The interest for endoscopic pulmonary anatomic resections has grown exponentially during the last decade. During thoracoscopic procedures surgeons cannot rely on digital handling and operative field is viewed on a two-dimensional video monitor, thus frequently encountering anatomical difficulties. The hypothesis is that foreknowledge of the anatomy of each patient would greatly contribute to the safety and accuracy of the operation.
The aim of the study was to evaluate the effectiveness of three-dimensional multidetector computed tomography (3D-MDCT) software to identify the pulmonary artery branching pattern during the preoperative study of endoscopic lobectomies and segmentectomies.
MethodsDescriptive prospective study of 25 consecutive patients scheduled from November 2015 to July 2016 in a tertiary referral academic hospital for VATS lobectomy or segmentectomy and evaluated about branching pattern of the pulmonary artery with preoperative 16-row 3D-MDCT angiography. Intraoperative findings of the pulmonary branching pattern were compared with the preoperative 3D-MDCT angiography images.
ResultsAccording to the intraoperative findings, 67 out of 68 (98%) of pulmonary artery branches were well defined in the 3D-MDCT angiography images. There was a unique 2mm undetected branch. No conversion to open thoracotomy was needed because of intraoperative bleeding.
Conclusion3D-MDCT angiography imaging is useful for preoperative identification of the pulmonary artery branching pattern.
El número de resecciones pulmonares mayores endoscópicas ha presentado un incremento exponencial durante la última década. La realización de la videotoracoscopia (VTC) puede ocasionar dificultades para la correcta interpretación de la anatomía torácica debido a la ausencia de exploración manual y de la visión en profundidad en el caso de trabajar con monitores bidimensionales. En consecuencia, el hecho de conocer con exactitud la anatomía de cada paciente contribuiría enormemente a la realización de una cirugía segura y precisa. El objetivo del estudio es analizar la eficacia de las reconstrucciones volumétricas realizadas mediante angiotomografía computarizada multidetector para identificar el patrón de ramificación de la arteria pulmonar en el preoperatorio de lobectomías y segmentectomías endoscópicas.
MétodosEstudio descriptivo prospectivo de 25 pacientes seleccionados de noviembre de 2015 a julio de 2016 para realización de lobectomía/segmentectomía VTC en un hospital de tercer nivel. En todos los casos se realizó una reconstrucción volumétrica de la arteria pulmonar mediante angiotomografía computarizada multidetector de 16 coronas. Se analizaron comparativamente el número de ramas arteriales identificadas mediante reconstrucción volumétrica y las observadas durante la resección pulmonar.
ResultadosEn total 67 de las 68 (98%) ramas de la arteria pulmonar fueron correctamente identificadas mediante la reconstrucción volumétrica preoperatoria. La única rama no objetivada mediante la reconstrucción volumétrica presentaba un diámetro menor a 2mm. No fue precisa ninguna conversión a toracotomía abierta debido a accidente vascular.
ConclusionesLa reconstrucción volumétrica es útil como herramienta diagnóstica preoperatoria para la correcta identificación del patrón de ramificación de la arteria pulmonar.
Video-assisted thoracoscopic (VATS) lobectomies and segmentectomies have been established as surgical techniques of choice for the treatment of lung cancer, pulmonary metastases and benign lung diseases.1–3 The efficacy of the endoscopic approach has been described on numerous occasions in the medical literature, providing the following advantages: lower morbidity, better postoperative respiratory function, and oncological results similar to those obtained by thoracotomy.4–9 The branching pattern of the pulmonary vessels presents great interindividual variability, a characteristic requiring detailed knowledge of the locoregional anatomy. The need to know the anatomy of the patient is accentuated in endoscopic surgery because it lacks manual exploration and depth perception when working with two-dimensional monitors. Computed tomography angiography provides volumetric reconstructions (3D-CTA) easily and quickly, while providing very practical visual information.10–14 The hypothesis of this study is that the preoperative identification of the pulmonary artery branching pattern would effectively contribute to the prevention of vascular accidents. The aim of the study is to analyze the efficacy of 3D-CTA for the preoperative planning of VATS lobectomies and segmentectomies.
