbidity and mortality, and because they believe that patient survival will be longer.30 They also associate DG with a higher rate of recurrence and, therefore, reoperations. Other surgeons, on the other hand, are more familiar with DG, since TG would be associated with a significantly higher morbidity and mortality rate (close to double).31,32 According to the latter, the Japanese Gastric Cancer Association defines standard gastrectomy as that which has a curative purpose, which would imply a D2 lymphadenectomy and, at least, the resection of 2/3 parts of the stomach, provided a sufficient margin is achieved (3 cm in expansive growth tumors and 5 cm in infiltrative growth tumors).33
Characteristics of the studies includedA total of 2 randomized prospective studies, 3 non-randomized prospective and 10 retrospective studies have been included in this study, with a total of 6303 patients (3641 DG and 2662 TG).
In general, it has always been stated that undifferentiated or diffuse stomach cancer according to Lauren’s classification, regardless of location, should always be treated with a TG. Surprisingly, we found that in all the series included in this meta-analysis except one (the Gockel et al study)16 Lauren’s undifferentiated or diffuse cancers were not the reason for excluding DG. Even in some series, such as that by Lin et al,21 up to 84% of DG were in patients with undifferentiated or diffuse cancers. Although all series consider adequate surgical margin an inclusion criterion, only 5 series16,18,19,22,29 defined a margin ranging between 3 and 6 cm as valid.
Laparoscopic surgery for gastric cancer is gaining presence. The same results are obtained in terms of oncological radicality and survival, with better hospital stay parameters.34 However, they are complex techniques and, therefore, few groups incorporate them into their routine work in gastric cancer. It is not surprising that only 3 of the included studies carried out laparoscopic resections.17,20,21
Postoperative complicationsOur meta-analysis confirms that after DG the patient is 1.72 times less likely to present complications in the postoperative period. This data is not surprising when verifying that, in all series except one (Gouzi et al15), complications were lower in the DG group.
It should be noted that Gouzi et al (14% complications in DG and 12% complications in TG – almost similar) defined inclusion criteria for their very restrictive patients: they rejected patients with chronic kidney or heart failure, poorly controlled diabetes, arteritis, body weight greater than 20% of the mean weight adjusted for age and sex, and liver cirrhosis. In short, the inclusion of patients who are technically simpler (not obese) or have a greater functional reserve (without comorbidities) could justify a similar range of complications, even in more laborious and complex technical procedures such as TG (in this same series, although the datum is not collected, there is a comment that there was a greater number of necessary splenectomies in the TG group).
Anastomotic fistulaThe reviewed data from our meta-analysis reflect that the chances of having an anastomotic fistula are 3 times lower after DG. The higher probability of dehiscence after esophagojejunal anastomosis is not surprising, since it has always been argued that this anastomosis has a higher risk of dehiscence related to ischemia or tension in the anastomosis. When it occurs early, it is attributed to technical errors, especially due to the suturing of the esophageal wall around the stem. It can be prevented by adequate thoroughness in technical steps during surgery. A greater and better vascular supply of the remaining stomach compared to the terminal esophagus in the anastomosis is, according to Gouzi et al15 (incidence of anastomotic fistula close to 10%), the determining cause of the lower incidence of fistula in patients with DG. Ambrosetti et al,28 who reported an incidence of fistula close to 20% in the TG group, consider it essential that total gastrectomy be referred to a surgeon with a large number of cases per year, implying that fistulae are largely due to technical errors.
Postoperative mortalityPostoperative mortality in our meta-analysis was 2.27 times lower in the DG group. Among those selected, 2 series stand out in which mortality was clearly lower in the DG group.19,28 Both registered the highest rate of anastomotic fistulae in the TG group (9% and 19%). It is evident that mortality in these 2 series was directly related to the anastomotic fistula. However, in the series by Gouzi et al,15 despite a high incidence of anastomotic fistula in the TG group (close to 10%), mortality in both groups was similar (2.4%). The reason for this apparent disparity is that more than 50% of the fistulae were subclinical, diagnosed on radiological follow-up studies, and all were medically managed successfully, with no mortality. Indeed, an anastomotic fistula after a total gastrectomy does not always imply the death of the patient. In fact, in 4 series consulted,15,19,20,28 the number of deaths in the postoperative period of TG was significantly lower than the number of fistulae that occurred. This emphasizes the importance of early and multidisciplinary treatment of this complication to avoid the death of the patient.
Radical oncological surgery after TG has been related to higher mortality rates and is one of the reasons why European groups have discouraged extended D2 lymph node dissections, so often recommended among Japanese surgeons.33 Along this line, the series by Gockel et al,16 with a mortality of 10% in the TG group, showed that pancreatic fistula (8.8%) was the main cause of death, surpassing anastomotic fistula (3.8%). In their series, lymphadenectomy was routinely D2, and splenectomy and left pancreatectomy were performed in 63.7% and 3.7% of their patients, respectively.
