Several clinical studies analyze axillary treatment in women with early-stage breast cancer because of changes in the indication for axillary lymph node dissection. The aim of the study is to analyze the impact of axillary radiotherapy in disease-free and overall survival in women with early breast cancer treated with lumpectomy.
MethodsRetrospective study in women with initial stages of breast carcinoma treated by lumpectomy. A comparative analysis of high-risk women with axillary lymph node involvement who received axillary radiotherapy with the group of women with low risk without radiotherapy was performed. Logistic regression was used to determine factors influencing survival and lymphedema onset.
ResultsA total of 541 women were included in the study: 384 patients (71%) without axillary lymph node involvement and 157 women (29%) with 1-3 axillary lymph node involvement. Patients with axillary radiotherapy had a higher number of metastatic lymph node compared to non-irradiated (1.6±0.7 vs 1.4±0.6, P=.02). The group of women with axillary lymph node involvement and radiotherapy showed an overall and disease-free survival at 10 years similar to that obtained in patients without irradiation (89.7% and 77.2%, respectively). 3 lymph nodes involved multiplied by more than 7 times the risk of death (HR=7.20; 95% CI: 1.36 to 38.12). The multivariate analysis showed axillary lymph node dissection as the only variable associated with the development of lymphedema.
ConclusionThe incidence of axillary relapse on stage I and II breast cancer is rare. In these patients axillary radiotherapy does not improve overall survival, but contributes to regional control in those patients with risk factors.
Diversos estudios clínicos analizan el tratamiento axilar en el cáncer de mama temprano debido a los cambios actuales en la indicación de la linfadenectomía axilar. El objetivo de este estudio fue analizar el impacto de la radioterapia axilar en la supervivencia global y libre de enfermedad en mujeres con un carcinoma de mama en estadio inicial tratadas mediante cirugía conservadora.
MétodosEstudio retrospectivo en mujeres con un carcinoma infiltrante de mama en estadios iniciales tratadas mediante cirugía conservadora. Análisis comparativo de las mujeres con afectación ganglionar y factores de riesgo asociados que recibieron radioterapia axilar frente a un grupo con afectación ganglionar de bajo riesgo sin tratamiento radioterápico. Se utilizó una regresión logística para determinar los factores que influían en la supervivencia y en la aparición de linfedema.
ResultadosSe incluyó a 541 mujeres, 384 (71%) sin afectación de ganglios linfáticos axilares y 157 (29%) con afectación de 1-3 ganglios axilares. Las pacientes con radioterapia axilar tenían un mayor número de ganglios metastásicos respecto a las no irradiadas (1,6±0,7 vs. 1,4±0,6; p=0,02). El grupo de mujeres con afectación ganglionar y radioterapia axilar tuvo una supervivencia global y libre de enfermedad a los 10 años similar a las pacientes sin irradiación de la axila (89,7 y 77,2%, respectivamente). La afectación de 3 ganglios incrementó 7 veces el riesgo de fallecer (HR=7,20; IC 95%: 1,36-38,12). En el estudio multivariante, la linfadenectomía axilar fue el único factor de riesgo independiente de aparición de linfedema (HR=22,22; IC 95%: 4,71-105,59; p<0,001).
ConclusiónLa recidiva axilar en el cáncer de mama en estadios I y II es un evento poco frecuente. En las enfermas con afectación axilar y factores de riesgo asociados, la radioterapia regional contribuye al control locorregional de la enfermedad con igual supervivencia global.
Breast-conserving surgery in breast cancer is based on the use of radiotherapy as adjuvant therapy to surgery. Several studies have demonstrated that local breast resection in association with radiation guarantees a survival rate similar to mastectomy,1–3 although the actual repercussion of its application in the axilla is unknown. Radiation of the thoracic wall and axilla in female patients after mastectomy has demonstrated a benefit in disease-free periods, especially in women with more than 3 affected lymph nodes.4,5 Currently, there is controversy about the indication for axillary radiotherapy in patients treated with breast-conserving surgery and involvement of 1–3 axillary lymph nodes (N1) due to 2 circumstances. First of all is the change in the indication for axillary lymph node dissection (ALND) in women with metastatic involvement of the sentinel lymph node after the publication of clinical trial ACOSOG Z0011.6 This circumstance has generated a group of N1 patients without ALND who receive breast radiotherapy, whose tangential fields include axillary level Iand in whom there is debate about whether radiotherapy is needed at all axillary levels. The second is the publication of the Canadian study MA.20,7 which demonstrated a reduction of axillary recurrences in patients treated with lumpectomy and axillary radiotherapy, without any repercussions in overall survival. Although international clinical guidelines8 accept the criteria of trial Z0011 in breast-conserving surgery, there continues to be a controversy of whether this patient group's treatment should be complemented with axillary radiotherapy.
The objective of this study was to analyze the impact of axillary radiotherapy on overall and disease-free survival of women with initial-stage breast cancer treated with breast-conserving surgery. Likewise, potential risk factors for lymphedema were analyzed in this group of patients.
