Mortality associated with pancreaticoduodenectomy (PD) has drastically decreased in the last decade, but morbidity figures continue to be high (30%-50%)1. Pancreatic fistula (PF) is one of the complications with the highest morbidity rates after this procedure, with an incidence of 3%-45%2,3, in spite of the new techniques and advances developed for its prevention, many of which continue to be the subject of debate today. The use of intraoperative pancreatic stents is generally defended due to a lower overall rate of PF4. Other noteworthy complications are biliary fistula (BF), delayed gastric emptying (DGE) or postoperative bleeding. Postoperative hemorrhage can reach mortality rates of up to 38%5. Severe postoperative bleeding that causes hemodynamic instability requires rapid and effective management, and a minimally invasive endovascular approach currently offers the best available treatment option. Isolated biliary fistula can cause severe abdominal sepsis, although this is rare (1%-3%)6. DGE is one of the most frequent complications (3.2%-59%), which causes great discomfort for patients, prolonged hospital stays, and increased hospital costs as a result7.
In order to analyze the morbidity of PD and its management, we have conducted a retrospective study using the prospective database of a tertiary care hospital. Complications were classified according to the definitions of the ISGPS2,4–6.
The inclusion criteria were: a) patients who underwent PD between January 2010 and November 2020, with Clavien-Dindo ≥ II complications; b) complete records for amylase in drained intra-abdominal fluid on the 3rd postoperative day; c) follow-up for 90 postoperative days.
The exclusion criteria were: a) other pancreatic resections, b) Clavien-Dindo complications < II; c) surgical protocols where the use of pancreatic stent was not reflected; d) absence of amylase registry in drain fluid; e) absence of follow-up.
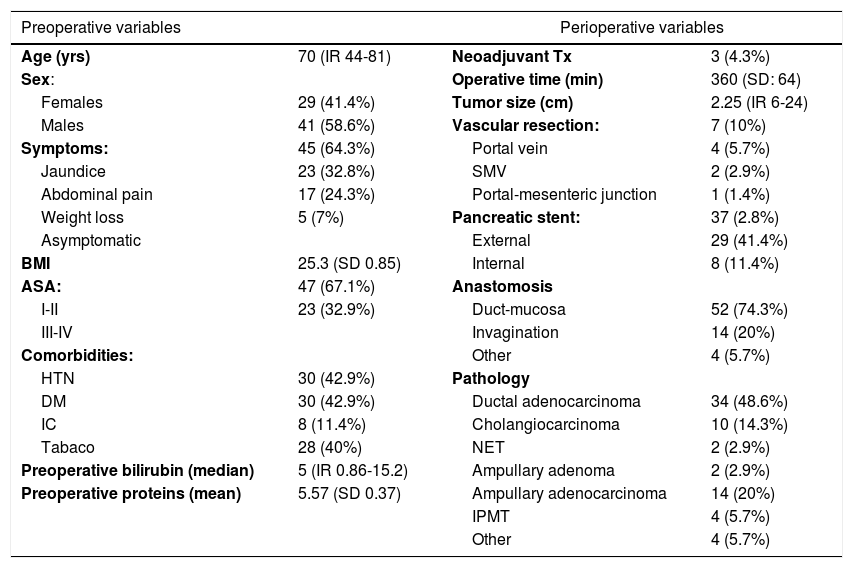
Out of a total of 159 patients operated on in this period, in the end 70 were analyzed according to the inclusion criteria, and 67% of the patients were ASA I-II. Biliary stents were placed by ERCP in 32.9% of patients and external-internal catheter in 8.6% in the preoperative period. During surgery, a pancreatic stent was used in 37 patients (52.8%), with an externalized drain tube in 71.4% of the cases. The most widely used pancreatic anastomosis was duct-to-mucosa in 52 patients (74.3%), while invagination anastomosis was used in 14 (20%). Pancreatic adenocarcinoma was the most frequent indication for surgery (48.6%). Other indications were ampuloma (22.9%), cholangiocarcinoma (14.3%), intraductal papillary mucinous tumor (5.7%) or neuroendocrine tumor (2.9%). The remaining preoperative and perioperative variables are represented in Table 1.
Preoperative and perioperative variables.
