The BRAF V600E mutation in papillary thyroid cancer (PTC) has been associated with resistance to 131I. Our aim was to quantify the response to 131I after surgery in patients who had the mutation (BRAF+) and those who did not have the mutated gene (BRAF−).
MethodA prospective cohort study was designed, from September 2015 to February 2016, which included patients with PTC receiving therapy after surgical treatment. Variables were described for age, gender, histology, tumor stage, thyroglobulin values before, 48h after and 6 months after 131I; absorbed dose and % activity on days 2 and 7 and elimination time.
Results41 patients giving in total 67 thyroid remnants were included. 61% were BRAF+. In stages III and IV, 80% were BRAF+. In lateral resection, 100% were BRAF+. The number of nodes was higher in BRAF+: 3.4 vs 1.2 (P=.01). The classic variant was predominant in BRAF+ (91.7% vs 8.3%, P=.03). 85.7% vs 14.3% of BRAF+ had desmoplastic reaction (P=.02). The BRAF+ had a lower absorbed dose than the administered activity (5.4Gy/MBq vs 20Gy/MBq, P=.02); lower% activity with respect to the unit of mass at 2 (0.046%/g vs 0.103%/g, P=.02) and at 7 days (0.006%/gr vs 0.034%/gr, P=.04).
ConclusionsThe mutation of the BRAF V600E gene is related with greater resistance to postoperative treatment with 131I since the onset of the disease.
La mutación BRAF V600E en el cáncer papilar de tiroides (CPT) parece asociarse a una resistencia al 131I. Nuestro principal objetivo fue cuantificar la respuesta al 131I tras la cirugía tanto en pacientes que presentaban la mutación (BRAF+) como en los que no presentaban el gen mutado (BRAF−).
MétodoEstudio prospectivo de los CPT intervenidos y tratados con 131I desde septiembre de 2015 hasta enero de 2017. Variables: edad, género, estadio tumoral, histológicas, tiroglobulina antes de 131I, a las 48h y a los 6meses; dosis absorbida y % de actividad a los 2 y a los 7días y tiempo de eliminación.
ResultadosCuarenta y un pacientes y 67 restos tiroideos. El 61% eran BRAF+. En los estadios III y IV, el 80% eran BRAF+. En el vaciamiento ganglionar terapéutico, el 100% eran BRAF+. El número de ganglios fue superior en BRAF+: 3,4 vs 1,2 (p=0,01). La variante clásica fue predominante en BRAF+ (91,7% vs 8,3%; p=0,03). El 85,7% vs 14,3% de los BRAF+ tuvieron reacción desmoplásica (p=0,02). Los BRAF+ presentaban menor dosis absorbida respecto a la actividad administrada (5,4 vs 20Gy/MBq; p=0,02); menor % de actividad respecto a la unidad de masa a los 2 (0,046 vs 0,103%/g; p=0,02) y a los 7días (0,006 vs 0,034%/g, p=0,04).
ConclusionesLa mutación del gen BRAF V600E se relaciona con una mayor resistencia al tratamiento posquirúrgico con 131I desde el inicio de la enfermedad.
The mutation of the BRAF V600E gene is the most frequent genetic alteration in papillary thyroid cancer (PTC) (from 23 to 83%).1,2 The role of this mutation as a prognostic marker in the staging of molecular risk for thyroid cancer is promising.3 In addition, it has been associated with more aggressive clinical–pathological characteristics,4 increased recurrence,5 resistance to 131I in metastatic disease6 and even mortality.7 However, in their latest update, the American Thyroid Association (ATA),8 while recognizing its role as a marker for the risk of recurrence, still does not recommend the routine study of the mutational state of the tumor.
