The objective of this study was to determine which image test used to measure the size of pre-operative primary breast cancer (mammography, ultrasound or magnetic resonance imaging [MRI]) correlated best with the size of the tumor in the postoperative surgical specimen.
MethodsA retrospective analysis was conducted of women diagnosed with breast cancer for which primary surgical treatment was indicated and who underwent surgical intervention between January 2014 and December 2016. Sociodemographic, imaging and histological variables were collected. The results are presented by age group, tumor size and histological type.
ResultsIn the 224 women studied, mammography and MRI tumor sizes were compared with pathology study tumor measurements, revealing no significant differences, both overall and based on histologic type or age. However, both significantly underestimated large tumors and significantly overestimated small tumors. Ultrasound significantly underestimated tumor size, especially in large tumors, older patients and in infiltrating ductal carcinoma (IDC) and infiltrating ductal carcinoma with associated ductal carcinoma in situ (IDC + DCIS). MRI correlated best with histological tumor size, although with no statistically significant differences.
ConclusionsMRI is the best predictor of tumor size in breast cancer. Histologic type and tumor size are key parameters when estimating tumor size and should be taken into account when planning surgery. Patient age does not interfere with the interpretation of imaging tests.
El objetivo de este estudio fue evaluar qué prueba de imagen de las empleadas para medir el tamaño del cáncer de mama primario preoperatorio (mamografía, ecografía o resonancia magnética (RM)) se correlacionó mejor con el tamaño del tumor en la pieza quirúrgica postoperatoria.
MétodosAnálisis retrospectivo de mujeres con diagnóstico de cáncer de mama y con indicación de tratamiento quirúrgico primario operadas desde Enero de 2014 a Diciembre de 2016. Se recogieron variables sociodemográficas, vinculadas a técnicas de imagen e histológicas. Los resultados se presentaron según edad, tamaño tumoral y tipo histológico.
ResultadosSe estudiaron 224 mujeres. Al comparar el tamaño mamográfico y de la RM con el histológico final no se encontraron diferencias significativas, tanto de forma global como teniendo en cuenta el grupo histológico o la edad, sin embargo, ambas infraestimaron significativamente los tumores grandes y sobrestimaron significativamente los pequeños. La ecografía infraestimó significativamente el tamaño del tumor, especialmente en tumores grandes, pacientes mayores y en los grupos de carcinoma ductal infiltrante (CDI) y carcinoma ductal infiltrante con carcinoma ductal in situ asociado (CDI + CDIS). La RM se correlacionó mejor con el tamaño tumoral histológico aunque sin diferencias estadísticamente significativas.
ConclusionesLa RM parece ser el mejor predictor del tamaño del tumor en el cáncer de mama. El grupo histológico y el tamaño del tumor fueron claves en la estimación de la medida del tumor, por lo que se deben tener en cuenta en la planificación de la cirugía. La variable edad no interfirió en la interpretación de las imágenes.
Breast cancer tumor size is a main prognostic indicator and a determining factor for planning surgical treatment. Therefore, accurate tumor size prediction by imaging studies at the time of diagnosis is essential to plan proper patient management. Imaging test results are classified using the Breast Imaging Reporting and Data System (BI-RADS) classification.1,2
Surgical treatment of breast cancer has evolved greatly in recent decades. The possibility of breast-preserving management is being increasingly offered, which depends significantly on the relationship between the size of the tumor and the breast. In addition, the indication for primary systemic treatment is made, among other things, based on tumor size.
In this scenario, MRI has become a great tool for the additional evaluation of tumor extension and synchronous tumors. Although it offers the advantage of high sensitivity, it also has disadvantages, such as false positives that require additional biopsies, patient anguish, prolonged time to surgery, cost and the possible overestimation of tumor size.3–5
The objective of this study was to analyze which of the current imaging methods is the most accurate for defining pre-therapeutic tumor size in primary breast cancer, using histological size as the gold standard in patients with newly diagnosed breast cancer.
The accuracy of the imaging tests was also analyzed by different tumor sizes, histological subgroups and patient age.