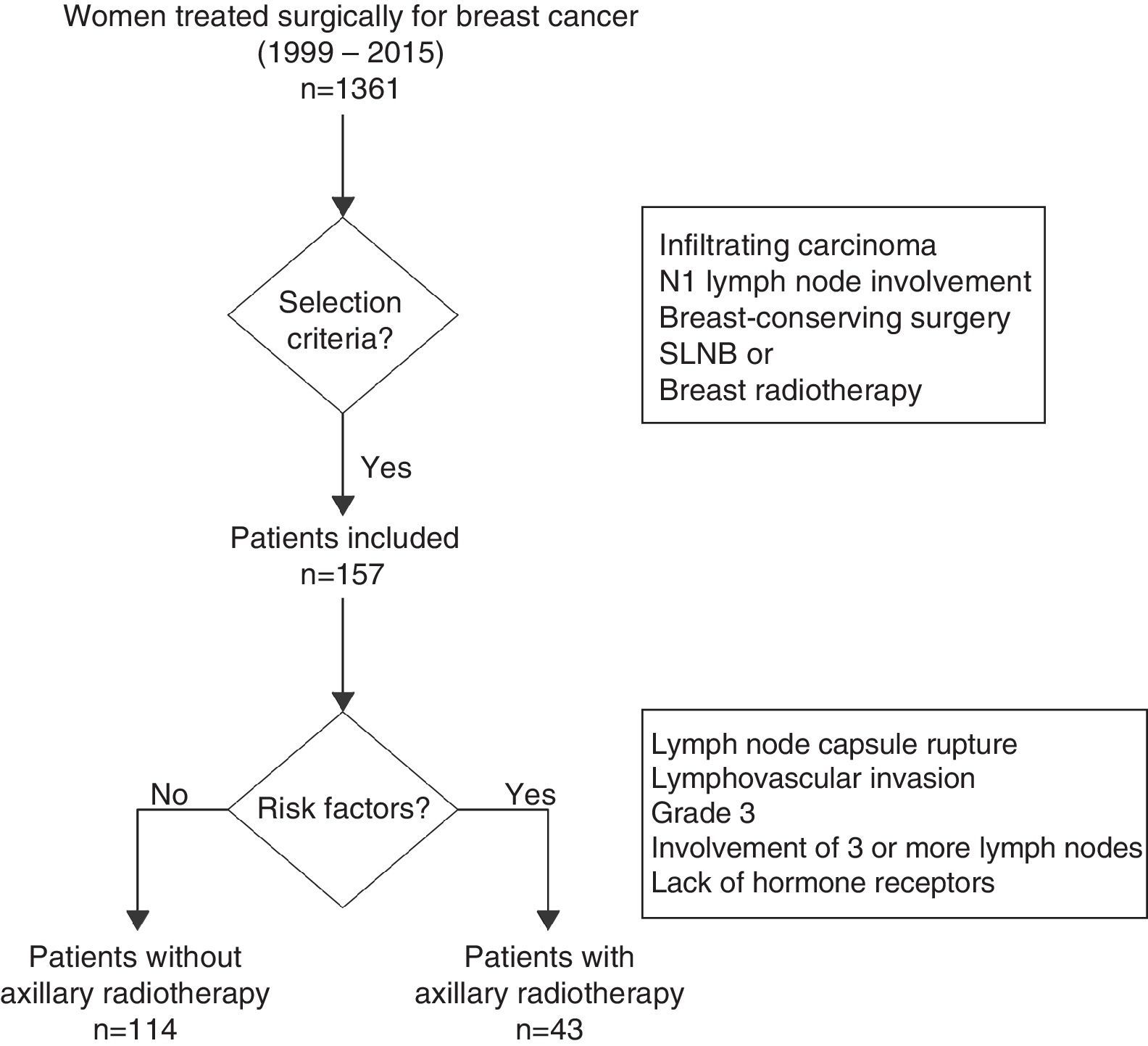
MethodsPatientsThis retrospective study was done between October 1999 and July 2015 and included women with invasive breast cancer in initial stages treated with breast-conserving surgery and axillary staging by sentinel lymph node biopsy (SLNB) or ALND. Tumors in initial stages were defined as those in stages I and II according to the 7th edition of the TNM Classification of the American Joint Committee on Cancer,9 which corresponds with tumors less than 5cm in size with no axillary involvement or with involvement of 1–3 lymph nodes.
Excluded from the study were those patients with involvement of 4 or more axillary lymph nodes, T3-T4 tumors, distant metastasis upon diagnosis, in situ carcinoma or a metachronous carcinoma in the same breast. Likewise, we excluded patients treated with primary systemic chemotherapy, mastectomy, no breast radiotherapy or lack of information about the radiotherapy regimen used.
The extension study of the axilla was done in accordance with the Breast Unit protocol in each period. From October 1999 to December 2001, this was done with ALND; after December 2001, SLNB was used and only one ALND in patients with sentinel gland involvement. Finally, from February 2010 to July 2015, criteria from the ACOSOG Z0011 clinical trial were applied,6 and only women with metastasis in 3 or more sentinel lymph nodes or capsular rupture were treated with ALND.
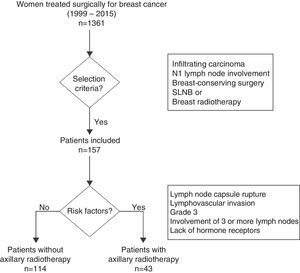
The analysis of the impact of axillary radiotherapy on the overall survival of women with initial-stage breast cancer was focused on the subgroup of patients with N1 axillary involvement (1–3 lymph nodes) (Fig. 1). The lymphedema study included all patients with breast cancer in initial stages (N0 and N1), and risk factors associated with its appearance were calculated. This analysis included N0 patients, as it is a patient group without lymph node involvement treated with ALND and no axillary radiotherapy, which enables us to calculate the true impact of ALND in the development of lymphedema, without the association of other risk factors.
The study was approved of by the Regional Research Ethics Committee (code Sentina 00-14).
Surgical TreatmentLumpectomy with local reconstruction was indicated in women with tumors smaller than 3cm and an oncoplastic pattern adapted to the type of breast and tumor localization in patients in whom moderate/severe deformity was expected. ALND was done in levels I/II with preservation of the neurovascular pedicle of the latissimus dorsi and long thoracic nerve.
Radiation TherapyAll patients included in the study received breast radiotherapy using tangential fields at a dose of 50Gy in 25 fractions of 2Gy. In cases with boost radiation of the tumor site, additional doses of between 8 and 10Gy were applied over 4 or 5 sessions. The women who received axillary or supraclavicular radiotherapy were administered doses of 50Gy in 25 sessions, at a depth of 3cm. Axillary radiotherapy was indicated in women with at least one of the following criteria: ruptured lymph node capsule, lymphovascular invasion, grade 3 on the Scarf–Bloom–Richardson grading system, involvement of 3 or more lymph nodes or lack of hormone receptor expression (Fig. 1). The internal breast chain was not radiated in any of the patients. The radiation therapy regimen was retrospectively reviewed for all patients.
Systemic TreatmentPatients with hormone receptor expression received hormone therapy for 5 or 10 years: premenopausal women received tamoxifen, and postmenopausal patients were administered aromatase inhibitors. Adjuvant chemotherapy was indicated according to the decision of the Tumor Committee at our hospital, based on the clinical guidelines for each period.8 In most cases, those who required chemotherapy received a sequential dosage of 4 cycles of adriamycin and cyclophosphamide every 3 weeks, followed by weekly paclitaxel for 12 cycles. From October 2002 on, in patients with HER2 overexpression, trastuzumab was prescribed every 3 weeks for one year.