The mutation involves the substitution of valine for glutamate in position 600 of the B-raf protein. It has been associated with the reduced uptake of 131I, due in part to the activation of the protein kinase signaling pathway activated by mitogens (MAPK).9 This signaling pathway, in which the oncogene BRAF takes part, with others such as RET/PTC and RAS, is essential for the homeostasis of thyroid cells. The constitutive activation of the MAPK pathway through these oncoproteins induces abnormal growth and resistance to proapoptotic signals.10
The molecular bases that explain the relationships between the various components, although complex and controversial, are of great clinical relevance because of their direct relationship with both the dedifferentiation of thyroid tumors and resistance to 131I therapy. This resistance is mediated fundamentally by BRAF by stimulating the induction of tumor growth factor (TGF-B) secretion, which represses the expression of the sodium/iodide symporter (NIS) that is necessary for the uptake of iodine in the cell, in addition to promoting changes necessary for migration and cellular invasion.11,12 This loss of expression is the basis of refractoriness to treatment with 131I. As a consequence, mutated PTC show a decrease in avidity for 131I,13 so it would be reasonable to think that, for this group of patients, more aggressive surgery would be the best therapeutic option.14
In this context, most studies measure this resistance to treatment with 131I in the tumor recurrence phase or in metastatic cancers, not at the beginning of postoperative therapy. The assessment of this response is obtained exclusively with indirect measurement of thyroglobulin serum values, and there are no studies in the literature that quantify the effect of the mutation in the images obtained by CT or SPECT-CT. However, there are other methods to estimate the quantification of the response to treatment, such as dosimetry. Through this methodology, important variables like absorbed dose can be obtained, a parameter closely related to cell destruction and, therefore, to the effect of treatment.15 A more detailed measurement of the absorption of 131I and the behavior of the BRAF V600E mutation after postoperative treatment with the radiopharmaceutical can definitively establish the prognostic value of the mutation and, therefore, a modification of the therapeutic strategy.
The objectives of this study are: (1) to describe the characteristics of patients with PTC according to their mutational status; (2) to quantify the response of 131I administered after surgery; and (3) to obtain a predictive model of risk stratification that defines patients with a higher risk of resistance to 131I.
MethodsWe designed a prospective analytical observational cohort study. The study population consisted of patients histologically diagnosed with papillary thyroid carcinoma treated with surgery and postoperative 131I from September 2015 to January 2017.
The inclusion criteria were: (1) histological diagnosis of PTC; (2) surgical treatment with total thyroidectomy performed by the Endocrine Surgery Division of the Basurto University Hospital, and (3) postoperative 131I treatment. The exclusion criteria were: (1) incidental cancer; (2) relapse of PTC; (3) having previously received therapy with I131, and (4) having had surgery at another medical center or by another surgical team.
Prior to surgery, the BRAF V600E mutation status was analyzed by immunohistochemistry analysis of the sample obtained by core needle biopsy, following the unit protocol.16 The surgical technique involved complete resection of the thyroid gland (with no macroscopic preservation of thyroid remnants) and/or prophylactic central lymph node dissection or therapeutic dissection of the affected compartments. In addition, in patients with preoperative ultrasound staging of N0, the sentinel lymph node technique was carried out according to the protocol of another study that was being carried out in the unit.
The following variables were studied: (1) demographics (gender, age); (2) surgical technique; (3) tumor staging after the study of the surgical piece; and (4) pathological features: (a) number of affected lymph nodes; (b) histological type (classical, follicular or mixed); (c) desmoplastic reaction; (d) bilateral disease; (c) extrathyroid extension; and (d) multiple foci.
Based on these obtained variables, patients were classified according to the risk of recurrence as low, intermediate or high risk, following the recommendations of the 2009 ATA guidelines in place the year of the beginning of the study,17 so that they were administered an oral dose of 30, 100 or 150mCi of 131I after stimulation of recombinant TSH.
