Pancreatic cancer surgery represents a challenge for surgeons due to its technical complexity, the potential complications that may appear, and ultimately because of its poor survival. The aim of this article is to summarise the scientific evidence regarding the surgical treatment of pancreatic cancer in order to help surgeons in the decision making process in the management of these patients. Here we will review such fundamental issues as the need for a biopsy before surgery, the type of pancreatic anastomosis leading to better results, and the need for placement of drains after pancreatic surgery will be discussed.
La cirugía del cáncer de páncreas es un reto para el profesional debido a su complejidad técnica, las posibles complicaciones derivadas y, en último término, por la mala supervivencia. El objetivo de este artículo es resumir toda la evidencia científica en torno al tratamiento quirúrgico del cáncer de páncreas para poder facilitar al cirujano la toma de decisiones en el manejo de estos pacientes. En él se abordan cuestiones tan fundamentales como la necesidad de practicar una biopsia antes de la intervención, el tipo de anastomosis pancreática con mejores resultados, o la necesidad de la colocación de drenajes tras la cirugía pancreática.
In the field of pancreatic surgery, pancreatic cancer is one of the most predominant pathologies. Its frequency has increased notably over the course of the last 20 years. The American Cancer Society estimated approximately 46420 new pancreatic cancer diagnoses in 2014. Furthermore, in spite of the advances both in surgery as well as oncologic treatment, expected deaths as a consequence of the disease for the same year were 39590,1 and the correlation between new diagnoses and deaths was 0.85.
Surgical resection is the main treatment of pancreatic cancer. Resections should be complete, with wide margins, and associated with appropriate lymph node resection. The high complexity of the surgery and the frequency and importance of its complications make it essential to have a solid knowledge of perioperative patient management. It is therefore necessary for surgeons who treat patients with pancreatic cancer to have the maximum amount of information possible about how to adjust patient management.
Recently, 4 consensus articles have been published by the International Study Group of Pancreatic Surgery (ISGPS), which have dealt with important topics, such as resection in borderline patients, lymph node dissection and extended pancreatectomy, and the need for preoperative pancreatic biopsy.2–5 In this article, it has been our intention to respond to these and other frequent questions that surgeons are confronted with when treating a patient who should undergo pancreatic resection in general and as a consequence of pancreatic cancer in particular, based on current scientific evidence reported in the literature. Furthermore, for each point we have summarised with classifications based on levels of evidence and degrees of recommendation (Table 1).
Levels of Evidence and Grades of Recommendation.
| Levels of evidence | |
| I | Evidence from systematic reviews of well-designed randomised controlled trials |
| II-1 | Evidence from at least one randomised controlled trial |
| II-2 | Evidence from at least one well-designed non-randomised controlled study |
| II-3 | Evidence from at least one well-designed not completely experimental study, such as cohort studies. This refers to the situation in which the application of an intervention is beyond the control of the researchers, but whose effect can be evaluated. |
| III | Evidence from well-designed non-experimental descriptive studies, such as comparative studies, correlation studies or case/control studies |
| IV | Evidence from documents or expert committee opinions or clinical experiences of prestigious authorities or case series |
| Grades of recommendation | |
| A | Good scientific evidence; suggests that the benefits of treatment substantially outweigh the potential risks. |
| B | Fair scientific evidence; suggests that the benefits of treatment outweigh potential risks. |
| C | Fair scientific evidence; suggests that the treatment provides benefits, but the balance between the benefits and the risks is too close to make general recommendations. |
| D | Fair scientific evidence; suggests that the treatment risks are greater than the potential benefits. |
| I | Deficient, poor quality or conflicting scientific evidence; suggests that the risk/benefit correlation cannot be evaluated. |
The levels of evidence and grades of recommendation of this article are adapted from the U.S. Department of Health and Human Services’ Agency for Healthcare Research and Quality (http://www.ahrq.gov).
Classically, the high morbidity and mortality associated with pancreatic surgery meant that the preoperative diagnosis of malignant diseases was a premise for their surgical treatment. Nonetheless, the morbidity and mortality of surgically treated patients has recently experienced a notable reduction thanks to the evolution of pancreatic surgery. Likewise, the improvements in radiological techniques have resulted in better non-invasive diagnostic capability. Right now, the basic radiological technique for the diagnosis of pancreatic cancer is computed tomography (CT), both helical as well as multislice, with a sensitivity of between 76% and 100% according to the data published in the literature.6 The negative consequence of the lack of a histological diagnosis before surgery is, of course, an incorrect diagnosis. It is estimated that between 5% and 10% of cases with clinical and radiological suspicion for malignancy have benign pathology results; meanwhile some 10% of patients with benign preoperative diagnosis will have a positive pathologic result for malignant cells.7–12
In this context, the consensus document by the ISGPS states that routine biopsy is not necessary for suspicious masses in the head of the pancreas. Thus, strong suspicion based on clinical and radiological studies should be sufficient to indicate surgery, and histologic confirmation prior to surgery should be reserved for those cases in which the therapeutic management could change depending on the specific diagnosis. Moreover, in certain pathologies, such as autoimmune pancreatitis, other strategies can be useful, such as IgG4 dosage or short-term corticosteroid treatment.