MethodsRetrospective review of a case series including women diagnosed with breast cancer (invasive and in situ) with indication for primary surgical treatment, operated on from January 2014 to December 2016.
All patients met the following inclusion criteria: women with primary breast cancer, no neoadjuvant chemotherapy, indication for primary surgical treatment, final histology information, stage Tis-T3, with the diagnosis made by imaging tests at the same hospital and always using MRI. The size reference chosen in each case was the largest tumor diameter.
The information was collected from electronic medical records, radiology reports and pathology reports.
Mammography was conducted with a full-field high-definition digital mammogram and tomosynthesis system (Selenia Dimensions, Hologic GmbH, Belgium, Germany).
Ultrasound was performed using a high-definition linear transducer with a width of 60 mm and a frequency of 15 MHz. We used the Logiq E9 model (GE Healthcare, Waunatosa, WI, USA). The tumor size measurement took into account the hypoechoic center of the lesion and the echogenic halo, when visible.
The MRI was performed with a 1.5 Tesla system (Aera, Siemens, Munich, Germany) with a T1-weighted dynamic gradient echo sequence (T1w-FFE fast field echo) used with one baseline series and 4 subsequent post-contrast series. A bolus of 0.20 mmol of gadolinium per kilogram of body weight was injected intravenously, followed by 20 mL of saline solution. The subsequent image processing involved the generation of subtraction series and the reconstruction of a maximum intensity projection (MIP). The image analysis was carried out using the digital data with the help of a suitable workstation (SYNGO via, Siemens, Munich, Germany).
All these imaging techniques were performed by radiologists specialized in breast diagnoses.
Statistical AnalysisThe qualitative variables were expressed as percentages and the quantitative variables as mean (standard deviation [SD]).
The difference in means between the image size (MRI, mammography and ultrasound) and the histological results was calculated with the paired sample t-test and related to the interval in which 95% of the calculated differences were found. The paired sample t-test was also used to compare tumor size by tumor group (IDC, DCIS, ILC, IDC + DCIS), size (≤20 mm and >20 mm) and age (≤40 and >40 yrs).
The Pearson correlation coefficients were calculated in the form of pairs between the histological measurement and MRI, mammogram and ultrasound, respectively.
The level of significance was defined as a P value <.05. The statistical analysis was done with SPSS® for Windows (version 15.0; IBM, Chicago, USA).
No objections were raised against the study by the hospital Research and Ethics Committee.
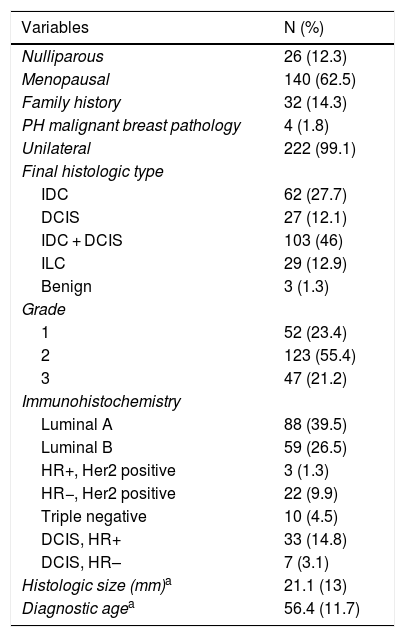
ResultsDescription of the Study Population and Findings From Imaging StudiesWe studied 224 women diagnosed with breast cancer, with a mean (SD) age of 56.4 (11.7) years. The main histological group was IDC + DCIS (46%), and 66% of the tumors presented a luminal phenotype (luminal A was somewhat more frequent) (Table 1).