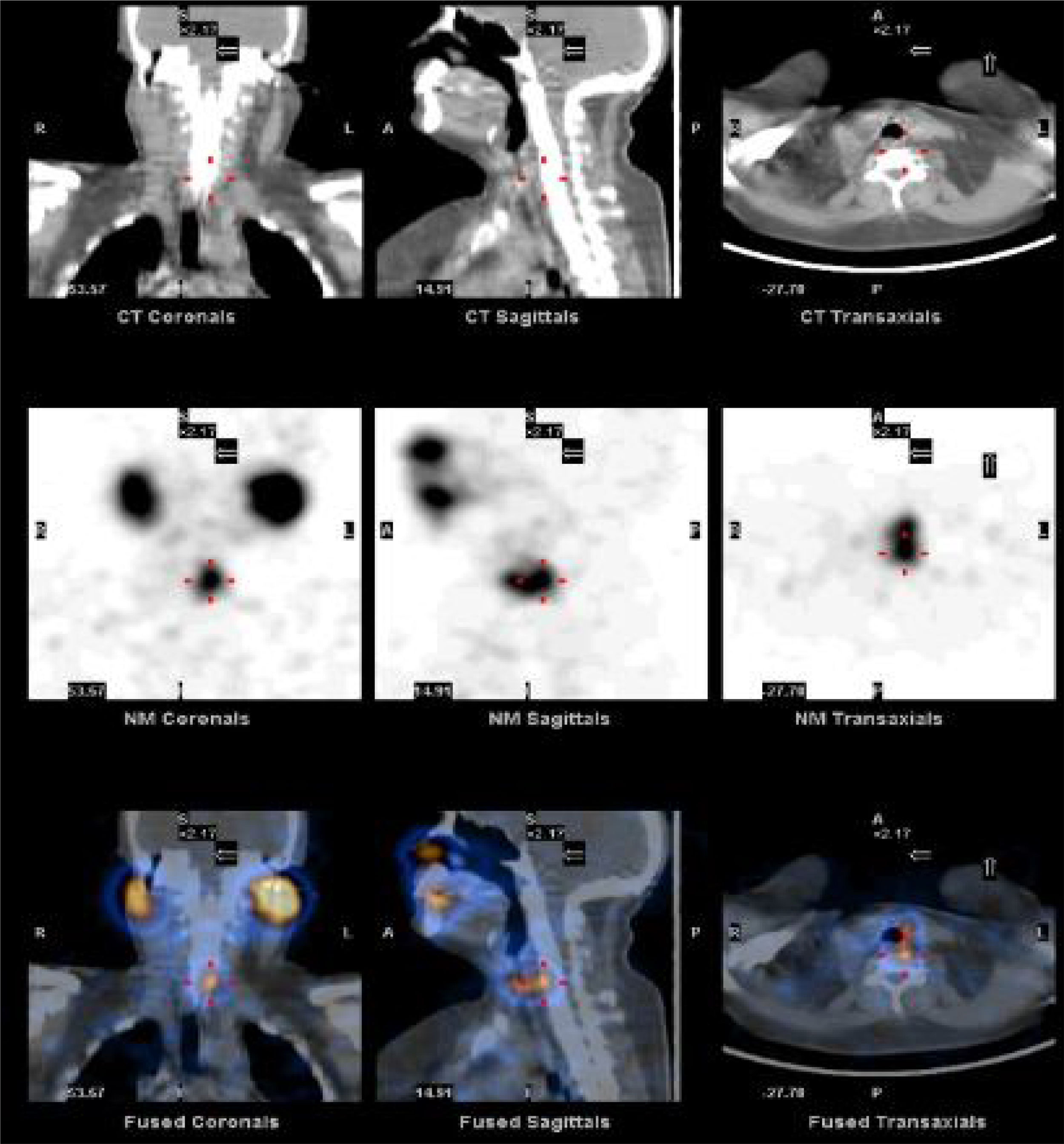
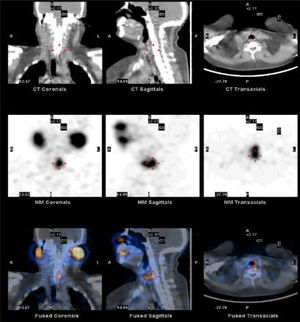
To quantify the response of 131I administered after surgery, we studied the SPECT-CT images of postoperative thyroid remains from each patient both 48h and 7 days after 131I treatment. After 2 days, the uptake of the remnants in the surgical bed had values close to their maximum value, while after 7 days the activity of 131I was considerably smaller and insignificant in places other than the thyroid bed. The analysis of the SPECT-CT images in the work station involved the identification of the thyroid remains and the differentiation of the physiological accumulation of the radiopharmaceutical in other anatomical areas, as shown in Fig. 1. For the quantification of the activity of the thyroid remains, previously a calibration factor was applied, according to recently published recommendations.18 Using dosimetry, the following variables were analyzed: (1) absorbed dose vs administered dose; (2) percentage of activity at 2 and 7 days in terms of the unit of mass; and (3) 131I elimination time. The calculation of the values according to the dose administered in the first measurement was made based on the mass calculated in the activity measurements after 2 and 7 days; this eliminated the possible influence of the different doses administered or volumes of thyroid remains obtained. To this end, the formalisms obtained for a medical dose of internal radiation were used.19
The serum values of thyroglobulin and anti-thyroglobulin antibodies were measured: (1) prior to the administration of 131I; (2) 48h after treatment; and (3) 6 months after.