Follow-upThe follow-up was done in the Breast Unit at our hospital for the first 5 years, which continued annually in Primary Care. Physical examinations and lab work were carried out every 3–4 months of the first 3 years and every 6 months the fourth and fifth years. Mammograms were done annually. During physical examination of the patients, the upper extremities were measured only in those patients with symptoms or clinical evidence of lymphedema. Lymphedema was defined as an increase of at least 10% the circumference of the arm or forearm or an increase of more than 2cm compared with the contralateral limb at the same moment. Overall survival was defined as the percentage of patients alive 10 years after diagnosis (the time at which they were included in the study) until death for whatever cause. Disease-free survival was defined as the percentage of patients who were alive and had had no recurrences (local recurrences, contralateral tumors or distant metastases) 10 years after diagnosis. All relapses were confirmed histologically.
Statistical AnalysisTo determine the power of the study, the sample size was calculated, estimating an incidence of axillary recurrences of 2% after 5 years6,7,10,11 with a precision of 1.5% and a confidence interval of 95%; 334 women were necessary for this study. A descriptive analysis was done for all the patients included in the study. We then conducted a comparative analysis of women with axillary involvement and risk factors (according to the aforementioned criteria), due to which they received axillary or supraclavicular radiotherapy, versus the group of women with low risk for axillary lymph node involvement, in whom regional radiotherapy was not indicated.
The descriptive analysis of all the variables expressed quantitative variables as mean±standard deviation and the qualitative variables as absolute value and percentage. A univariate study was completed of the variables associated with axillary radiotherapy, locoregional and distant recurrences, and lymphedema. The association of the qualitative variables was done with the chi-squared test. The comparison of means, after determining normal distribution, was done with Student's t test or Mann–Whitney U, as necessary. Multivariate logistic regression models were created to determine risk factors for lymphedema.
We studied the probability for recurrence (locoregional or distance) in the follow-up and overall survival for breast cancer with Kaplan–Meier curves and log rank tests. Cox multivariate and univariate regression analysis models were used for overall survival and lymphedema variables.
ResultsA total of 1361 women were treated during the study period, 541 of whom met the inclusion criteria and 820 of whom were excluded. In the study group, 384 patients (71.0%) did not present involvement of the axillary lymph nodes, versus 157 women (29.0%) who showed involvement of 1–3 axillary lymph nodes. A total of 47 patients (8.7%) had received axillary radiotherapy, out of which 43 patients (91.5%) presented axillary involvement (N1). The mean follow-up of the group studied was 7.3 years (standard deviation±4.5) and 5.8 (±4.8) years for N1 women.
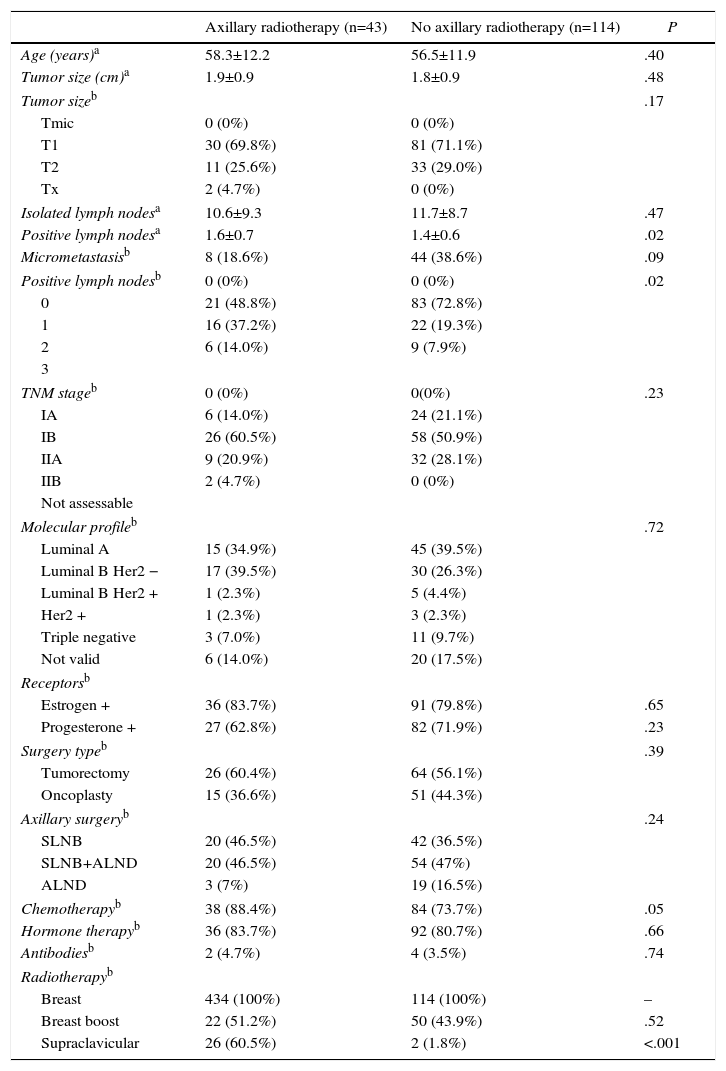
Patient CharacteristicsAxillary radiotherapy was indicated in 43 of the 157 patients with lymph node involvement (27.3%) (Table 1) and in 4 of the 384 women (1.0%) without axillary involvement. The patients with radiation therapy of the axilla presented larger tumor size, more advanced tumor stage, and higher rates of isolated and positive axillary lymph nodes compared to non-irradiated patients; these differences were statistically significant. The study of the N1 patients demonstrated a higher number of metastatic lymph nodes in irradiated versus non-irradiated patients (1.6±0.7 vs 1.3±0.6; P=.02). The most common indications for radiation therapy of the axilla were rupture of the lymph node capsule (39.5%) and elevated tumor grade (32.6%). Half of the irradiated patients (46.9%) presented an affected sentinel lymph node without ALND and corresponded with patients in whom criteria from the Z0011 study were used. However, most patients with affected sentinel lymph nodes who did not undergo axillary radiotherapy or ALND presented micrometastasis (33 out of 44 women); only one patient with micrometastasis had ALND and axillary radiotherapy due to capsular rupture of the sentinel lymph node in the area of the micrometastasis.