Clinical-epidemiological Characteristics.
| Variables | N (%) |
|---|---|
| Nulliparous | 26 (12.3) |
| Menopausal | 140 (62.5) |
| Family history | 32 (14.3) |
| PH malignant breast pathology | 4 (1.8) |
| Unilateral | 222 (99.1) |
| Final histologic type | |
| IDC | 62 (27.7) |
| DCIS | 27 (12.1) |
| IDC + DCIS | 103 (46) |
| ILC | 29 (12.9) |
| Benign | 3 (1.3) |
| Grade | |
| 1 | 52 (23.4) |
| 2 | 123 (55.4) |
| 3 | 47 (21.2) |
| Immunohistochemistry | |
| Luminal A | 88 (39.5) |
| Luminal B | 59 (26.5) |
| HR+, Her2 positive | 3 (1.3) |
| HR−, Her2 positive | 22 (9.9) |
| Triple negative | 10 (4.5) |
| DCIS, HR+ | 33 (14.8) |
| DCIS, HR– | 7 (3.1) |
| Histologic size (mm)a | 21.1 (13) |
| Diagnostic agea | 56.4 (11.7) |
PH: personal history; IDC: invasive ductal carcinoma; DCIS: ductal carcinoma in situ; ILC: invasive lobular carcinoma; LCIS: lobular carcinoma in situ; HR: hormone receptors.
Most patients were treated conservatively (31.3% mastectomies), and only 7.6% required another surgery to extend the margins, with demonstrated tumor persistence in 5 patients.
With regard to diagnostic tests, mammography was performed in 99.5% of the patients, ultrasound in 96.4% and MRI in 100%.
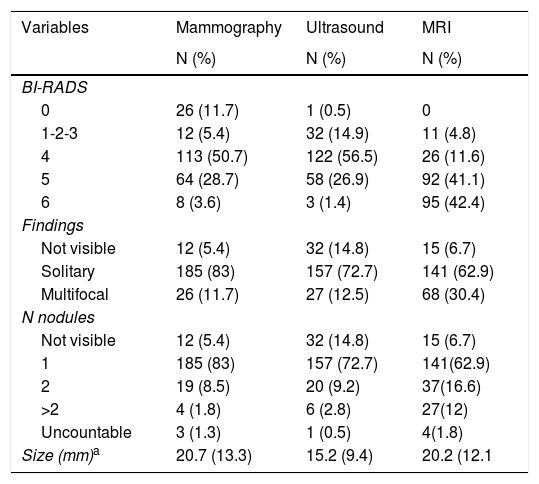
Table 2 shows how the mammogram was considered an incomplete evaluation in 11.7%, and breast cancer was not observed in 12 cases (5.4%). Ultrasound was considered benign or very likely to be benign in 14.9% of the study population, and did not visualize cancer in 32 cases (14.8%). In both mammography and ultrasound, a solitary lesion was the most common finding. On MRI, the tumor was not visible in 15 cases (6.7%) but multifocal involvement was observed more frequently, which supports the theory that MRI detects a greater number of accessory nodes, both in the ipsilateral and contralateral breasts.
Imaging Tests.
| Variables | Mammography | Ultrasound | MRI |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| BI-RADS | |||
| 0 | 26 (11.7) | 1 (0.5) | 0 |
| 1-2-3 | 12 (5.4) | 32 (14.9) | 11 (4.8) |
| 4 | 113 (50.7) | 122 (56.5) | 26 (11.6) |
| 5 | 64 (28.7) | 58 (26.9) | 92 (41.1) |
| 6 | 8 (3.6) | 3 (1.4) | 95 (42.4) |
| Findings | |||
| Not visible | 12 (5.4) | 32 (14.8) | 15 (6.7) |
| Solitary | 185 (83) | 157 (72.7) | 141 (62.9) |
| Multifocal | 26 (11.7) | 27 (12.5) | 68 (30.4) |
| N nodules | |||
| Not visible | 12 (5.4) | 32 (14.8) | 15 (6.7) |
| 1 | 185 (83) | 157 (72.7) | 141(62.9) |
| 2 | 19 (8.5) | 20 (9.2) | 37(16.6) |
| >2 | 4 (1.8) | 6 (2.8) | 27(12) |
| Uncountable | 3 (1.3) | 1 (0.5) | 4(1.8) |
| Size (mm)a | 20.7 (13.3) | 15.2 (9.4) | 20.2 (12.1 |
The evaluation of malignancy by imaging studies was done with the BI-RADS1 classification system, where 84.8% of the ultrasound results, 83% of the mammographic results and 95.1% of the MRI were classified preoperatively as BI-RADS 4 or higher (Table 2). MRI in up to 42.4% of cases was categorized as BI-RADS 6, given that it was performed as a complementary study in patients already diagnosed with breast cancer.