Statistical AnalysisFrequencies and percentages were used for the descriptive analysis of the qualitative variables, and means and standard deviation (SD) were used for the quantitative variables.
Patient characteristics were compared between BRAF+ and BRAF− patients. The qualitative variables were compared with the chi-squared or Fisher's exact tests, while the quantitative variables were compared with t-test or the nonparametric Wilcoxon test.
Furthermore, iodine biokinetics were studied in the thyroid remnants using the nonparametric Wilcoxon test. In addition, within each group (BRAF+ and BRAF−), the change in thyroglobulin from before the administration of 131I to 48h afterwards was analyzed by the non-parametric Wilcoxon signed rank test for paired data.
In the end, we analyzed the possible association of the different characteristics with the absorbed dose regarding the activity administered by the general linear model. First, bivariate analyses were performed to identify the variables that individually were found to have a significant association with the absorbed dose. The multivariate analysis was then carried out to observe the joint influence of these variables, considering independent variables to be those with a P value <.15 in the bivariate analyses. In the final models, only the variables with P<.05 were considered. Due to the non-normality of the absorbed dose variable, a logarithmic transformation was carried out. Therefore, for the interpretation of the results, the exponential of the beta parameters was considered.
For all the analyses, a P<.05 was considered a statistically significant result. The analyses were performed with the SAS for Windows Statistical Software, version 9.2 (SAS Institute, Inc., Carey, NC).
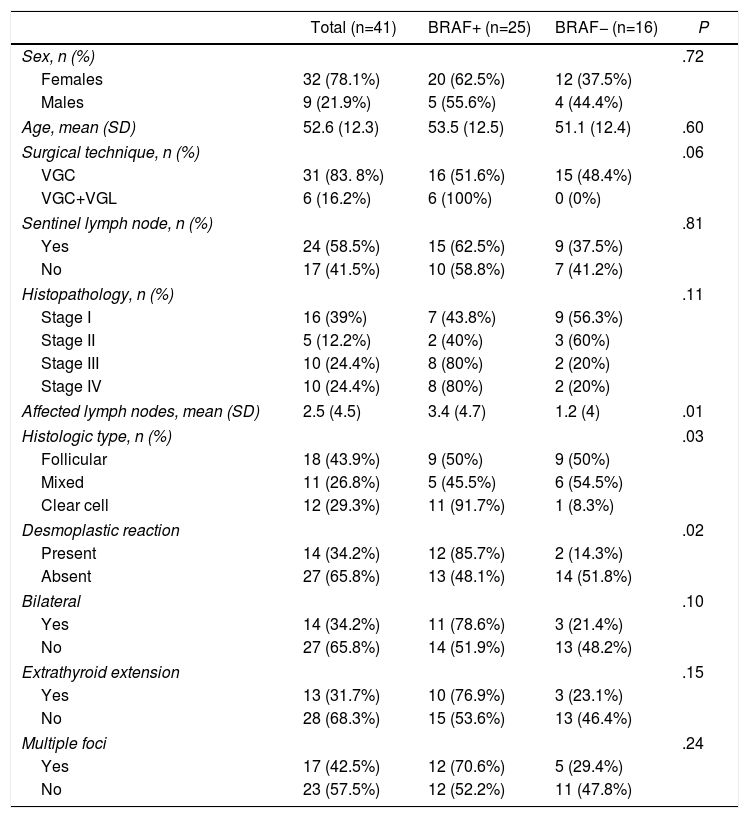
ResultsCharacteristics of the Patients With Papillary Thyroid Cancer According to Mutational StateThe epidemiological and pathological results as well as the surgical technique are presented in Table 1. Out of the 41 patients treated surgically for PTC who were administered 131I ablation between September 2015 and January 2017, 25 (61%) were positive for the mutation. The frequency of the mutation in women was somewhat higher than in men: 62.5% vs 55.5%, P=.72 (non-significant). Mean patient age was 52.6 (SD 12.3).