MethodsThe following is a descriptive, prospective study of 25 patients selected for lobectomy or VATS segmentectomy from November 2015 to July 2016 at the only third-level hospital in the corresponding geographical area. The inclusion criteria for VATS lobectomy or segmentectomy included adult patients with stage I lung cancer, central lung metastases and benign lung disease (lobar emphysema). In lung cancer, mediastinal staging guidelines of the European Thoracic Surgery Society were applied.15 In all cases of lung cancer, systematic lymphadenectomy was carried out after lung resection according to the recommendations established by the European guidelines: en bloque resection of mediastinal lymphadenopathies together with adjacent tissue/fat of at least 3 lymph node regions (among which the subcarinal region was always included), accompanied by the resection of hilar and intrapulmonary lymphadenopathies.16 The exclusion criteria of the study were lung cancer stages II and III, body mass index >30, peripheral pulmonary metastases (defined as metastases in the external third of the lung parenchyma), previous thoracic surgery, previous thoracic radiotherapy and known history of tuberculosis. Informed consent was obtained from all patients, and the Declaration of Helsinki guidelines for data confidentiality were followed. As part of the preoperative study, volumetric reconstructions were obtained for all patients using multidetector computed tomography with intravenous contrast, in accordance with our hospital protocol.
All patients underwent 16-crown multidetector computed tomography (Philips Ingenuity Flex™ V3.6.6.13104, Philips Healthcare, Netherlands) with the following parameters: rotation speed 0.5s; collimation 16×0.75; and voltage 120kV. The 1-mm thick digital images were transferred to a workstation with software designed to create multiplanar and volumetric reconstructions. CTA provided a very precise three-dimensional map of the pulmonary artery in 15–25min.