Tumor size measurement was not specified equally in the different imaging techniques and was available in 38.9% of mammograms, 95.6% of ultrasounds and 88.4% of MRI.
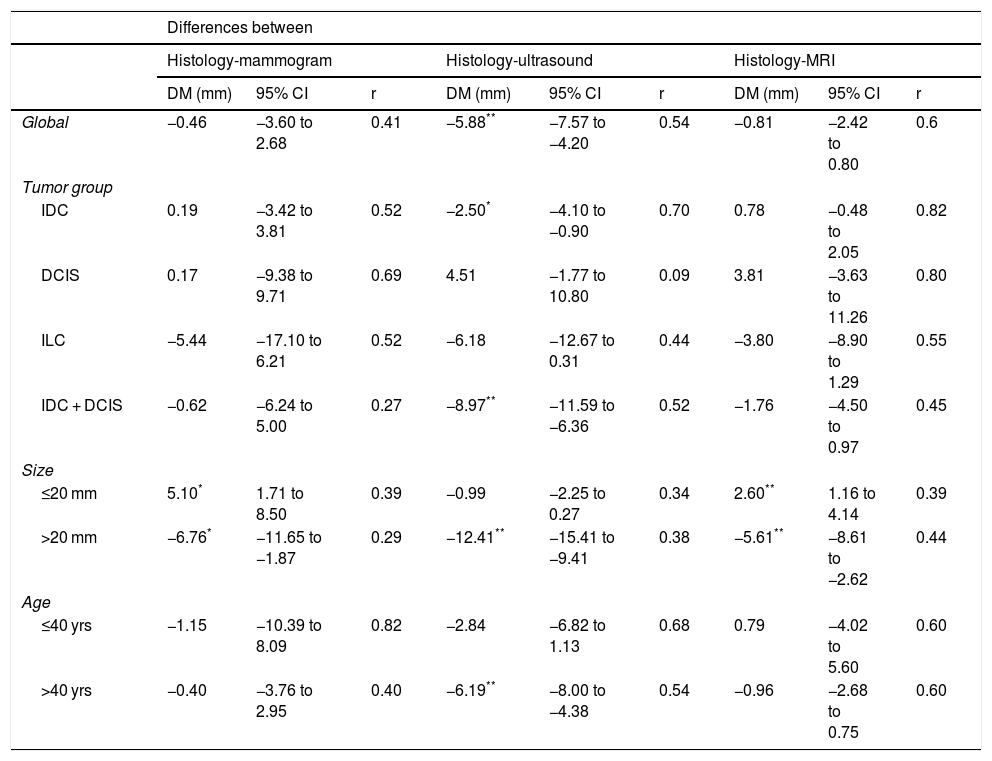
Correlation of the Histological Size With the Ultrasound, Mammogram and Magnetic Resonance Imaging ResultsTable 3 compares the sizes from the different imaging tests compared to the histology results.
Correlation Between Tumor Size on Imaging Studies and the Histologic Tumor Size.