Patient Characteristics (n=41).
| Total (n=41) | BRAF+ (n=25) | BRAF− (n=16) | P | |
|---|---|---|---|---|
| Sex, n (%) | .72 | |||
| Females | 32 (78.1%) | 20 (62.5%) | 12 (37.5%) | |
| Males | 9 (21.9%) | 5 (55.6%) | 4 (44.4%) | |
| Age, mean (SD) | 52.6 (12.3) | 53.5 (12.5) | 51.1 (12.4) | .60 |
| Surgical technique, n (%) | .06 | |||
| VGC | 31 (83. 8%) | 16 (51.6%) | 15 (48.4%) | |
| VGC+VGL | 6 (16.2%) | 6 (100%) | 0 (0%) | |
| Sentinel lymph node, n (%) | .81 | |||
| Yes | 24 (58.5%) | 15 (62.5%) | 9 (37.5%) | |
| No | 17 (41.5%) | 10 (58.8%) | 7 (41.2%) | |
| Histopathology, n (%) | .11 | |||
| Stage I | 16 (39%) | 7 (43.8%) | 9 (56.3%) | |
| Stage II | 5 (12.2%) | 2 (40%) | 3 (60%) | |
| Stage III | 10 (24.4%) | 8 (80%) | 2 (20%) | |
| Stage IV | 10 (24.4%) | 8 (80%) | 2 (20%) | |
| Affected lymph nodes, mean (SD) | 2.5 (4.5) | 3.4 (4.7) | 1.2 (4) | .01 |
| Histologic type, n (%) | .03 | |||
| Follicular | 18 (43.9%) | 9 (50%) | 9 (50%) | |
| Mixed | 11 (26.8%) | 5 (45.5%) | 6 (54.5%) | |
| Clear cell | 12 (29.3%) | 11 (91.7%) | 1 (8.3%) | |
| Desmoplastic reaction | .02 | |||
| Present | 14 (34.2%) | 12 (85.7%) | 2 (14.3%) | |
| Absent | 27 (65.8%) | 13 (48.1%) | 14 (51.8%) | |
| Bilateral | .10 | |||
| Yes | 14 (34.2%) | 11 (78.6%) | 3 (21.4%) | |
| No | 27 (65.8%) | 14 (51.9%) | 13 (48.2%) | |
| Extrathyroid extension | .15 | |||
| Yes | 13 (31.7%) | 10 (76.9%) | 3 (23.1%) | |
| No | 28 (68.3%) | 15 (53.6%) | 13 (46.4%) | |
| Multiple foci | .24 | |||
| Yes | 17 (42.5%) | 12 (70.6%) | 5 (29.4%) | |
| No | 23 (57.5%) | 12 (52.2%) | 11 (47.8%) | |
There was a total of 31 (75.6%) patients with bilateral prophylactic central lymph node dissection. Of these, 16 (51.6%) were BRAF+. In 6 cases (16.2%), therapeutic lymph node dissection was performed due to lymph node metastasis, and 100% were BRAF+.
A total of 15 patients (62.5%) who presented the mutation underwent the sentinel lymph node technique. In contrast, the other 10 patients with the mutation (58.8%) did not have this procedure. These differences were not statistically significant (P=1.0).
The number of affected lymph nodes was significantly higher in the BRAF+ patients, at 3.4 (SD 4.7) vs 1.2 (SD 3.9) (P=.01). The mutation was higher among patients with the classic type, with a total of 11 cases (91.7%), compared to follicular with 9 patients (50%), or mixed with 5 (45.4%) (P=.03). In the BRAF+ group, 12 patients (85.7%) had a desmoplastic reaction, while 13 (48.1%) did not present this characteristic (P=.02). There were no differences between the two groups in terms of extrathyroid extension, bilaterality or the presence of more than one carcinoma site.