Surgeries were performed under general anesthesia, selective intubation and in the lateral decubitus position. VATS lobectomies and segmentectomies were performed with 2 or 3 ports, according to the surgeon's preferences: one 10.5mm trocar in the 8th intercostal space anterior to the axillary line for 30° optics; another 10.5mm trocar in the 7th/8th intercostal space posterior to the axillary line; and a 3cm utility incision in the 4th intercostal space anterior to the axillary line. We used instruments designed for major endoscopic lung resection. Rib spreaders were not used in any of the patients.
The three-dimensional vascular map was interpreted jointly by two thoracic surgeons and a specialized chest radiologist. Arterial branches were considered “undetected” if they were observed during pulmonary resection but had not been identified in the previous radiological study.
Statistical AnalysisThis is a prospective, descriptive study. Demographic variables, 3D-CTA information and pulmonary resection data were compiled in a standardized Microsoft Excel v.15.17 template. Absolute frequency measures and percentages were used to describe the nominal variables. The quantitative variables were expressed by means of centralization statistics.
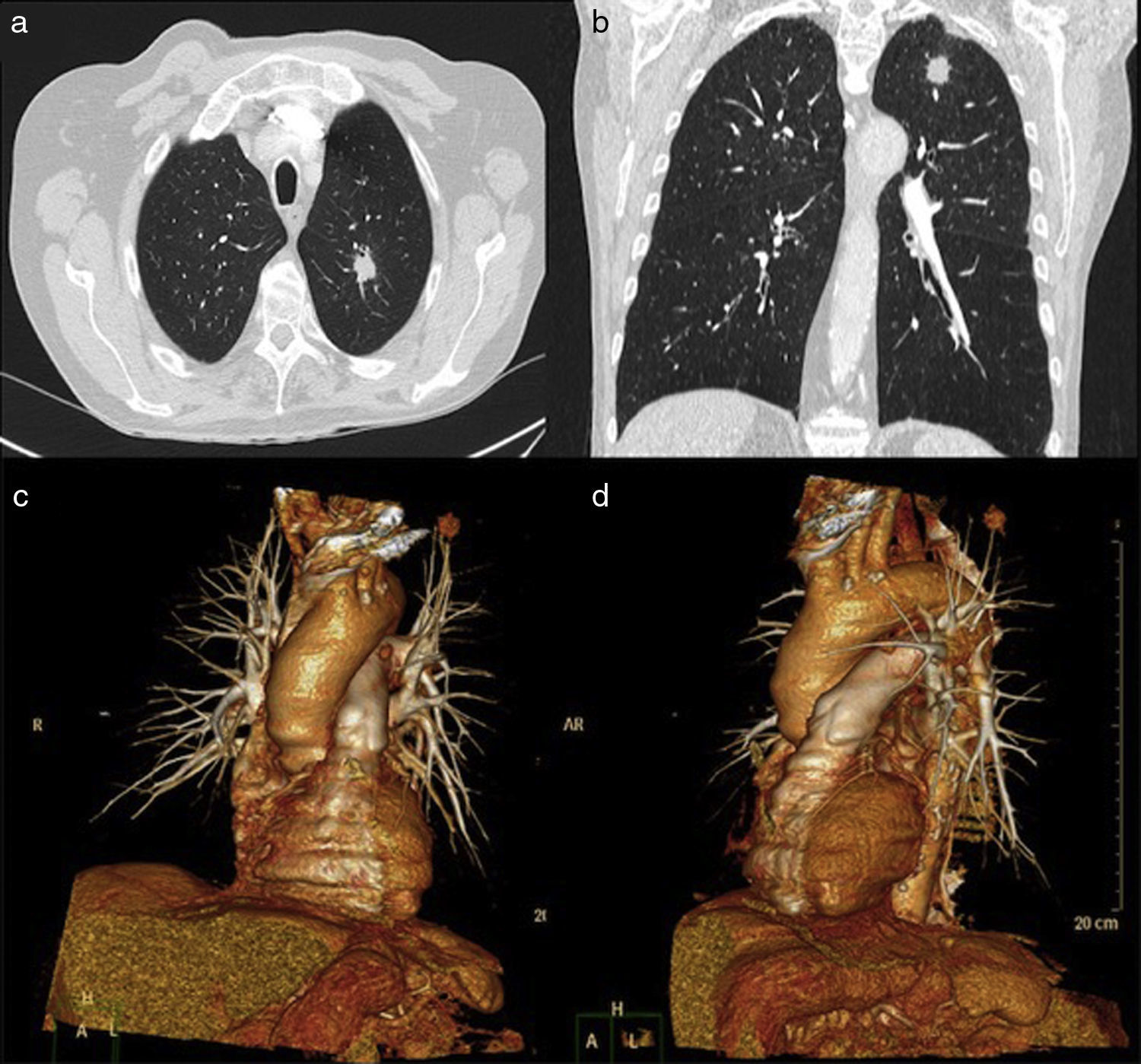
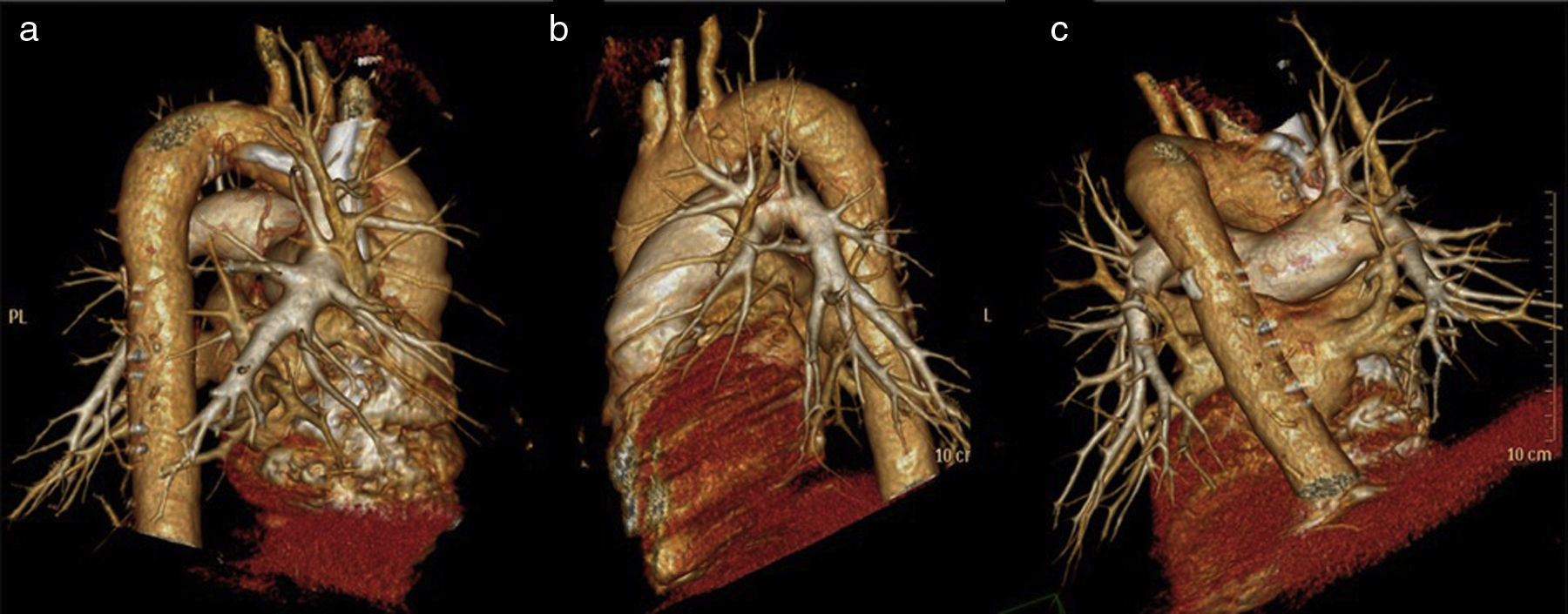
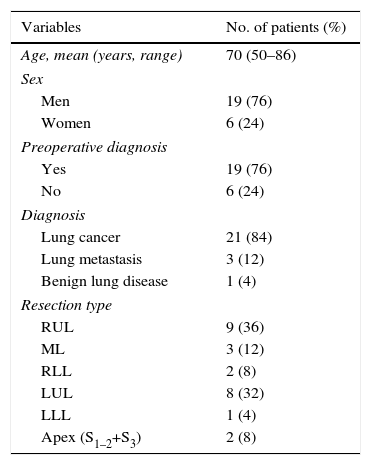
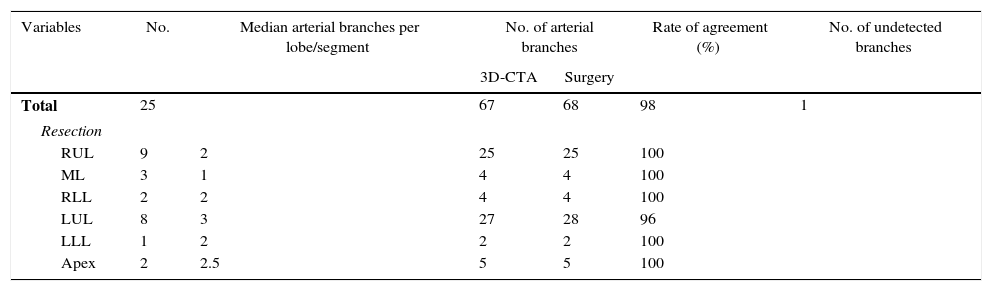
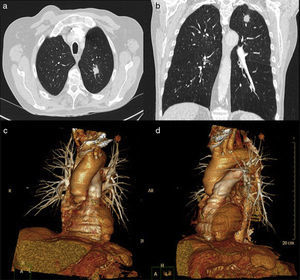
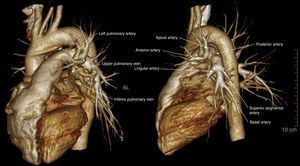
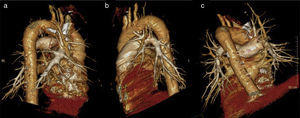