Clinical–Pathological Characteristics of Patients With Involvement of 1–3 Axillary Lymph Nodes (N1).
| Axillary radiotherapy (n=43) | No axillary radiotherapy (n=114) | P | |
|---|---|---|---|
| Age (years)a | 58.3±12.2 | 56.5±11.9 | .40 |
| Tumor size (cm)a | 1.9±0.9 | 1.8±0.9 | .48 |
| Tumor sizeb | .17 | ||
| Tmic | 0 (0%) | 0 (0%) | |
| T1 | 30 (69.8%) | 81 (71.1%) | |
| T2 | 11 (25.6%) | 33 (29.0%) | |
| Tx | 2 (4.7%) | 0 (0%) | |
| Isolated lymph nodesa | 10.6±9.3 | 11.7±8.7 | .47 |
| Positive lymph nodesa | 1.6±0.7 | 1.4±0.6 | .02 |
| Micrometastasisb | 8 (18.6%) | 44 (38.6%) | .09 |
| Positive lymph nodesb | 0 (0%) | 0 (0%) | .02 |
| 0 | 21 (48.8%) | 83 (72.8%) | |
| 1 | 16 (37.2%) | 22 (19.3%) | |
| 2 | 6 (14.0%) | 9 (7.9%) | |
| 3 | |||
| TNM stageb | 0 (0%) | 0(0%) | .23 |
| IA | 6 (14.0%) | 24 (21.1%) | |
| IB | 26 (60.5%) | 58 (50.9%) | |
| IIA | 9 (20.9%) | 32 (28.1%) | |
| IIB | 2 (4.7%) | 0 (0%) | |
| Not assessable | |||
| Molecular profileb | .72 | ||
| Luminal A | 15 (34.9%) | 45 (39.5%) | |
| Luminal B Her2 − | 17 (39.5%) | 30 (26.3%) | |
| Luminal B Her2 + | 1 (2.3%) | 5 (4.4%) | |
| Her2 + | 1 (2.3%) | 3 (2.3%) | |
| Triple negative | 3 (7.0%) | 11 (9.7%) | |
| Not valid | 6 (14.0%) | 20 (17.5%) | |
| Receptorsb | |||
| Estrogen + | 36 (83.7%) | 91 (79.8%) | .65 |
| Progesterone + | 27 (62.8%) | 82 (71.9%) | .23 |
| Surgery typeb | .39 | ||
| Tumorectomy | 26 (60.4%) | 64 (56.1%) | |
| Oncoplasty | 15 (36.6%) | 51 (44.3%) | |
| Axillary surgeryb | .24 | ||
| SLNB | 20 (46.5%) | 42 (36.5%) | |
| SLNB+ALND | 20 (46.5%) | 54 (47%) | |
| ALND | 3 (7%) | 19 (16.5%) | |
| Chemotherapyb | 38 (88.4%) | 84 (73.7%) | .05 |
| Hormone therapyb | 36 (83.7%) | 92 (80.7%) | .66 |
| Antibodiesb | 2 (4.7%) | 4 (3.5%) | .74 |
| Radiotherapyb | |||
| Breast | 434 (100%) | 114 (100%) | – |
| Breast boost | 22 (51.2%) | 50 (43.9%) | .52 |
| Supraclavicular | 26 (60.5%) | 2 (1.8%) | <.001 |
SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
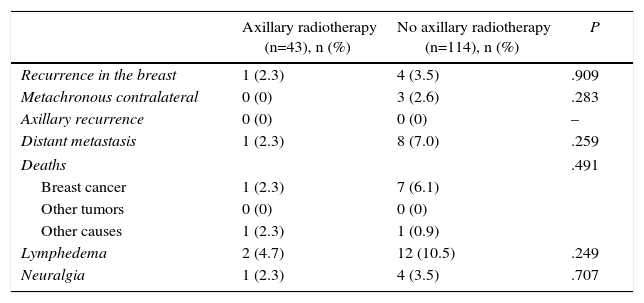
Table 2 shows the events that occurred during follow-up in the N1 patients. During follow-up, breast recurrences were diagnosed in 11 patients (5 patients in the N1 group and 6 N0 patients) and metachronous tumors of the contralateral breast were observed in 14 women (3 in the N1 group and 11 patients in the N0), which represent a 10-year actuarial incidence of 2.1% and 3.3%, respectively. The incidence of recurrences and contralateral tumors was similar for N1 patients with and without axillary radiotherapy. No axillary recurrences were observed during the study period.
Events During the Follow-up of N1 Patients.
| Axillary radiotherapy (n=43), n (%) | No axillary radiotherapy (n=114), n (%) | P | |
|---|---|---|---|
| Recurrence in the breast | 1 (2.3) | 4 (3.5) | .909 |
| Metachronous contralateral | 0 (0) | 3 (2.6) | .283 |
| Axillary recurrence | 0 (0) | 0 (0) | – |
| Distant metastasis | 1 (2.3) | 8 (7.0) | .259 |
| Deaths | .491 | ||
| Breast cancer | 1 (2.3) | 7 (6.1) | |
| Other tumors | 0 (0) | 0 (0) | |
| Other causes | 1 (2.3) | 1 (0.9) | |
| Lymphedema | 2 (4.7) | 12 (10.5) | .249 |
| Neuralgia | 1 (2.3) | 4 (3.5) | .707 |
Variables presented in number of patients and percentage.