Regarding the tumor stage after the pathology analysis of the surgical piece, 80% of the patients who were in a more advanced stage (III and IV) had the mutated gene. More than half (57.1%) of the patients who were in the earliest stages (I and II) did not have the mutation.
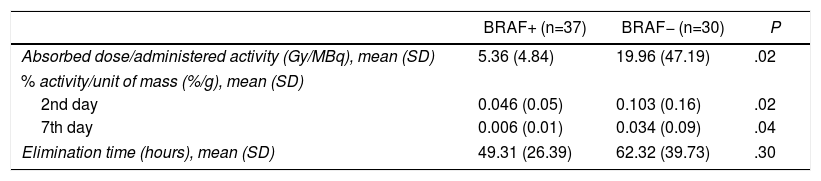
Quantification of the Postoperative Response to 131IAs for the effect of the mutation on the 131I treatment, iodine biokinetics were studied in a total of 67 thyroid remnants because, even though the majority of patients had a single thyroid remnant, there were cases of 2 and even 3 remnants in the same patient. The BRAF+ patients had a lower absorbed dose than the administered activity (5.4 vs 20Gy/MBq; P=.02); lower % of activity compared to the unit of mass after 2 days (0.046 vs 0.103%/g; P=.02) and 7 days (0.006 vs 0.034%/g; P=.04). Although there were no significant differences, a faster elimination time was observed in the patients with the mutation (49.3 vs 62.3h; P=.30) (Table 2).
Quantification of the Postoperative Response to 131I (n=67).
| BRAF+ (n=37) | BRAF− (n=30) | P | |
|---|---|---|---|
| Absorbed dose/administered activity (Gy/MBq), mean (SD) | 5.36 (4.84) | 19.96 (47.19) | .02 |
| % activity/unit of mass (%/g), mean (SD) | |||
| 2nd day | 0.046 (0.05) | 0.103 (0.16) | .02 |
| 7th day | 0.006 (0.01) | 0.034 (0.09) | .04 |