| Differences between | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Histology-mammogram | Histology-ultrasound | Histology-MRI | |||||||
| DM (mm) | 95% CI | r | DM (mm) | 95% CI | r | DM (mm) | 95% CI | r | |
| Global | −0.46 | −3.60 to 2.68 | 0.41 | −5.88** | −7.57 to −4.20 | 0.54 | −0.81 | −2.42 to 0.80 | 0.6 |
| Tumor group | |||||||||
| IDC | 0.19 | −3.42 to 3.81 | 0.52 | −2.50* | −4.10 to −0.90 | 0.70 | 0.78 | −0.48 to 2.05 | 0.82 |
| DCIS | 0.17 | −9.38 to 9.71 | 0.69 | 4.51 | −1.77 to 10.80 | 0.09 | 3.81 | −3.63 to 11.26 | 0.80 |
| ILC | −5.44 | −17.10 to 6.21 | 0.52 | −6.18 | −12.67 to 0.31 | 0.44 | −3.80 | −8.90 to 1.29 | 0.55 |
| IDC + DCIS | −0.62 | −6.24 to 5.00 | 0.27 | −8.97** | −11.59 to −6.36 | 0.52 | −1.76 | −4.50 to 0.97 | 0.45 |
| Size | |||||||||
| ≤20 mm | 5.10* | 1.71 to 8.50 | 0.39 | −0.99 | −2.25 to 0.27 | 0.34 | 2.60** | 1.16 to 4.14 | 0.39 |
| >20 mm | −6.76* | −11.65 to −1.87 | 0.29 | −12.41** | −15.41 to −9.41 | 0.38 | −5.61** | −8.61 to −2.62 | 0.44 |
| Age | |||||||||
| ≤40 yrs | −1.15 | −10.39 to 8.09 | 0.82 | −2.84 | −6.82 to 1.13 | 0.68 | 0.79 | −4.02 to 5.60 | 0.60 |
| >40 yrs | −0.40 | −3.76 to 2.95 | 0.40 | −6.19** | −8.00 to −4.38 | 0.54 | −0.96 | −2.68 to 0.75 | 0.60 |
DM: difference of means; CI: confidence interval; r: correlation coefficient; MRI: magnetic resonance.
Mammography and MRI underestimated the tumor size globally by 0.46 mm and 0.81 mm, respectively. Both techniques significantly overestimated small tumors and also significantly underestimated larger tumors. No significant differences were observed depending on the tumor group or patient age.
Ultrasound significantly underestimated overall tumor size (5.88 mm) and also lesions in women over the age of 40, tumors larger than 20 mm, and in the IDC and IDC + DCIS tumor groups.
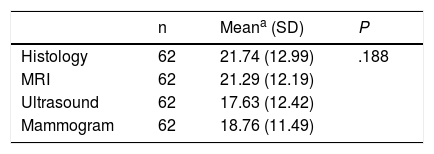
Overall, when we compared the mean sizes of the 3 imaging tests with the actual histological size (Table 4), including in this calculation only those patients who have data for the 3 diagnostic tests (62 patients), no statistically significant differences were found. The similarity of MRI and histology seemed clinically relevant, although the sample size was small.
Comparison of Mean Tumor Size by Imaging Tests and Histological Tumor Size.
| n | Meana (SD) | P | |
|---|---|---|---|
| Histology | 62 | 21.74 (12.99) | .188 |
| MRI | 62 | 21.29 (12.19) | |
| Ultrasound | 62 | 17.63 (12.42) | |
| Mammogram | 62 | 18.76 (11.49) |
| n | Meana (SD) | P | |
|---|---|---|---|
| Histology | 169 | 21.07 (13.12) | <.0001 |
| MRI | 169 | 19.17 (10.63) | |
| Ultrasound | 169 | 15.30 (9.54) |
When we excluded mammography from this comparison and we considered the 169 patients who had complete histological, ultrasound and MRI information, we observed that MRI remained similar to histology, finding significant differences between the two measurements and ultrasound
DiscussionTo properly plan the surgical management of patients diagnosed with breast cancer, it is essential to accurately predict tumor size at the time of diagnosis, as evaluated by imaging tests. Therefore, many studies have tried to assess the degree of correlation between imaging test findings and the final histological size, with differing results.
In our study, mammography underestimated tumor size, although not significantly. This finding coincides with the study by Hieken et al.,6 which also showed underestimated tumor size by mammogram that was attributed to the high compression of the breast during the examination.
We must bear in mind that precise tumor size measurements using this technique can also be negatively affected by increased breast density.7–9 Higher density is usually seen in younger patients, and that is why we include the age variable when interpreting our results. We observed that the mammogram size estimation was not negatively affected by patient age and, therefore, neither by breast density, which coincides with Leddy et al.10 However, these results may be limited by the low rate of tumor size specification by mammogram (38.9%). Hung-Wen et al.11 attributed this low report (18.9%) to the high breast density that makes it difficult to detect and measure tumor size. Thus, breast density could be affecting our low number of measurements and, therefore, our results.