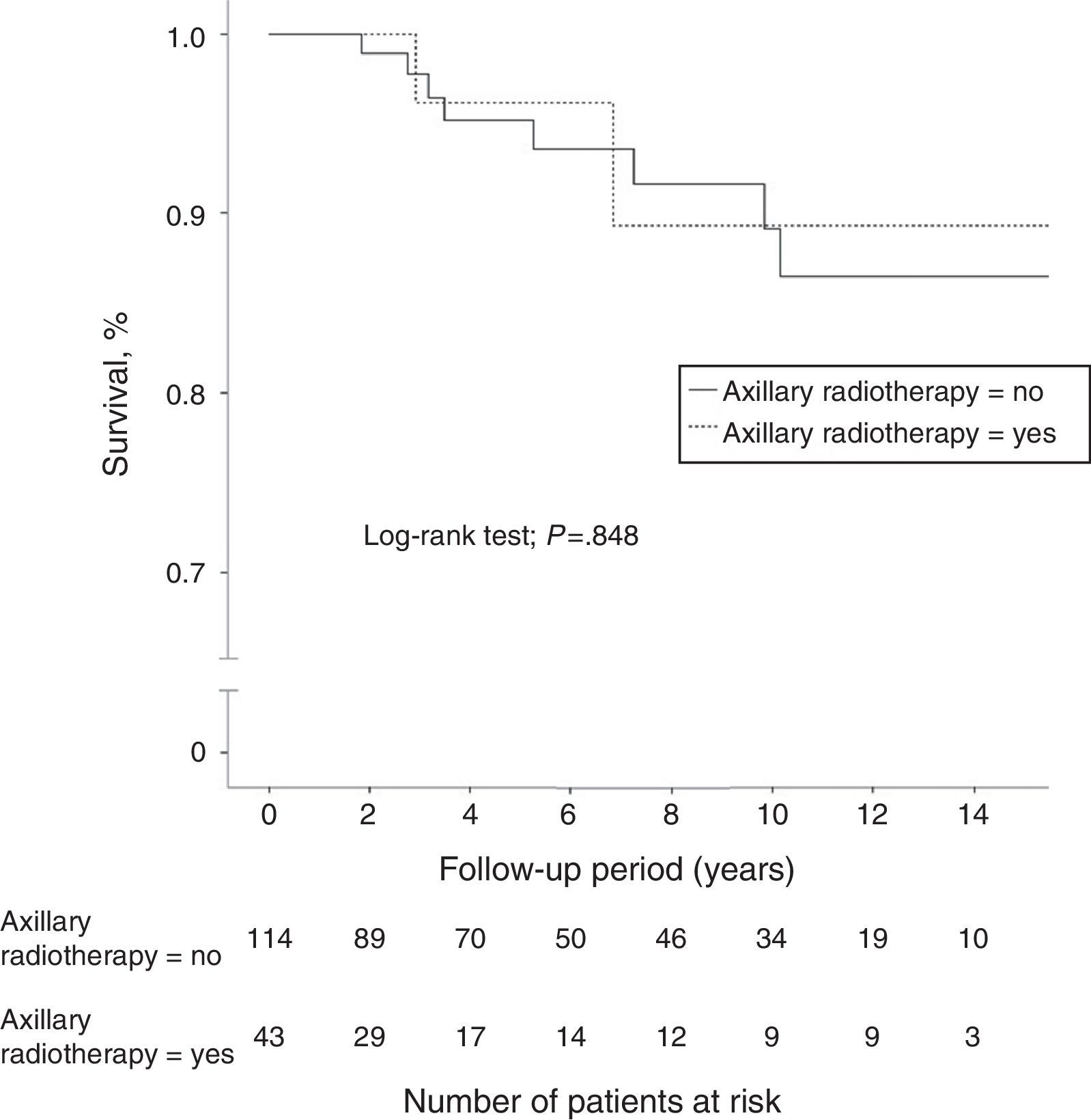
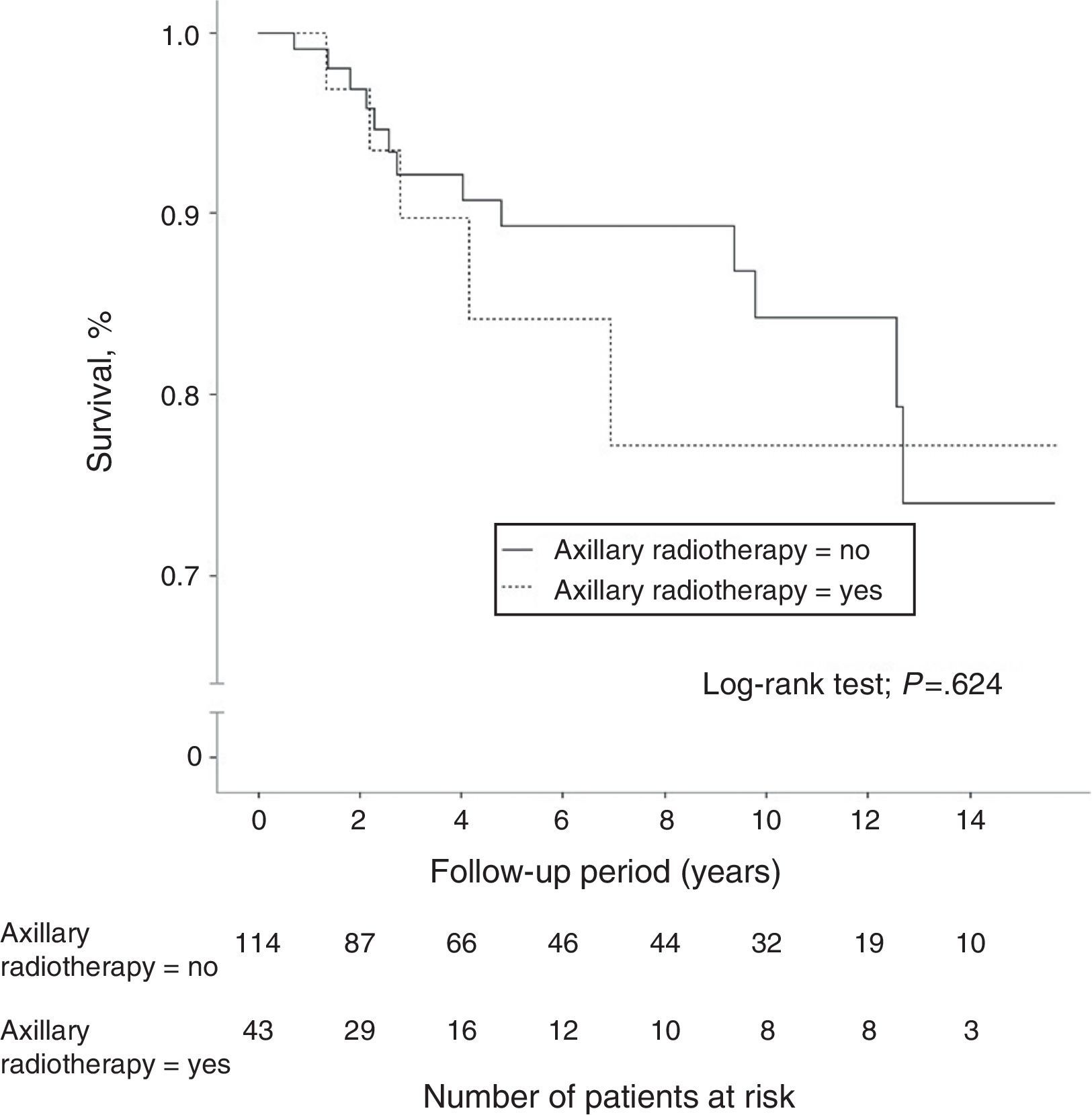
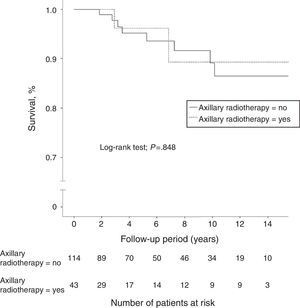
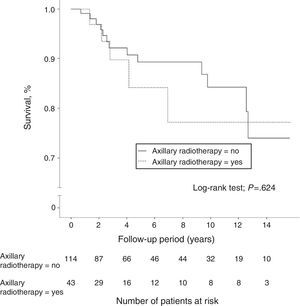
A total of 40 women (7.4%) died during follow-up, although breast cancer was the cause of death in only 18 patients (3.3%), 8 of whom (5.1%) were in the N1 group. Another 5 women died due to tumors not related to their senology process. Overall 10-year survival was 95.9% (95%CI: 94.6–97.2) in the N0 patients and 89.1% (95%CI: 82.3–96.5) in the N1 patients. The group of women with lymph node involvement and axillary radiotherapy showed an overall 10-year survival of 89.7% (95%CI: 85.4–94), similar to that of patients without radiation therapy of the axilla (Fig. 2). In patients with lymph node involvement without axillary radiotherapy, there was a higher incidence of distant metastasis and breast cancer deaths, although these differences were not statistically significant. In the women with lymph node involvement, no statistically significant differences were demonstrated in 10-year disease-free survival between the group with axillary irradiation (77.2%; 95%CI: 67.5–86.9) and the group without radiotherapy of the axillary (84.3%; 95%CI: 79.6–89) (Fig. 3).
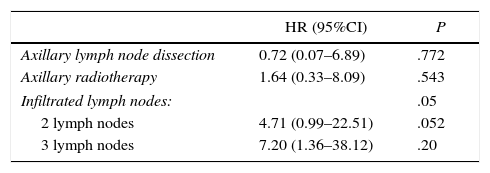
Once the univariate study was finished, a multivariate Cox regression model was used to estimate overall survival of the patients with axillary involvement that included the variables for ALND, axillary radiotherapy and number of affected lymph nodes. No significant differences in risk of death were seen during follow-up in the