| Elimination time (hours), mean (SD) | 49.31 (26.39) | 62.32 (39.73) | .30 |
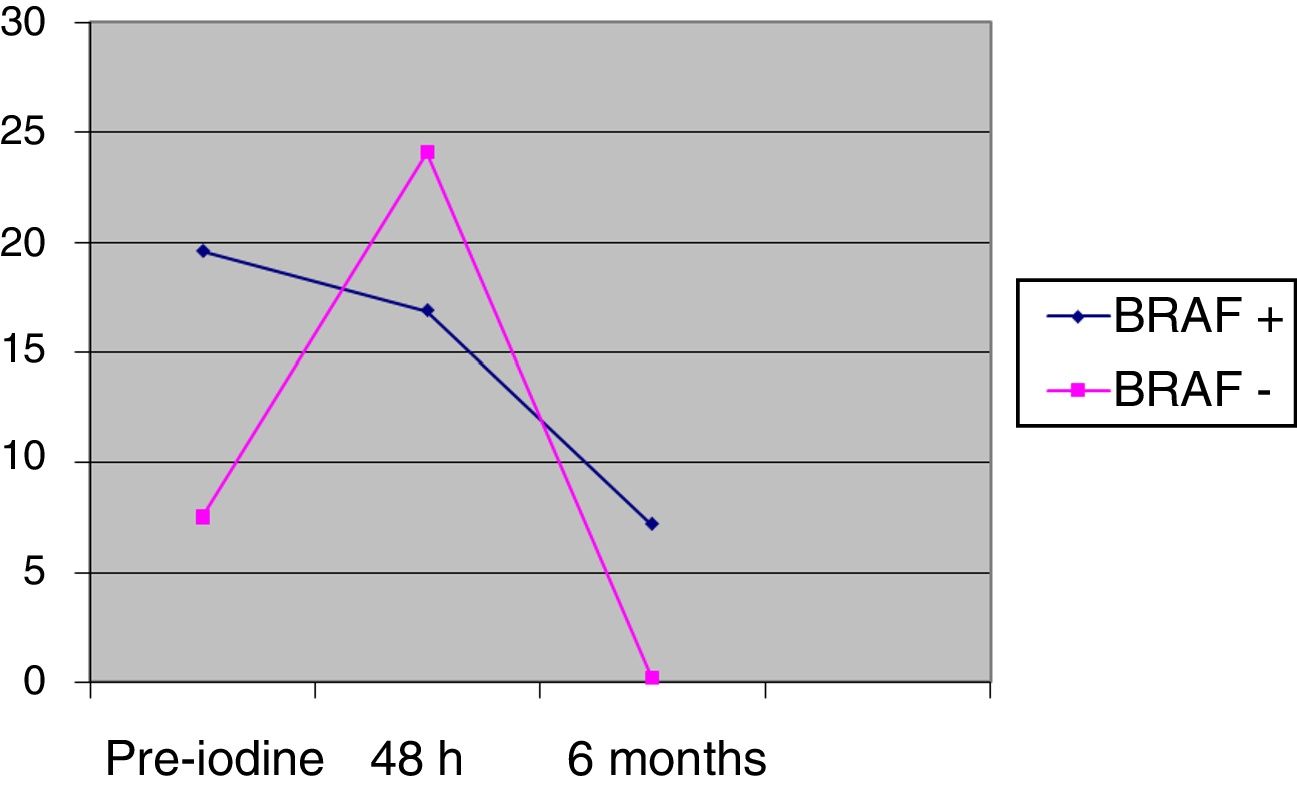
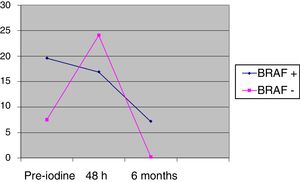
The differences between BRAF+ and BRAF− in thyroglobulin values prior to iodine (pre-iodine) were 20.2 (SD 85.7) vs 7.6 (SD 17.9), and after 48h 17.6 (SD 69.4) vs 24.1 (SD 65.6), without being significant. The change of the pre-iodine after 48h with regards to the baseline value is significant in the BRAF− group (P=.0023), whereas the change from the pre-iodine after 48h is not statistically significant in the BRAF+ group (P=.0560), as shown in Fig. 2. After 6 months of follow-up, there were no significant differences between the two groups: 7.2 (SD 28.2) vs 0.2 (SD 0.3); (P=.74).
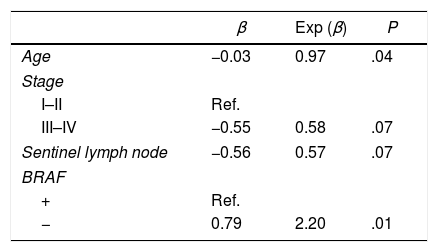
Predictive Model for Patients With a Greater Risk of Resistance to 131ITable 3 shows the results from the bivariate analyses of the association of different characteristics on the dose absorbed, showing only those characteristics with a P<.15. The variables that were found to be significantly associated with the absorbed dose were age and BRAF mutation. In the case of age, for each one-year increase, the absorbed dose was 0.97 times greater (P=.04). As for the mutation, the dose absorbed in the BRAF− was 2.20 times that of BRAF+ patients (P=.01). In the multivariate analysis, only the mutation variable was significant.