Nonetheless, in cases in which a histologic diagnosis is considered necessary, what is the best option? Around 70%13–15 of patients with neoplasias located in the head of the pancreas present with elevated bilirubin levels. In cases in which a plastic biliary stent is necessary to ensure correct biliary drainage by means of endoscopic retrograde cholangiopancreatography (ERCP), brush cytology is an option to reach a histologic diagnosis. This technique has shown low sensitivity (in the best cases up to 50%) but high specificity (up to 100% in some series).16,17 It should be remembered that the placement of a preoperative biliary drain in patients with pancreatic cancer is not routinely justified, either because it provides no advantage18,19 or because it increases postoperative complications and should be used only in selected cases.20–23 If brush cytology of the biliary tract is not used, histologic diagnostic methods involve using percutaneous pancreatic biopsy or endoscopic ultrasound (EUS)-guided transduodenal biopsy. Of the two methods, EUS-guided biopsy provides better histologic diagnostic capability while providing a more detailed examination of both the pancreatic gland and the duodenum because of its high capability to detect small-sized lesions.24,25 Its sensitivity is around 90%–100%, with a specificity of 94%–100%.26–30 A controlled randomised study comparing the sensitivity and specificity of EUS-guided or percutaneous (computed tomography/abdominal ultrasound) biopsy showed better results with EUS-guided biopsy, although no significant differences were found between the EUS or percutaneous methods.31 A recent meta-analysis on the risk for pancreatic cancer with atypia results reported global results from 25% to 100% (mean: 58%; 95% CI: 47–69%), but the heterogeneity of the studies used makes it difficult to interpret this factor of this study.32 Finally, an on-site cytologist during EUS is a great asset because direct examination of the histologic samples during the procedure can confirm the diagnosis immediately; if necessary, additional samples can be taken, which ultimately has the capability to raise the already-high sensitivity and specificity of the technique.33
Consequently, the conclusion of the ISGPS5 consensus is that if biopsy is necessary (due to uncertain diagnosis or before initiating neoadjuvant treatment), ERCP with brush cytology can be used when biliary stent placement is necessary. In other cases, EUS-guided biopsy should be the technique of choice whenever possible.
- -
A high level of clinical and radiological suspicion is sufficient in most cases, and preoperative biopsy is reserved for those patients with uncertainties or whose treatment would differ depending on the histologic results. Level of evidence: IV; Grade of recommendation: I.
- -
The test with the best diagnostic performance is EUS-guided biopsy. The presence of a pathologist increases the predictive value of the test. Level of evidence: II; Grade of recommendation: B.
When treating a patient with suspected pancreatic cancer, it is extremely important to clearly determine tumour resectability. This is defined by the absence of tumour involvement of the peripancreatic vascular structures (mesenteric portal axis, superior mesenteric artery, celiac trunk, and corresponding branches). Unfortunately, nowadays it is difficult to confirm vascular infiltration based on imaging tests, and there are publications that have reported up to 40% false positives when true infiltration of the venous wall was later not found during the histological study.34
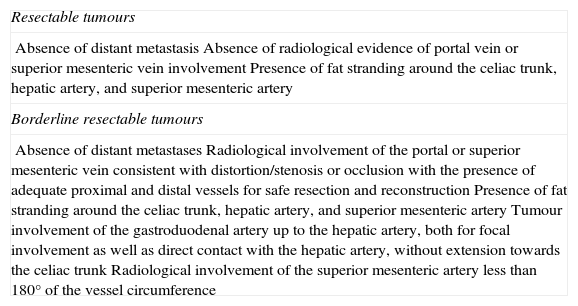
According to preoperative radiological findings, 3 categories of resectability have been defined for pancreatic tumours: resectable, intermediate or borderline resectability, and unresectable (Table 2).35 Both in the case of resectable as well as unresectable patients, the classic approach of surgeons has been evident; what has been more problematic is the management of borderline patients. The consensus declaration of the ISGPS about patients with borderline tumours2 establishes surgical indication in these patients in order to at least confirm whether there is vascular infiltration, especially of the arteries.