ResultsThe demographic characteristics of patients who underwent VATS lobectomy or segmentectomy during the study are shown in Table 1. The cohort of 25 patients included 19 (76%) men and 6 (24%) women, with a mean age of 70 years (r: 50–86). In 19 of the cases (76%), the histological diagnosis was obtained in the preoperative extension study. The remaining 6 cases (24%) required intraoperative biopsy for pulmonary nodule determination. The definitive diagnosis was 21 cases (84%) of lung cancer, 3 (12%) lung metastases and one case (4%) of lobar emphysema. Out of the 21 cases of lung cancer, the pathology diagnosis was 12 adenocarcinomas (57%), 7 squamous carcinomas (33%), one carcinoid tumor (5%) and one small-cell lung cancer (5%). The surgically treated stages were: 14 cases pT2aN0M0 (66%), 5 pT1aN0M0 (24%), one pT3satN0M0 (5%) and one pT2bN2M0 (5%). Volumetric reconstructions were created in all cases (Figs. 1–3). There was no case of contraindication for intravenous contrast due to allergy or comorbidity. The VATS resections performed were: right upper lobectomy (9; 36%), left upper lobectomy (8; 32%), middle lobectomy (3; 12%), right lower lobectomy (2; 8%), segmentectomy S1–2+S3 (2; 8%) and left lower lobectomy (1; 4%). Regarding the intraoperative findings, 67 of the 68 branches of the pulmonary artery were correctly identified in the preoperative 3D-CTA (Table 2), resulting in an overall agreement rate of 98%. The only branch not observed by 3D-CTA was tributary of the left upper lobe and had a diameter of less than 2mm. The median number of arterial branches per lobe, confirmed by surgery, was as follows: left upper lobe 3 (r: 2–5); apex 2.5 (r: 2–3); right upper lobe 2 (r: 1–5); right lower lobe 2 (r: 2–3); left lower lobe 2; and middle lobe 1 (r: 1–2). No conversion to open thoracotomy was required due to vascular accident.