When we analyzed the histological group and mammogram, no significant differences were found in the accuracy of the tumor measurement, coinciding with Gruber et al.12 However, we should point out that mammography more notably underestimated the ILC group, without being significant. These results lean towards the same direction as the publication by Leddy et al.,10 where this underestimation was significant. The lack of significance in our study is probably due to the small sample size in this group.
Regarding tumor size in the context of previous publications, it is very important to consider lesion size when interpreting mammogram images, as we observed that mammography significantly underestimates the size of tumors greater than 20 mm and overestimates those that are smaller.
When we analyzed the ultrasound results, we found that they very significantly underestimate tumor size. This underestimation was greater in the measurement of larger tumors, in older patients. Bosch et al.13 related the underestimation of large tumors with technical difficulties, as, in many cases the image exceeds what is technically possible to measure with the transducer. The size of the ultrasound device probe, which is generally less than 5 cm wide, is too small to accurately estimate T3, or larger tumors. Therefore, for tumors larger than T2, some authors recommend MRI, mammography.11
The ultrasound underestimation was also more evident in the IDC and IDC + DCIS subgroups. This finding coincides with previously published studies.6,11–14 Hieken et al.6 attributed it to the fact that ultrasound does not show precise margins in tumor groups with extensive ductal in situ components. Other studies,15–17 such as the one published by Soo et al.,17 have found that in histological types like DCIS (or in some cases of IDC), which present with microcalcifications, these are difficult to identify and measure by ultrasound. One difference versus the published studies is that while Gruber et al.12 and Print et al.18 observed the maximum underestimation in the ILC group (due to its difficult measurement caused by the diffuse and infiltrating growth pattern),19 we have observed this maximum underestimation in the IDC + DCIS group. This is justified by our previous statements that they are less identifiable lesions and have less precise margins on ultrasound.
According to the results of our study, MRI underestimates tumor size globally, without becoming significant. This finding does not coincide with what was previously published,20 as Gruber et al.12 and Leddy et al.10 reported overestimated tumor size on MRI.
When the patients were classified by tumor size, we observed that MRI significantly overestimated tumors smaller than 20 mm. On the other hand, it also significantly underestimated tumors larger than 20 mm. These results do not coincide with those published by Onesti et al.,20 which describe a significant overestimation especially for tumors >2 cm. In general, MRI has been shown to have higher agreement rates for small tumors than for large tumors.11,21 Further studies would be necessary to determine the accuracy of MRI measurements based on tumor size.
Regarding the tumor type, we can affirm that MRI has not demonstrated significant differences in measuring tumor size, although it is true that it seems to overestimate the size of DCIS and IDC in a non-significant manner, something also shown in Hung-We et al.11 When compared to other imaging techniques, it more precisely measures ILC, as shown by Rodenko et al.,22 Leddy et al.10 and Hung-We et al.11 In addition, these types of tumors tend to be multifocal due to the formation of peritumoral satellite foci, which are more accurately visualized with MRI. Therefore, in these cases, MRI is justifiable for surgical planning.
In short, studies that have comparatively analyzed the accuracy of diagnostic measurements by mammogram, ultrasound and MRI have reached diverse conclusions.10,12,23,24 However, most coincide with our results25–29 and have observed better correlation between MRI and histological tumor size compared to the other diagnostic tests.
Therefore, we can conclude that, according to our results, MRI is the best predictor of tumor size in breast cancer, and it is very important to individualize each case while considering the tumor characteristics. Both the histological subtype and tumor size can vary the accuracy of the size estimation of each of the imaging tests, which must be considered when planning the best treatment for the patient. In contrast, the age variable does not seem to interfere with the accuracy of the tumor measurement. These results should be taken with caution, as more prospective studies with larger series are needed.
Conflict of InterestsNone.
Please cite this article as: Cuesta Cuesta AB, Martín Ríos MD, Noguero Meseguer MR, García Velasco JA, Matías Martínez M, Bartolomé Sotillos S, Abreu Griego E. Precisión de la resonancia magnética, ecografía y mamografía en la medida del tamaño tumoral y su correlación con el tamaño histopatológico en el cáncer de mama primario. Cir Esp. 2019;97:391–396.