Pancreatic Cancer Resectability Criteria.
| Resectable tumours |
| Absence of distant metastasisAbsence of radiological evidence of portal vein or superior mesenteric vein involvementPresence of fat stranding around the celiac trunk, hepatic artery, and superior mesenteric artery |
| Borderline resectable tumours |
| Absence of distant metastasesRadiological involvement of the portal or superior mesenteric vein consistent with distortion/stenosis or occlusion with the presence of adequate proximal and distal vessels for safe resection and reconstructionPresence of fat stranding around the celiac trunk, hepatic artery, and superior mesenteric arteryTumour involvement of the gastroduodenal artery up to the hepatic artery, both for focal involvement as well as direct contact with the hepatic artery, without extension towards the celiac trunkRadiological involvement of the superior mesenteric artery less than 180° of the vessel circumference |
| Unresectable tumours | |
| Head | Distant metastasisInvolvement of the superior mesenteric artery of more than 180°; any involvement of the celiac trunkNon-reconstructable occlusion of the portal/superior mesenteric veinInvasion or inclusion of the inferior vena cava or aorta |
| Body | Distant metastasisInvolvement of the superior mesenteric artery of the celiac trunk of more than 180°Non-reconstructable occlusion of the portal/superior mesenteric veinInvasion of the aorta |
| Tail | Distant metastasisInvolvement of the superior mesenteric artery or celiac trunk of more than 180° |
| Lymph node involvement | Lymph node metastases beyond the resection field should be considered unresectable |
Adapted from the NCCN Guidelines (version 1.2014).
Initially, the presence of venous involvement was a contraindication for resection because of its poor results.36 Over time, the appearance of patient series with venous resections have demonstrated their safety and long-term results comparable with those of patients without venous resections,37–42 even though some authors see a greater proportion of patients with R1 after venous resection.40,43 Other literature reviews have shown that reported cases favour vein resection, with results similar to those of patients without venous involvement and 5-year patient survival between 5% and 18%.44,45 Finally, a recent meta-analysis comparing the published results between patients with and without portal vein involvement did not show differences in perioperative morbidity (OR: 0.95; 95% CI: 0.74–1.21; P=.67), mortality (OR: 1.19; 95% CI: 0.73–1.96; P=.48) or overall 5-year survival (OR: 0.57; 95% CI: 0.32–1.02; P=.06) between patients who had and those who had not undergone venous resection.46 The consensus declaration of the ISGPS is that vein involvement does not contraindicate resection, as long as the vascular reconstruction is feasible and done at hospitals with a high volume and experience in this type of interventions.
Patients with arterial involvement, however, present a clearly inferior survival compared with patients without this involvement. Artery resection is accompanied by important morbidity and mortality rates as a consequence of post-surgical complications. Despite the existence of series that do not show a poorer short-term prognosis in patients who have required artery resection47 and isolated reports of cases of artery resection in cases of neoplasias of the body/tail of the pancreas,48,49 current evidence does not support this opinion. A recent meta-analysis of patients with artery resection showed a significantly higher risk for perioperative mortality (OR: 5.04; 95% CI: 2.69–9.45; P<.0001) and lower 1-year and 3-year postoperative survival rates compared with patients without artery resection.50 The ISGPS consensus document2 concludes that there is no clear evidence that artery resection in cases of cancer of the head of the pancreas provides any benefits and should be done in the context of clinical trials and only for exceptional cases. Therefore, artery resection is not recommended in cases of pancreatic cancer.
It has been demonstrated that one of the prognostic factors of patients with pancreatic cancer is the complete resectability of the tumour. Consequently, it is extremely important to obtain the maximum amount of anatomopathologic information from the surgical specimen to be able to establish a prognosis and be able to adjust the complementary treatment for these patients. The ISGPS supports the histologic criteria of the British Royal College of Pathologists (RCPath)51 for pancreatic cancer, and urges pathologists to provide information about all the margins of the surgical specimen, including the depth of the vascular invasion.
- -
Whenever technically possible, tumour invasion of the venous vascular axis is not a contraindication for surgical treatment. Level of evidence: I; Grade of recommendation: A.
- -
Although there are reports of isolated cases in the literature, current evidence does not recommend artery resection in cases of pancreatic cancer. Level of evidence: I; Grade of recommendation: A.
Given the poor survival of patients with pancreatic cancer, several authors have proposed extended surgical resection with the aim to prolong patient survival. This extended resection can be interpreted as a lymphadenectomy that goes beyond the classical technique or also organ resection outside of conventional resectability criteria.