Characteristics of Patients who Were Treated With VATS After Obtaining 3D-CTA Reconstruction (n=25).
| Variables | No. of patients (%) |
|---|---|
| Age, mean (years, range) | 70 (50–86) |
| Sex | |
| Men | 19 (76) |
| Women | 6 (24) |
| Preoperative diagnosis | |
| Yes | 19 (76) |
| No | 6 (24) |
| Diagnosis | |
| Lung cancer | 21 (84) |
| Lung metastasis | 3 (12) |
| Benign lung disease | 1 (4) |
| Resection type | |
| RUL | 9 (36) |
| ML | 3 (12) |
| RLL | 2 (8) |
| LUL | 8 (32) |
| LLL | 1 (4) |
| Apex (S1–2+S3) | 2 (8) |
3D-CTA: three-dimensional computed tomography angiography; RLL: right lower lobe; LLL: left lower lobe; ML: middle lobe; RUL: right upper lobe; LUL: left upper lobe; VATS: video-assisted thoracoscopic surgery.
Identification Rate of Pulmonary Artery Branches by 3D-CTA and VATS Surgery.
| Variables | No. | Median arterial branches per lobe/segment | No. of arterial branches | Rate of agreement (%) | No. of undetected branches | |
|---|---|---|---|---|---|---|
| 3D-CTA | Surgery | |||||
| Total | 25 | 67 | 68 | 98 | 1 | |
| Resection | ||||||
| RUL | 9 | 2 | 25 | 25 | 100 | |
| ML | 3 | 1 | 4 | 4 | 100 | |
| RLL | 2 | 2 | 4 | 4 | 100 | |
| LUL | 8 | 3 | 27 | 28 | 96 | |
| LLL | 1 | 2 | 2 | 2 | 100 | |
| Apex | 2 | 2.5 | 5 | 5 | 100 | |
3D-CTA: three-dimensional computed tomography angiography; RLL: right lower lobe; LLL: left lower lobe; ML: middle lobe; RUL: right upper lobe; LUL: left upper lobe; VATS: video-assisted thoracoscopic surgery.
The aim of the study was to analyze the effectiveness of 3D-CTA to determine the pulmonary artery branching pattern as a preoperative study protocol. With this technique, a total of 67 arterial branches (98%) were correctly identified.
According to articles published in the medical literature, 95%–98% of pulmonary artery branches were properly identified in patients who had preoperative 3D-CTA before major lung resection (either VATS or open).12–14 These results are identical to those obtained in our study. In addition, the identification of the anatomical characteristics of each patient before surgery has also been related to a lower rate of postoperative complications and shorter surgical times.17–19 In this context, we have proposed a second phase of the study in patients undergoing VATS anatomic lung resection in order to perform a comparative analysis between patients with preoperative 3D-CTA and those with conventional multiplanar CT.
Although conventional CT provides detailed information about vascular and bronchial patterns in 2D, in practice it is difficult to achieve a composition of the dimensions, direction, length and angle of the vessels. 3D-CTA provides more accurate and detailed information on the regional anatomy of each patient and, consequently, helps surgeons to perfect the anatomic orientation, especially during VATS surgery.