variables ALND and axillary irradiation. Contrarily, the number of affected lymph nodes was shown to be an independent risk factor for a shorter survival. Thus, the involvement of 2 axillary lymph nodes increased the risk of death by 4.7 (HR=4.71; 95%CI: 0.99–22.51), and the involvement of 3 lymph nodes multiplied the risk of death by 7 compared to only one positive lymph node (HR=7.20; 95%CI: 1.36–38.12) (Table 3).
Cox Multivariate Regression Model for the Analysis of Overall Survival in N1 Patients.
| HR (95%CI) | P | |
|---|---|---|
| Axillary lymph node dissection | 0.72 (0.07–6.89) | .772 |
| Axillary radiotherapy | 1.64 (0.33–8.09) | .543 |
| Infiltrated lymph nodes: | .05 | |
| 2 lymph nodes | 4.71 (0.99–22.51) | .052 |
| 3 lymph nodes | 7.20 (1.36–38.12) | .20 |
CI, confidence interval; HR, hazard ratio.
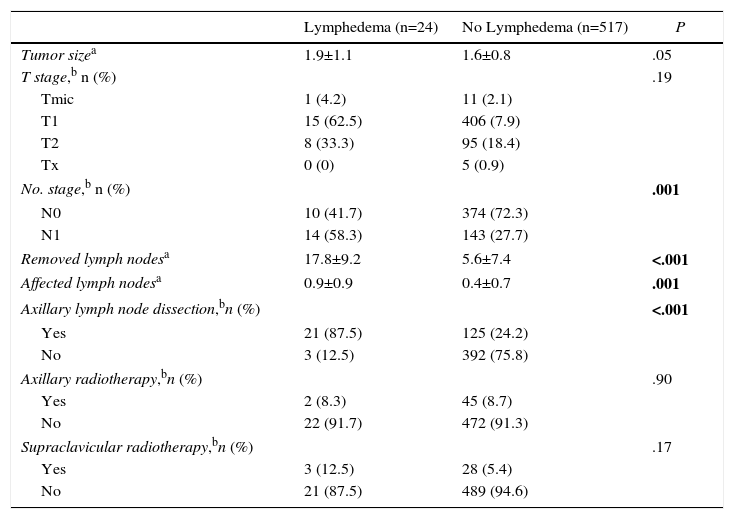
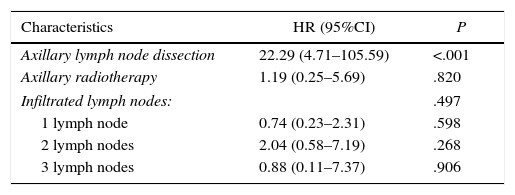
A total of 24 (4.45%) women presented lymphedema of the upper extremity during follow-up (Table 4). Mean time transpired from surgery until the diagnosis of lymphedema was 24.6 months (±6.0). The univariate study identified lymph node involvement, ALND, the number of axillary lymph nodes removed and the number of affected lymph nodes as variables related with the appearance of lymphedema. A multivariate Cox regression model was used to estimate the incidence of lymphedema that included the variables of ALND, axillary radiotherapy and number of affected lymph nodes. This study identified ALND as the only risk factor for developing lymphedema (HR=22.22; 95%CI: 4.71–105.59; P<.001) (Table 5).