Extended LymphadenectomyStandard lymphadenectomy in pancreatic cancer includes resection of the anterior and posterior pancreaticoduodenal, pyloric, bile duct, suprapancreatic and infrapancreatic lymph nodes (N1).52 The consensus declaration of the ISGPS4 defines the so-called standard lymphadenectomy for each type of resection: pancreaticoduodenectomy (lymph nodes from stations 5, 6, 8a, 12b1, 12b2, 12c, 13ª, 13b, 14a, and 14b in the right lateral margin) and distal pancreatectomy (10, 11, 18, and 9 if the tumour is in the body of the pancreas, in addition to splenectomy). Likewise, the minimum number of resected lymph nodes should be 15.
Early observations based on retrospective Asian series53,54 that found that lymphadenectomy did not reach areas affected by the neoplasia suggested the idea of an extended lymphadenectomy (extended to the lymph nodes of the hepatic hilum, celiac trunk, superior and inferior mesenteric artery, para-aorta, and both renal hila (N2).
Several studies have proven that an extended lymphadenectomy does not guarantee an evident improvement in patient survival and can entail postsurgical complications, such as profuse diarrhoea as a consequence, fundamentally, of the lymphadenectomy of the superior mesenteric artery, or a higher rate of delayed gastric emptying. There are studies that do not show any type of advantage in patients with extended lymphadenectomy (EL), such as the retrospective study published by Mukaiya et al.55 with 500 patients, or the most recent randomised study by Nimura et al.56 with 112 patients. Other studies show a slight advantage in patients with EL.43,57 Afterwards, another prospective, randomised study of 79 patients showed no advantage in terms of survival, although it also did not show poorer morbidity or mortality, even though the patients presented more complications like diarrhoea 4 months after surgery.58 Recently, Schwarz et al.59 have published an article demonstrating that the tumour involvement of the para-aortic lymph nodes entails an ill-fated prognosis for the patient and, according to the authors, would contraindicate pancreatic resection.
Last of all, 2 meta-analyses60,61 that reviewed studies with a total of 1900 patients concluded that extended lymphadenectomy is not justified due to the lack of differences in patient survival and due to the presence of higher morbidity in the postoperative period. Thus, the ISGPS consensus does not recommend performing EL.
Extended PancreatectomyInfiltration of pancreatic tumours to the surrounding organs has also been the object of debate. What results are offered by the resection of these structures together with the tumour? Are there data to indicate extending the resection limits or, contrarily, do the results contraindicate this?
Several studies have tried to shed light on this subject in spite of the fact that, due to its nature, there are no prospective randomised studies.62–72 Said studies are homogeneous, and there are numerous different organs and vascular structures involved as well as mixed pancreatic resections, a fact that makes their interpretation difficult. In general and according to the literature, extended pancreatectomies involve increased patient morbidity rates, greater intraoperative haemorrhage, higher rates of blood product transfusion and longer ICU stay. Nonetheless, several authors coincide in indicating that the long-term prognosis of these patients is similar to patients who undergo standard pancreatic resection, which is clearly superior to that obtained with palliative treatment using chemotherapy.
The ISGPS3 consensus clearly defines what is considered extended pancreatectomy in cases of cephalic, distal or total pancreatectomy, and it considers that complete macroscopic tumour resection is feasible in most cases. The conclusion of the authors is to recommend extended pancreatectomy in selected cases, given the results obtained, and always within the framework of a hospital with extensive experience in pancreatic surgery.
- -
It has not been demonstrated that EL contributes to improving long-term results, although it may involve increased postoperative morbidity. Level of evidence: I; Grade of recommendation: A.
- -
In selected patients and in hospitals with experience in pancreatic surgery, it is feasible to associate resections of neighbouring organs directly affected by the neoplasia in order to obtain complete macroscopic resection. Although increased postoperative morbidity can be observed, extended pancreatectomy is justified by long-term results compared to treatment with chemotherapy. Level of evidence: III; Grade of recommendation: C.
In 1974, Traverso and Longmire73 published the use of pylorus-preserving (PP) pancreaticoduodenectomy (PD) in patients with cancer of the head of the pancreas. The idea behind this technical modification was to make enteric reconstruction more physiological, respect the pyloric sphincter and avoid the rapid transit of the bolus towards the intestine. Afterwards, there have been reports that the technique favoured postoperative patient weight gain and better quality of life when compared with the classic intervention.74,75 However, there was the concern that it would be a less radical intervention from an oncologic standpoint and that it would therefore have repercussions on patient survival.