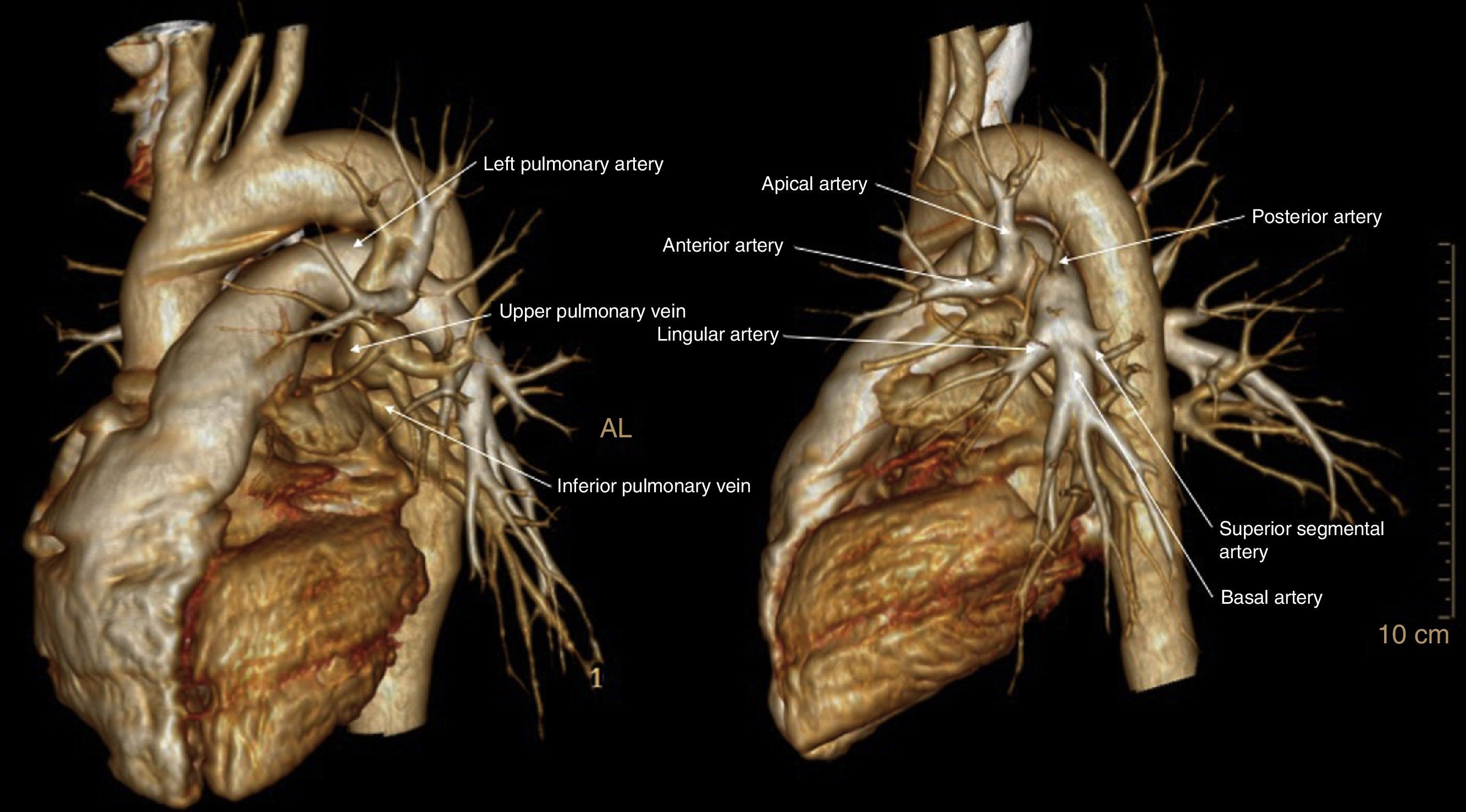
Firstly, 3D-CTA is a minimally invasive and less costly technique than conventional angiography. Second, the images can be rotated, providing dynamic interaction from the desired angle (Figs. 1 and 2). The ability to observe the vascular structures from the point of view of the surgeon is an added advantage. In this manner, its contribution to the teaching and learning of surgical anatomy is immense. As for the disadvantages of 3D-CTA, it is difficult to discern the arterial and venous branches of the upper lobes because they usually overlap in a complex anatomical maze (Fig. 3). The posterior ascending artery and the anterior arterial trunk may be confused with the apical segmental vein or with interlobar venous branches. This fact may be due to the anatomy of the upper lobes, which is more complex than that of the lower lobes. However, the software employed is able to remove unwanted structures and obtain separate images of the pulmonary artery, pulmonary veins and tracheobronchial tree.