Univariate Analysis of the Factors Associated With the Appearance of Lymphedema.
| Lymphedema (n=24) | No Lymphedema (n=517) | P | |
|---|---|---|---|
| Tumor sizea | 1.9±1.1 | 1.6±0.8 | .05 |
| T stage,b n (%) | .19 | ||
| Tmic | 1 (4.2) | 11 (2.1) | |
| T1 | 15 (62.5) | 406 (7.9) | |
| T2 | 8 (33.3) | 95 (18.4) | |
| Tx | 0 (0) | 5 (0.9) | |
| No. stage,b n (%) | .001 | ||
| N0 | 10 (41.7) | 374 (72.3) | |
| N1 | 14 (58.3) | 143 (27.7) | |
| Removed lymph nodesa | 17.8±9.2 | 5.6±7.4 | <.001 |
| Affected lymph nodesa | 0.9±0.9 | 0.4±0.7 | .001 |
| Axillary lymph node dissection,bn (%) | <.001 | ||
| Yes | 21 (87.5) | 125 (24.2) | |
| No | 3 (12.5) | 392 (75.8) | |
| Axillary radiotherapy,bn (%) | .90 | ||
| Yes | 2 (8.3) | 45 (8.7) | |
| No | 22 (91.7) | 472 (91.3) | |
| Supraclavicular radiotherapy,bn (%) | .17 | ||
| Yes | 3 (12.5) | 28 (5.4) | |
| No | 21 (87.5) | 489 (94.6) | |
P in bold are variables with statistically significant differences.
Cox Multivariate Regression Analysis of Predictive Factors for Lymphedema.
| Characteristics | HR (95%CI) | P |
|---|---|---|
| Axillary lymph node dissection | 22.29 (4.71–105.59) | <.001 |
| Axillary radiotherapy | 1.19 (0.25–5.69) | .820 |
| Infiltrated lymph nodes: | .497 | |
| 1 lymph node | 0.74 (0.23–2.31) | .598 |
| 2 lymph nodes | 2.04 (0.58–7.19) | .268 |
| 3 lymph nodes | 0.88 (0.11–7.37) | .906 |
CI, confidence interval; HR, hazard ratio.
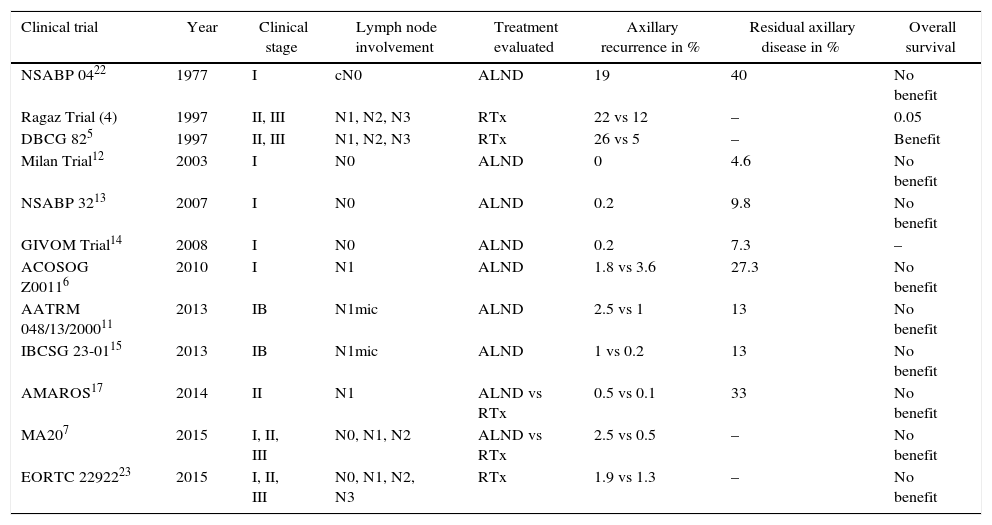
Several clinical trials have analyzed the impact of axillary treatment in women with initial-stage breast cancer (Table 6). From these studies, 3 main conclusions can be drawn.
Results of Clinical Trials That Have Analyzed the Impact of Axillary Treatment on Overall and Disease-Free Survival.
| Clinical trial | Year | Clinical stage | Lymph node involvement | Treatment evaluated | Axillary recurrence in % | Residual axillary disease in % | Overall survival |
|---|---|---|---|---|---|---|---|
| NSABP 0422 | 1977 | I | cN0 | ALND | 19 | 40 | No benefit |
| Ragaz Trial (4) | 1997 | II, III | N1, N2, N3 | RTx | 22 vs 12 | – | 0.05 |
| DBCG 825 | 1997 | II, III | N1, N2, N3 | RTx | 26 vs 5 | – | Benefit |
| Milan Trial12 | 2003 | I | N0 | ALND | 0 | 4.6 | No benefit |
| NSABP 3213 | 2007 | I | N0 | ALND | 0.2 | 9.8 | No benefit |
| GIVOM Trial14 | 2008 | I | N0 | ALND | 0.2 | 7.3 | – |
| ACOSOG Z00116 | 2010 | I | N1 | ALND | 1.8 vs 3.6 | 27.3 | No benefit |
| AATRM 048/13/200011 | 2013 | IB | N1mic | ALND | 2.5 vs 1 | 13 | No benefit |
| IBCSG 23-0115 | 2013 | IB | N1mic | ALND | 1 vs 0.2 | 13 | No benefit |
| AMAROS17 | 2014 | II | N1 | ALND vs RTx | 0.5 vs 0.1 | 33 | No benefit |
| MA207 | 2015 | I, II, III | N0, N1, N2 | ALND vs RTx | 2.5 vs 0.5 | – | No benefit |
| EORTC 2292223 | 2015 | I, II, III | N0, N1, N2, N3 | RTx | 1.9 vs 1.3 | – | No benefit |
First of all, axillary recurrence is an uncommon event in patients without lymph node involvement (N0) or with limited involvement in the axilla (N1); its incidence ranges between 0% and 3.6%.6,12 These results contrast with trials that included women with massive axillary involvement (N2, N3), in whom the risk for locoregional recurrence increases to 22%–26%, and decreases to 5%–12% with axillary radiotherapy, as reported by Canadian4 and Danish5 studies.