A prospective, randomised study, although with only 33 patients,76 showed identical survival results between groups but also an increased delay in gastric emptying in the PP group. Other randomised and prospective studies74,77–79 have comprehensibly shown that there were no statistically significant differences in either the complications between the two techniques (either post-op or in the long term) or survival. These facts have later been confirmed with the appearance of 3 meta-analyses and a systematic Cochrane review.80–83
- -
Based on current evidence, we can conclude that both techniques are equivalent for oncological and postoperative morbidity results, and that the choice of one of the two techniques depends on the preferences of the surgeon. Level of evidence: I; Grade of recommendation: A.
Although the classic anastomosis for pancreatic reconstruction after resection surgery has been done with the jejunum, there is a growing debate about which anastomosis can provide better short- and long-term results. Today, the problem of postoperative pancreatic fistula is far from being resolved. Moreover, there are also personal reasons to prefer one type of pancreatic anastomosis over another. Probably, the reasons why pancreaticojejunal (PJ) anastomosis is more widely used are diverse. It all began with the initial descriptions of the surgical technique and gained inertia to the point that it is now feasible to utilise the 3 reconstruction anastomoses after PD with the same jejunal loop. In recent years, however, there has been growing evidence about the comparison of PJ with pancreatic-gastric (PG) anastomoses.
In the scientific literature, there are currently several prospective, randomised studies and meta-analyses that deal with this subject. The first prospective, randomised study was published by the Yeo group84 in 1995, which compared the results of 145 patients at Johns Hopkins, divided into two groups: PG vs PJ. The results showed no differences between the two techniques and had a similar rate of fistulas (12.3% vs 11.7%). Five years later, Takano et al.85 published their second article, which was a two-centre study where one hospital used PJ in 69 patients and the other hospital used PG in 73, although it was the same surgical team that performed all the procedures. The results of this study showed PG to be superior, with a lower fistula rate than PJ (0% vs 13%, P=.014); the remaining complications were comparable between the two groups. The following two studies were also published 5 years later. Bassi et al.,86 from the University of Verona, analysed the results of 151 patients who underwent PD and compared PG and PJ groups. The results of their study did not show any differences in the pancreatic fistula rate between groups; however, the patients assigned to PG showed a significantly lower frequency of biliary fistula, postoperative collections and delayed gastric emptying, as well as a lower frequency of other postoperative complications, so they concluded that the results of PG were superior to PJ. That same year, Duffas et al.,87 from the French Association for Surgical Research, reported the results from a randomised multicentre study of 149 patients, with no differences between the two treatment groups. In 2008, Fernandez-Cruz et al.88 showed the results of a prospective randomised study with 108 patients divided between conventional PJ (n=55) and modified PG after gastric partition (PG-GP) (n=53). In this study, the results of the PG-GP were superior to those of PJ in terms of overall complications (23% vs 44%; P<.01) and pancreatic fistula (4% vs 18%; P<.01). Another study published by Wellner et al.89 that included 116 patients was not able to show differences in the pancreatic fistula rate. In this study, PG entailed less operative time and hospital stay, but higher delayed gastric emptying (27% vs 17%; P=.246) and postoperative bleeding (PG vs PJ, 7% vs 2%; P=.364). More recently, in 2013, two randomised prospective studies were published, comparing both techniques. In a multicentre study, Topal et al.90 analysed both techniques in 167 patients who underwent PD, with the particularity of stratifying the patients at each centre according to the Wirsung duct diameter (greater or less than 3mm). Even though the overall number of complications between groups was not different, the severity of the complications (equal or superior to Clavien grade 3a) was higher in the PJ group and, furthermore, the pancreatic fistula rate was lower in the PG group (8% vs 19.8%; P=.002). Last of all, in 2013, Figueras et al.91 published the results of the latest known prospective, randomised study, which is also multicentre and included 123 randomised patients with conventional PJ vs PG done by the invagination technique. The results show PG to be superior in terms of incidence of pancreatic fistulas (34% vs 15%; P=.014) and clinical importance, defined based on ISGPS criteria, lower rate of re-admittances, less weight loss and less loss of pancreatic exocrine function.