The use of segmentectomies is becoming increasingly extended for the resection of lesions with premalignant ground-glass characteristics, initial stages of lung cancer in fragile or elderly patients, and for resection of central lung metastases adjacent to the origin of the vascular branches.20–22
The possibility to visualize the anatomical relationships between the arterial/venous branches and segmental bronchi is very useful for planning VATS segmentectomies due to the difficulty inherent in this type of resection, since they require more extensive and precise dissection of the pulmonary bronchovascular network than lobectomies. In fact, the identification of the segmental vein has been used as a reference to locate the intersegmental plane.11,17 In this way, in the preoperative study the location of the lesion and the margin between the lesion and the intersegmental plane are determined jointly. This fact is particularly applicable to endoscopic segmentectomies, since assistance minithoracotomy is not used and digital palpation cannot be used to identify the location of the lesion23–26 and the existing margin versus the intersegmental plane.
In our series, 2 lung apex resections (S1–2+S3) were performed without incident. The 3D-CTA was able to determine the location of the lesion and its relation with bronchovascular components. As described by Shimizu18 and Oizumi,19 during surgery the intersegmental plane was determined by the insufflation/deflation technique after clamping the corresponding segmental bronchus.
In our experience, we consider it fundamental to evaluate the reconstructions together with the radiologist in order to compare opinions, clarify doubts and perform a study directed at the surgery that we have in mind. It seems reasonable to come upon 3%–5% of non-detectable branches, especially considering that, according to our series, the size of these branches is less than 2mm. The small size of these vessels makes any lacerations or vascular ruptures manageable by VATS. In this sense, Akiba et al.14 reported 2 cases of 2mm arterial branches that had not been identified by the 3D-CTA study. In their series, both cases presented lymph node metastases; therefore, it was suggested that these branches had not been identified, not because of their size, but because of the reduced arterial flow caused by the metastatic infiltration of the lymphadenopathies, leading to arterial compression. In our series, the unidentified 2mm branch corresponded to the pN2 positive patient, so Akiba's explanation would be plausible.
The frequency of conversion from VATS lobectomies to open surgery varies from 2 to 23%, depending on the series.27–29 In our series, there were no conversions due to surgical accidents, probably due to the rigorous selection of cases and the accuracy of 3D-CTA to identify pulmonary vessels, which allowed the surgical team to anticipate potential complications. In this context, we excluded patients with conditions that could potentially make it difficult to perform VATS resection due to the presence of firm pleuropulmonary adhesions or lymphadenopathies adhered to the pulmonary artery, including: locoregionally advanced stages of lung cancer, thoracic radiotherapy, previous lung surgery, and history of known pulmonary tuberculosis.
It is surprising that a minimally invasive technique that does not entail additional economic costs has not been more widely used. It is possible that the need for a specialized radiologist to complete the volumetric reconstruction and the additional elaboration time (15–60min) may have been deterrents. Recently, new software has been released that is capable of performing volumetric reconstructions in a few minutes, without the need for involving or overloading a specialized radiologist.11,17,30 This new program will hopefully facilitate the use and diffusion of 3D technology by surgeons.
In conclusion, 3D-CTA is useful for understanding the patient's anatomy prior to surgery. It is effective in determining the pulmonary artery branching pattern and may contribute to safer VATS lobectomies and segmentectomies. Based on our experience, we recommend requesting a 3D-CTA for endoscopic anatomical pulmonary resections. The second phase of this study, designed as a comparative study, may further support this statement.
Authorship/CollaborationsStudy design: Amaia Ojanguren.
Data acquisition and collection: Amaia Ojanguren, José Luis Recuero, Marina Pardina, Lucía Milla, Maite Santamaría.
Analysis and interpretation of the results: Amaia Ojanguren, José Luis Recuero, Marina Pardina.
Article composition: Amaia Ojanguren.
Critical review: José Luis Recuero, Lucía Milla, Maite Santamaría.
Conflict of InterestsNone.
Please cite this article as: Ojanguren A, Recuero JL, Pardina M, Milla L, Santamaría M. Rentabilidad de la reconstrucción volumétrica de la arteria pulmonar para la planificación de lobectomías y segmentectomías endoscópicas. Cir Esp. 2017;95:102–108.