Resected lymph nodesToday, most surgeons lean towards D2 lymph node dissection, albeit without the enthusiasm of Japanese surgeons, who are credited with the most extensive lymph node dissections. It is possible that this radicality is also greatly influenced by the phenotype of Japanese patients, who are less frequently obese and, therefore, easier from a technical standpoint. In patients who are overweight or technically more complex due to another cause, the minimum quality standard required to achieve correct staging of the tumor involves resecting no fewer than 15 nodes. In addition, the latest chemotherapy and radiotherapy regimens provide additional treatments to surgery, which complement and support these dissections that are perhaps insufficient in the number of lymph nodes, according to the criteria of the Japanese school. In the series consulted, the DG group had a significantly lower number of nodes (OR: −7.07; 95% CI: [−9.54]-[−4.49]; I2: 93%), although its range of lymph nodes (15-40) in the meta-analysis can be considered adequate and, in fact, we have seen that this has not affected the long-term survival of this group.
Long-term survival75% of the series consulted (8/12) in this meta-analysis did not show differences in long-term survival between the 2 groups, DG and TG. Indeed, almost all of them establish N stage,15,16,19,22,23,25–29 T stage,15,16,22,25 and TNM stage,18,19,24,26–28 as fundamental predictive factors for poor survival in the multivariate analysis, as well as, in only one series, the extent of lymphadenectomy18 or neoadjuvant therapy,29 without mentioning the type of gastrectomy performed. In none of the series was the Lauren diffuse type or undifferentiated adenocarcinoma shown to be a predictor for poor prognosis. Now, the final result of the meta-analysis, including the 4 remaining series, concludes that the 5-year survival is 2 times longer in the DG group.
We will analyze these 4 series individually. In the series by Ogoshi et al,25 5-year survival favors DG (86.4% vs. 48%), and they argue that the greater margin obtained after TG and even the greater number of resected lymph nodes would not be relevant to survival. On the contrary, they consider that preserving the duodenum, with the consequent passage of food through it, after DG (75% Billroth 1) would be associated with better immunological conditions, less weight loss and better regulation of gastrointestinal hormones, all of which are parameters associated with improved quality of life and greater survival. In the series by Lee et al,24 the differences in favor of DG were also broad (69% vs. 38%), although with no statistical significance after the multivariate analysis. However, they also insisted on the better nutritional quality of life of these patients and therefore recommended DG, provided that the margin was adequate. Cenitagoya et al26 claim that the only reason to explain the better survival in DG (51% vs. 29%) is the location of the tumor. They merely describe how tumors of the middle third have a worse prognosis, although without relating this location to the other 2 variables, which were only significant in their series of poor prognosis after the multivariate analysis: lymph node involvement and TNM stage. Lastly, the survival rate, which was widely favorable for DG in the Mocan series19 (58% vs. 37%), was only significant in stage IB of the TNM classification. In the multivariate analysis of the global series, the type of gastrectomy was not significant, in favor of lymph node involvement and TNM stage.
Limitations of the meta-analysisAlthough this meta-analysis has been carried out following guidelines for quality, we found a series of limitations.
First, only 2 of the studies are randomized controlled clinical trials and, interestingly, they are the oldest 2 included in the meta-analysis. Furthermore, only 3 have been published in the last 5 years, so the impact on survival of neoadjuvant treatment has been analyzed in a single series. Second, the studies have been carried out in hospitals in countries such as Italy, Germany, Korea or China, but there are many other countries that have not been included in the study. Additionally, only articles written in English, French and Spanish were included, which may have left out articles on this topic that did not meet the language criterion. Third, the sample size of 15 series is small and, furthermore, 5 of them did not include morbidity, surgical mortality or 5-year survival among their study variables. For this reason, we feel that more controlled and randomized clinical trials with larger patient samples will be necessary in the future to determine the advantages or disadvantages that DG and TG may present in the treatment of distal stomach cancer.
ConclusionsOur meta-analysis concludes that DG is the ideal technique in middle-third and distal stomach cancer, regardless of whether it is undifferentiated or diffuse according to Lauren’s classification. Provided a sufficient margin can be obtained, DG is associated with lower postoperative morbidity and mortality rates. Although fewer lymph nodes are removed, the quality standard of 15 nodes is reached in the lymph node dissection, which is even associated with a longer 5-year survival rate. Unfortunately, the limited number of prospective randomized studies in this meta-analysis detracts from its results and, therefore, these conclusions must be considered with caution.
FundingThis study has received no specific funding from public, commercial or non-profit sources.
Conflict of interestNone.
Please cite this article as: Durán Giménez-Rico H, Diéguez Aguirre L, Ríos Pérez L, Cardinal-Fernández P, Caruso R, Ferri V, et al. Estudio comparativo entre la gastrectomía total y subtotal en el cáncer distal de estómago: metaanálisis de estudios prospectivos y retrospectivos. Cir Esp. 2020;98:582–590.