In addition, there are several meta-analyses to consider. The two oldest, which compiled the scientific evidence available at the time (2006 and 2007), have considered overall that the safest technique is PG and that PJ has poorer results, with a relative risk for presenting pancreatic fistula, overall morbidity and mortality rates of 2.62 (95% CI: 1.91–3.6), 1.43 (95% CI: 1.26–1.61) and 2.51 (95% CI: 1.61–3.91), respectively.92 They do admit, however, that many of the results are from comparative observational studies and not controlled randomised studies.93 The results of other more recent meta-analyses favour the results of PG over PJ with regards to the rate of overall postoperative complications (OR: 0.53; 95% CI: 0.30–0.95; P=.03), pancreatic fistulas (OR: 0.47; 95% CI: 0.22–0.97; P=.04) and intraabdominal collections (OR: 0.42; 95% CI: 0.25–0.72; P=.001), and in the end their conclusions are that the results of PG are as safe as those of PJ.94
- -
The results of prospective and randomised studies, as well as meta-analyses, conclude that both techniques are safe and that PJ is not superior to PG. Level of evidence: I; Grade of recommendation: A.
Another frequent manoeuvre in pancreatic surgery during pancreatic tract reconstruction is the placement of an intraductal stent, either left in the anastomosis or external to control pancreatic exocrine secretions. This manoeuvre is done with greater frequency in cases in which there is a pancreas with normal characteristics (soft in consistency and with a small-sized Wirsung duct).
In 2002, Poon et al.95 analysed, within the scope of a review article, the factors that may favour the appearance of postoperative pancreatic fistulas. One of the factors analysed that showed a protective effect against pancreatic fistulas was the utilisation of an external transductal pancreatic drain. In 2006, Winter et al.96 published the results of a prospective randomised study in which they first stratified the patients according to the characteristics of pancreas consistency (soft/normal vs hard), after which the patients were randomised for placement of a 6cm duct stent within the jejunal lumen after PD. The results did not show significant differences for pancreatic fistula either in the subgroup of patients with hard pancreas (1.7% vs 4.8%; P=.4) or in patients with normal pancreas (21.1% vs 10.7%; P=.1).
The effect of placing a tube outside the pancreatic duct was analysed in a randomised, prospective study from 2007.97 A total of 120 patients were randomised, with favourable pancreatic fistula results in the group with external pancreatic drains (6.7% vs 20%; P=.032), even though postoperative morbidity and mortality showed no differences between groups. Even better results were obtained in another French multicentre, prospective, randomised study that analysed the influence of the placement of an external pancreatic drain in 158 patients; there were significant differences favouring the group with external biliary drainage in terms of pancreatic fistulas (26% vs 42%; P=.034), morbidity (41.5% vs 61.7%; P=.01), and delayed gastric emptying (7.8% vs 27.2%; P=.001), even though the complication rates were higher than usual.98
Several recent meta-analyses have attempted to define the utility of pancreatic stents, either internal or external, for the prevention of complications in pancreatic surgery. The use of an external drain was the subject of a meta-analysis published in 2011, which showed a reduction in pancreatic fistulas (OR: 0.34; 95% CI: 0.23–0.15; P=.001) as well as better results for global postoperative morbidity, delayed gastric emptying and hospital stay.99 Likewise, a review of 13 articles with a total of more than 1.800 patients comparing the utility of internal vs external drains showed that, although the use of external drains resulted in a decrease in pancreatic fistulas (OR: 0.47; 95% CI: 0.31–0.71; P=.0004) and overall morbidity (OR: 0.64; 95% CI: 0.45–0.90; P=.01), the same was not true when internal drains were used, and the results were even worse (OR: 1.97; 95% CI: 1.05–3.69; P=.03).100 Another meta-analysis from 2013 confirmed the previous data in that the use of an internal pancreatic drain does not reduce the rate of pancreatic fistulas.101 Finally, a recent meta-analysis that evaluated only prospective randomised studies concluded that the utilisation of external pancreatic drains leads to a decrease in pancreatic fistulas (RR: 0.57; 95% CI: 0.41–0.80; P=.001), overall morbidity and duration of hospital stay compared with those patients in whom external drains were not used.102
- -
The use of external pancreatic drain tubes has not been shown to have a positive influence on postoperative morbidity. Level of evidence: I; Grade of recommendation: A.
- -
The placement of external pancreatic duct drains contributes to significantly improve the perioperative results of patients who have undergone PD. Level of evidence: I. Grade of recommendation: A.