DiscussionThe BRAF V600E mutation of the PTC is the most frequent genetic alteration (between 23 and 83%),1,2,20 which coincides with our results. If we take into account the patients undergoing therapeutic lymph node dissection, 100% of the patients were BRAF+, evidence of the aggressive nature of the mutation, as demonstrated in several studies.7,21,22 Likewise, both the more advanced stage and the greater number of affected lymph nodes in patients with the present mutation further reinforce the concept of worse prognosis when the mutation is present, as recently published.5 However, no differences have been found between other risk factors for recurrence, such as extrathyroid extension, multiple foci or bilateral involvement, probably due to the size of the sample or histopathological characteristics, which have correlated with the mutation in other studies.14 Among the patients who presented the mutation, the most frequently associated histopathologic type was classic, data similar to those published after the identification of the genome sequencing for PTC.23 The presence of desmoplastic reaction was also more frequent, as published in some studies.22
Regarding the quantification of postoperative 131I, this is the first study to evaluate the response of the mutation to treatment with the radiopharmaceutical in a quantitative manner by calculating parameters related to iodine biokinetics, with the dosimetry method already described in previous studies.24 The absorbed 131I dose levels were significantly lower in patients who presented the mutation, which correlates with experimental studies about the decrease in iodine uptake in cells due to the blockade of the symporter secondary to the effect of the mutated gene.9,13 Likewise, the percentage of radioiodine activity after 2 and 7 days was lower in BRAF+ patients. A shorter 131I half-life was observed in patients with the mutated gene, although without reaching statistical significance; therefore, not only has lower 131I uptake been shown in patients with the mutation, but 131I was also eliminated more quickly in these patients.
In the absence of a method that quantifies and evaluates the effect of postoperative 131I treatment that is compatible with the recommendations established in the initial treatment guidelines for PTC,8 we believe that dosimetry can be an alternative to be considered.
With regards to serum Tg values, an increase was observed 48h after the administration of 131I in patients who did not present the mutation; however, this peak concentration was not observed in patients with the mutated gene. This increase after ablation with 131I has been identified in the literature25 related with increased destruction of the thyroid remnant and the consequent release of said protein. In this context, the identification of a lower concentration of serum Tg in patients with the mutated gene after treatment with the radiopharmaceutical is interpreted as less cellular destruction, thereby supporting the theory of increased resistance to 131I. However, in the results at the 6th month follow-up, this difference was not observed, so it is necessary to evaluate longer-term results with a greater number of patients to define the clinical consequences.
We observed that the only variable that was significant in the multivariate analysis was the mutation of the BRAF gene, which, therefore, is a good prognostic marker to consider in order to define the most appropriate treatment. However, it was not possible to establish a predictive model to determine the profile of the patients with greater resistance to 131I therapy, probably because of the sample size. In contrast, what was identified were the patient characteristics that define a group with greater risk of resistance to 131I: age and BRAF mutation. In the case of age, as patients become older, 131I absorption increases. This fact, in addition to being novel, may be of interest for future studies that measure the actual benefit of the radiopharmaceutical in the older age group, since an increase in mortality in elderly patients and BRAF+ has recently been described.26
We are aware of the limitations of this study. One of them, mentioned above, is the sample size. In addition, the study is based on thyroid remnants, which may be remnants of both tumor and normal thyroid tissue, and therefore the direct nature of the tumor process is not represented. The results obtained suggest that the totality of the gland, through different signaling pathways that we still do not know of, may be influenced by the process of tumor carcinogenesis. However, more longer-term studies are needed with larger sample sizes to confirm these results.
In conclusion, (1) the mutation of the BRAF V600E gene is related to factors for a poor prognosis, such as a more advanced stage or greater lymph node involvement; (2) BRAF+ patients show that treatment with 131I is less efficient since the onset of the disease; and (3) the absorption of 131I is higher in older patients.
Conflict of InterestsThe authors have no conflicts of interests to declare.
Please cite this article as: Domínguez Ayala M, Expósito Rodríguez A, Bilbao González A, Mínguez Gabiña P, Gutiérrez Rodríguez T, Rodeño Ortiz de Zarate E, et al. Mutación del gen BRAF V600E en el cáncer papilar de tiroides y su efecto en la terapia con yodo radiactivo (131I) posquirúrgica: ¿deberíamos modificar nuestra estrategia terapéutica? Cir Esp. 2018;96:276–282.
Part of the results from this study were presented at the 29th Annual Congress of the European Association of Nuclear Medicine (EANM) 2016, held in Barcelona, 15–19 October, 2016; at the 6th Meeting of the Iberian Peninsula of the Endocrine Surgery Division held in Seville, 27–28 April, 2017, and the 21st National Conference on Surgery held in Malaga, 18–20 October, 2017.