| Preoperative variables | Perioperative variables | ||
|---|---|---|---|
| Age (yrs) | 70 (IR 44-81) | Neoadjuvant Tx | 3 (4.3%) |
| Sex: | Operative time (min) | 360 (SD: 64) | |
| Females | 29 (41.4%) | Tumor size (cm) | 2.25 (IR 6-24) |
| Males | 41 (58.6%) | Vascular resection: | 7 (10%) |
| Symptoms: | 45 (64.3%) | Portal vein | 4 (5.7%) |
| Jaundice | 23 (32.8%) | SMV | 2 (2.9%) |
| Abdominal pain | 17 (24.3%) | Portal-mesenteric junction | 1 (1.4%) |
| Weight loss | 5 (7%) | Pancreatic stent: | 37 (2.8%) |
| Asymptomatic | External | 29 (41.4%) | |
| BMI | 25.3 (SD 0.85) | Internal | 8 (11.4%) |
| ASA: | 47 (67.1%) | Anastomosis | |
| I-II | 23 (32.9%) | Duct-mucosa | 52 (74.3%) |
| III-IV | Invagination | 14 (20%) | |
| Comorbidities: | Other | 4 (5.7%) | |
| HTN | 30 (42.9%) | Pathology | |
| DM | 30 (42.9%) | Ductal adenocarcinoma | 34 (48.6%) |
| IC | 8 (11.4%) | Cholangiocarcinoma | 10 (14.3%) |
| Tabaco | 28 (40%) | NET | 2 (2.9%) |
| Preoperative bilirubin (median) | 5 (IR 0.86-15.2) | Ampullary adenoma | 2 (2.9%) |
| Preoperative proteins (mean) | 5.57 (SD 0.37) | Ampullary adenocarcinoma | 14 (20%) |
| IPMT | 4 (5.7%) | ||
| Other | 4 (5.7%) | ||
IR: interquartile range; min: minutes; cm: centimeters; BMI: body mass index; SMV: superior mesenteric vein; ASA: American Society of Anesthesiologists; HTN: hypertension; DM: diabetes mellitus; IC: ischemic cardiomyopathy; NET: neuroendocrine tumor; IPMT: intraductal papillary mucinous tumor; SD: standard deviation; IR: interquartile range
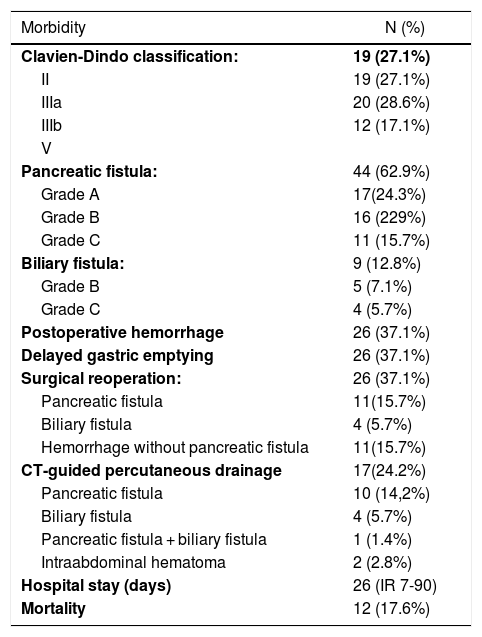
In terms of postoperative complications, 28.6% of the patients analyzed presented Clavien-Dindo grade IIIB postoperative complications. Complications are recorded in Table 2. Forty-four patients (62.9%) presented PF, including biochemical fistula, which accounted for 24.3%; type B 22.9% and type C 15.7%. The use of pancreatic stents significantly reduced the fistula rate (48% vs 75.8%; P = .02) in the patients analyzed, with no differences between external or internal use (P = .62). Postoperative bleeding occurred in 26 patients (37.1%), appearing a mean of 8 days after surgery (range 1-20 days). In 7 of the 26 patients who presented postoperative bleeding (30.7%), angiography with embolization was required, which was located in the hepatic artery in 4 patients (57.1%), the gastroduodenal in one patient (14.3%), and the left gastric and right gastric in one patient, respectively (14.3%). In 3 patients who presented postoperative bleeding (11.5%), the main sign was upper gastrointestinal bleeding, but oral panendoscopy was not able to visualize the bleeding point; 2 patients required angiography with embolization, while one required urgent surgery.
Morbidity-mortality after PD.
| Morbidity | N (%) |
|---|---|
| Clavien-Dindo classification: | 19 (27.1%) |
| II | 19 (27.1%) |
| IIIa | 20 (28.6%) |
| IIIb | 12 (17.1%) |
| V | |
| Pancreatic fistula: | 44 (62.9%) |
| Grade A | 17(24.3%) |
| Grade B | 16 (229%) |
| Grade C | 11 (15.7%) |
| Biliary fistula: | 9 (12.8%) |
| Grade B | 5 (7.1%) |
| Grade C | 4 (5.7%) |
| Postoperative hemorrhage | 26 (37.1%) |
| Delayed gastric emptying | 26 (37.1%) |
| Surgical reoperation: | 26 (37.1%) |
| Pancreatic fistula | 11(15.7%) |
| Biliary fistula | 4 (5.7%) |
| Hemorrhage without pancreatic fistula | 11(15.7%) |
| CT-guided percutaneous drainage | 17(24.2%) |
| Pancreatic fistula | 10 (14,2%) |
| Biliary fistula | 4 (5.7%) |
| Pancreatic fistula + biliary fistula | 1 (1.4%) |
| Intraabdominal hematoma | 2 (2.8%) |
| Hospital stay (days) | 26 (IR 7-90) |
| Mortality | 12 (17.6%) |
PD: pancreaticoduodenectomy.
BF was observed in 8 patients (12.8%), 5 of which were grade B. Biliary fistula was more frequent in patients treated for benign disease (75% vs 8.8%; P = .012). DGE was observed in 26 patients (37.1%) and successfully managed with prokinetics and a nasogastric tube in all cases.
Twenty-six patients (37.1%) required surgical reintervention: 11 (15.7%) for PF, 4 (5.7%) for BF, and 11 (15.7%) for hemorrhage unrelated to PF. In 17 patients (24.2%), percutaneous drainage of intra-abdominal abscess was performed, finding PF in 10 cases, BF in 4, PF associated with BF in one case, and superinfected hematoma in 2 patients.
The median hospital stay was 26 days (IR 7-90), and the recorded mortality was 17%.
PD is a complex procedure with high morbidity despite the new advances developed. Regarding PF, our results suggest that the use of pancreatic stents could reduce its overall rate, which is consistent with current evidence.
Please cite this article as: Pastor-Peinado P, Ocaña J, Lobo E, Fernández-Cebrían JM, Sanjuanbenito A. Análisis, impacto y manejo de las complicaciones moderadas y graves asociadas a la duodenopancreatectomía cefálica. Cir Esp. 2022;100:314.