Intraabdominal drain placement after pancreas surgery is, in most cases, a routine part of the surgical procedure that is done systematically in most hospitals as an unquestionable part of the intervention. Nonetheless, Petrowsky et al.103 in a systematic review and meta-analysis of the literature from 2004, showed that there are intraabdominal procedures, such as hepatic or colorectal, in which intraabdominal drains can be omitted (Grade of recommendation: A). In esophagogastric surgery, they are necessary due to the potentially fatal consequences of suture dehiscence, which has been corroborated in another later analysis of the literature.104 In fact, this is exactly the problem of pancreatic surgery, where a pancreatic fistula that is not drained can lead to serious consequences for the patient. Thus, in a study by Balzano et al.105 the policy of maintaining the intraoperative drain in cases of left pancreatectomy provided a low rate of fistula-related morbidity. A retrospective Japanese study from 2010 showed that a safe attachment technique of the intraabdominal drain improved pancreatic fistula results in the postoperative period.106
Nonetheless, in 2001, a randomised prospective study was published including179 cases of pancreatic resection surgery (139 PD and 40 distal pancreatectomies) comparing the results of postoperative complications amongst a group of patients assigned to standard intraabdominal drains (closed suction type) and another group of patients without drains. In spite of not finding differences in the number or type of complications between the groups, in the group with drains there was a greater probability of developing a symptomatic intraabdominal abscess, collection or fistula.107 Another non-randomised prospective study of 226 patients (179 with drains, 47 without drains) showed that the patients with intraabdominal drains presented a higher number of postoperative complications (65% vs 47%; P=.020), among them fewer gastric emptying delays. However, a greater proportion of patients had to be treated with the placement of an external drain.108 A retrospective review did not show a different rate of complications in patients with or without drains after distal pancreatectomy, although quick identification did help.109 In a case-control study of patients who had undergone PD in France, it was observed that the patients with low risk for presenting pancreatic fistulas during post-op had a tendency for lower postoperative morbidity and a statistically significant lower rate of pancreatic fistulas (0% vs 22%; P=.009) in the group of patients that were not drained prophylactically; therefore, the authors proposed that pancreatic drainage should not be systematic and that its use could reduce hospital stay after PD.110 A meta-analysis of the data from the literature referring to 494 patients showed a tendency for better results in the patient groups that did not have postoperative drains, but the study showed no statistically significant differences and concluded that the use of drains can result in a greater risk of complications, although the evidence is inconclusive.111 Finally, a recent prospective, randomised, multicentre study that analysed the results with and without drainage in 137 patients after PD showed that the patients without intraabdominal drains presented a greater number of postoperative complications; ultimately, the study was interrupted due to an increase in mortality from 3% to 12% in patients after PD without drains.112
Other studies have analysed how long drains should remain in place. A systematic review of the literature concluded that the prophylactic placement of a drain has not been shown to reduce post-op complications after pancreatic surgery (whether PD or distal pancreatectomy), but if a drain is inserted, it is safe to remove it on the first day post-op if postoperative amylase levels are lower than 5000U/L.113 Another prospective, randomised study of the group from Verona in patients who underwent pancreatic resection with a low probability for pancreatic fistula (amylase levels on the first day post-op below 5000U/L) compared patients after early (3rd day) or standard (after the 5th day) drain withdrawal. The group of patients with early drain withdrawal showed a lower rate of complications, including pancreatic fistula, shorter hospitalisation, and lower hospital costs.114 Likewise, a prospective Japanese study of 104 patients compared the infectious complications of patients who underwent PD and separated the patients into 2 groups: those with drain withdrawal on the 4th or 8th day post-op. The group with early withdrawal presented a lower rate of pancreatic fistulas and infectious intraabdominal complications (3.6% vs 23% and 8% vs 38%, respectively; P<.05).115 A German meta-analysis that included 2 prospective, randomised studies, and 2 prospective cohort studies showed that early drain withdrawal (days 3–4) compared with late withdrawal (>5 days) significantly reduced the presence of fistulas, intraabdominal collections, and hospitalisation.116
- -
Although there are articles with a high degree of evidence that show the safety of not inserting prophylactic intraabdominal drains after pancreatic surgery, other recently appearing articles demonstrate the opposite. Thus, the no-prophylactic-drain policy cannot be recommended. Level of evidence: I; Grade of recommendation: I.
- -
In the case of drain placement, early withdrawal is recommended in favourable cases as this manoeuvre has been shown to reduce the presence of postoperative complications. Level of evidence: I; Grade of recommendation: A.
Because somatostatin (SMT) or its analogue octreotide are able to satisfactorily inhibit pancreatic exocrine secretion,117 its postoperative use has been routine in many hospitals after pancreatic resection with the aim to minimise postoperative pancreatic fistulas.