The second conclusion is that the residual axillary disease does not necessarily progress to axillary recurrence. Two facts provide evidence of this circumstance. First, in SLNB validation studies, the rate of false negatives from SLNB does not concur with the predicted incidence of axillary recurrences, since the Milán,12 NSABP3213 and GIVOM14 trials report axillary relapses of 0.2%, despite presenting false negatives of 4.6%, 9.8% and 7.3%, respectively. The second fact refers to the low incidence of axillary recurrences in women with metastatic involvement of the sentinel lymph node in whom ALND was not carried out. The ACOSOG Z0011,6 AATRM 04811 and IBCSG 23-0115 studies have demonstrated axillary relapses lower than 2.5%, in spite of residual disease rates of 27%, 13% and 13%, respectively.
Finally, the third conclusion of these clinical trials is that ALND or radiotherapy do not influence the overall survival of women with breast cancer. Thus, in N0 and N1mic patients, ALND does not improve survival compared to SLNB.11,13 Meanwhile, in N1 patients ALND does not modify survival compared to SLNB,6 nor does axillary radiotherapy versus ALND.7 Our study reflects similar results: overall survival and 10-year disease-free survival (DFS) in the N1 patients was 89.7% and 82.6%, respectively, with no significant differences between irradiated and non-irradiated patients.
Treatment of the axilla in women with breast cancer is planned according to the clinical stage of the disease and histology findings in the primary tumor as well as the axillary lymph nodes. N0 patients require no complementary treatment in the axilla. In our experience, this group of patients did not undergo ALND or axillary radiotherapy, with no observed axillary recurrences, despite the fact that the rate of false negatives in our validation phase for SLNB (6.8%) allowed us to predict a total of 24 women with residual disease in the axilla that did not progress toward axillary recurrence. Patients with N2–N3 lymph node involvement, however, required radical lymphadenectomy and radiation therapy of the axilla to achieve adequate locoregional control.
Finally, in women with limited involvement of the axillary lymph nodes (N1), it is debatable whether treatments complementary to SLNB would be beneficial. For this group with involvement of the sentinel lymph node, 3 alternatives are proposed: follow-up, ALND or axillary radiation therapy. The first option is valid for patients with micrometastatic involvement of the sentinel lymph node (N1mic), since the Spanish AATRM04811 and the Italian IBCSG 23-0115 clinical trials, designed for this group of patients, have demonstrated a similar incidence of regional recurrences, with no impact on overall survival. In our study, most of the patients with involvement of the sentinel lymph nodes who did not undergo ALND or axillary radiotherapy were women with micrometastases, and no mid-term relapses were observed.
The regional management of patients with micrometastatic involvement of the sentinel lymph node is controversial. The results of the ACOSOG Z0011 study6 propose observation in this group of patients treated with breast-conserving surgery and macrometastases in up to 2 sentinel lymph nodes, based on the fact that the breast tangential fields include axillary level I and provide adequate control of the process, a circumstance that is not seen in women with a mastectomy. Nonetheless, the review of the radiotherapy regimen of the patients who participated in this clinical trial16 demonstrated that at least 17% of the patients received an additional field in the supraclavicular/axillary region, possibly due to risk factors related with the tumor and the patient. Meanwhile, the MA.20 study7 demonstrated improved regional control after axillary radiotherapy in women with involvement of 1–3 axillary lymph nodes and with risk factors for recurrence, although they did not demonstrate improvement in overall survival. Similar to the Z0011 and MA.206,7 trials, our study was focused on women with initial-stage disease and breast-conserving surgery, which comprise a homogeneous group for the analysis of locoregional control. The criteria for axillary radiotherapy in our patients with involvement of 1–3 axillary lymph nodes were similar to the MA.20 study,7 which provided adequate locoregional control for this group of patients, with no evidence of axillary recurrences during follow-up. In contrast, after the approval of the Z0011 criteria, in patients with sentinel lymph node involvement without risk factors for recurrence, observation has provided good mid-term evolution. In our opinion, axillary radiotherapy is appropriate in women with risk factors and sentinel lymph node involvement without ALND, as both the MA.207 as well as AMAROS17 trials support its use and demonstrate good regional control of the pathology and a lower rate of lymphedemas versus ALND.
Several studies18,19 report the appearance of most lymphedemas in the first 48 months post-treatment and identify axillary radiotherapy and ALND as risk factors for lymphedema, with an incidence close to 40% when both treatments are associated.20 Our study showed evidence of a lower incidence of lymphedemas (5%) due to its retrospective design, which undoubtedly leads to an underestimation of this consequence. In our experience, only ALND was identified as a risk factor for the appearance of lymphedema, but not axillary radiotherapy or the association of both treatments. The AMAROS17 study shows evidence of similar results with a lower rate of lymphedemas in patients with radiation therapy without ALND. It therefore seems logical to complement regional treatment of patients with limited axillary involvement with radiotherapy, thus reducing the indication of ALND and the appearance of lymphedemas.
In the coming years, the POSNOC study21 will randomize patients for observation, ALND and axillary radiotherapy, thereby providing information about the value of ALND and axillary radiotherapy in women with micrometastatic involvement of the sentinel lymph nodes.
Our study presents several limitations due to its observational character. On one hand, the lack of axillary relapses during the follow-up does not allow us to identify risk factors for their appearance and for disease-free survival. Likewise, the sample size is not sufficient to detect the relative risk of axillary recurrence presented by the group without axillary radiotherapy. Furthermore, the retrospective character of the study entails an underestimation in the incidence of lymphedemas in our series, which is lower to that seen in prospective studies.
In conclusion, women with breast cancer and N1 lymph node involvement treated with breast-conserving surgery present a low incidence of axillary recurrences. In this group of patients, axillary radiation therapy does not improve overall survival but does contribute to regional control in those patients with risk factors and limited involvement of the axillary lymph nodes.
Conflict of InterestThere are no conflicts of interest to declare.
Please cite this article as: García Novoa A, Acea Nebril B, Díaz I, Builes Ramírez S, Varela C, Cereijo C, et al. Radioterapia axilar en la cirugía conservadora del cáncer de mama en estadio temprano (estadio I y II). Cir Esp. 2016;94:331–338.