Several studies have shown advantages to the prophylactic use of SMT or analogues after pancreatic resection surgery. In a randomised prospective study by the University of Heidelberg, the prophylactic use of SMT correlated with a significant decrease in pancreatic fistulas, postoperative abscesses, sepsis, and postoperative pancreatitis.118 An Italian multicentre study of 33 hospitals reported that pancreatic complications significantly decreased after the use of octreotide compared with the placebo group.119 Another Italian study from the same period also showed that the prophylactic use of SMT reduced postoperative complications after pancreatic resection compared with the administration of placebo.120 Shan et al.121 analysed the results of patients with high-risk pancreas after PD. A total of 54 patients were studied, all of whom had been operated on by the same surgeon in order to eliminate the surgeon factor; it was observed that the patients who had received SMT had a reduction in overall morbidity and complications related with the pancreatic stump. Another French prospective randomised study was able to demonstrate a lower incidence of specific pancreatic complications and shorter hospitalisation in patients treated with SMT-14, in addition to a decrease in daily pancreatic secretion volume and amylase concentrations.122 Also from France is the study that randomised 230 patients to treatment groups either with or without octreotide. The treatment group with octreotide presented fewer intraabdominal complications. However, as a criticism to this study, there was a significantly higher number (68% vs 39%) of patients in the octreotide group who were treated with biological glue either within the Wirsung duct or around the anastomosis, a fact that could be a confounding factor when analysing the results.123
Contrarily, some studies demonstrate that the routine prophylactic use of SMT or its analogues does not reduce the number or importance of the specific complications for the pancreas. In a prospective randomised study of 62 patients, Fernandez-Cruz et al.124 showed that the postoperative administration of octreotide did not modify pancreatic secretions in the postoperative period or morbidity rates after pancreatic resection surgery. Likewise, another randomised prospective study of 381 patients after pancreatic resection in patients with chronic pancreatitis provides no benefit for the reduction of specific pancreatic complications.125 In a group of 105 patients, Hesse et al.126 from the University of Gante showed that the use of SMT for 7 consecutive days after pancreatic surgery did not reduce the presence of complications or the formation of fistulas. A randomised, double-blind, multicentre study of 275 patients evaluated the capability of vapreotide to reduce postoperative complications. The result of the study was that no differences were found between groups, so it was therefore concluded that vapreotide did not reduce specific complications between groups.127 Last of all, an American multicentre study evaluating the influence of subcutaneous octreotide use vs placebo showed that it provided no evident improvement and also evaluated the considerable healthcare costs associated with the product. The authors concluded that the prophylactic use of octreotide should be eliminated.128
A meta-analysis that comprised the results from 22 studies (1918 patients) showed that SMT and analogues do not reduce postoperative mortality or the rate of suture dehiscence, but they did significantly reduce overall morbidity, specific complications of the pancreas and the rate of biochemical pancreatic fistulas.129 Another meta-analysis and systematic review of the literature, including 7 studies and a total of 1359 patients, showed that the use of octreotide significantly diminished the risk of pancreatic fistulas after surgery, with a relative risk of 0.59 (95% CI: 0.41–0.85), although it does not have an effect on postoperative mortality.130 These results concur with another more recently published meta-analysis of 2143 patients from Cochrane studies in which we found fewer complications in the SMT group, including postoperative pancreatic fistulas.131 Lastly, a systematic Cochrane review that included 21 studies (although 19 had an elevated risk for bias) concluded that the prophylactic use of SMT reduces postoperative complications but it does not affect postoperative mortality, so the authors recommended its routine use after pancreatic resection.132 Last of all, a systematic review of the literature that studied the influence of SMT or octreotide in the prophylaxis of pancreatic fistula in 1686 patients and the treatment of enterocutaneous fistula in 301 concluded that, in units where pancreatic fistula exceeds 10%, the administration of SMT or analogues can reduce the rate of major postoperative complications, particularly pancreatic fistulas.133 Nonetheless, another meta-analysis that included the experiences of 8 studies did not find differences in the results of the patients after the administration of SMT or its analogues.134
The wide disparity found in the articles in the literature about pancreatic fistulas makes them difficult to interpret. Cases in which pancreatic surgery is less perfect could theoretically benefit from prophylactic treatment with SMT or its analogues to reduce the rate of fistulas after pancreatic resection surgery.
- -
There is an abundance of articles with a high level of evidence that report contradictory results for the efficacy of SMT and its analogues. This is probably due to the great heterogeneity of these studies with regards to SMT dosage and, above all, the different classifications of pancreatic fistula seen among the studies. Thus, we cannot recommend the prophylactic use of SMT or its analogues in all cases of pancreatic resection. Level of evidence: I; Grade of recommendation: A.
This manuscript has received no funding of any kind.
Conflict of InterestThe authors deny having any conflict of interests.
Please cite this article as: Sánchez Cabús S, Fernández-Cruz L. Cirugía del cáncer de páncreas: estrategias quirúrgicas según los datos basados en la evidencia. Cir Esp. 2015;93:423–435.