Breast conservative surgery after neoadjuvant chemotherapy intends to remove any residual tumor with negative margins. The purpose of this study was to analyze the preoperative clinical-pathological factors influencing the margin status after conservative surgery in breast cancer patients receiving neoadjuvant chemotherapy.
MethodsA retrospective study of 91 breast cancer patients undergoing neoadjuvant chemotherapy (92 breast lesions) during the period 2006–2013. A Cox regression analysis to identify baseline tumor characteristics associated with positive margins after breast conservative surgery was performed.
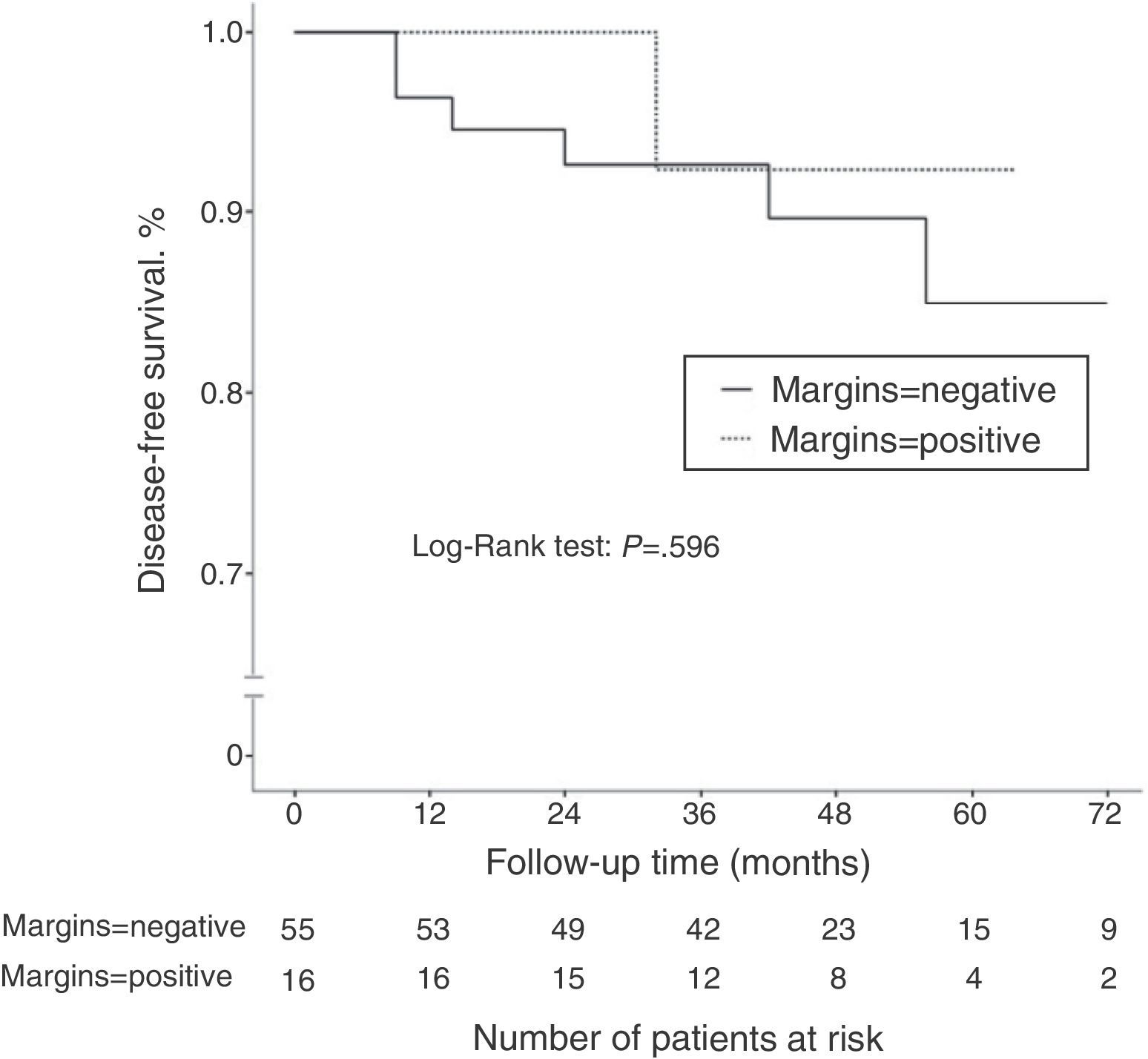
ResultsOf all cases, 71 tumors were initially treated with conservative surgery after neoadjuvant chemotherapy. Pathologic exam revealed positive margins in 16 of the 71 cases (22.5%). The incidence of positive margins was significantly higher in cancers with initial size >5cm (P=.021), in cancers with low tumor grade (P=.031), and in patients with hormone receptor-positive cancer (P=.006). After a median follow-up of 45.2 months, 7 patients of the 71 treated with conservative surgery had disease recurrence (9.8%). There was no significant difference in terms of disease-free survival according to the margin status (P=.596).
ConclusionsA baseline tumor size >5cm, low tumor grade and hormone receptor-positive status increase the risk for surgical margin involvement in breast conservative surgery after neoadjuvant chemotherapy.
La cirugía conservadora de mama tras la quimioterapia neoadyuvante pretende resecar cualquier tumor residual con unos márgenes negativos. El objetivo de este estudio fue analizar los factores clínico-patológicos preoperatorios que influyen sobre el estado de los márgenes de resección tras la cirugía conservadora en pacientes con cáncer de mama tratadas con quimioterapia neoadyuvante.
MétodosEstudio retrospectivo de 91 pacientes con cáncer de mama (92 tumores) tratadas con quimioterapia neoadyuvante durante el periodo 2006–2013. Se realizó un análisis de regresión de Cox para identificar las características basales del tumor asociadas con la afectación de los márgenes de resección tras cirugía conservadora de la mama.
ResultadosDel total de casos del estudio, 71 tumores se trataron inicialmente mediante cirugía conservadora tras la quimioterapia neoadyuvante. El examen patológico reveló afectación de márgenes en 16 de los 71 casos (22,5%). Se observó una mayor incidencia de márgenes positivos en los tumores con un tamaño inicial superior a 5cm (p=0,021), en los tumores de bajo grado histológico (p=0,031) y en los tumores con estatus positivo de los receptores hormonales (p=0,006). Tras un seguimiento medio de 45,2 meses, 7 de las 71 pacientes tratadas con cirugía conservadora presentaron recidiva de la enfermedad (9,8%). No se observaron diferencias estadísticamente significativas en la supervivencia libre de enfermedad según el estado de los márgenes quirúrgicos (p=0,596).
ConclusionesUn tamaño tumoral basal superior a 5cm, el bajo grado tumoral y el estatus positivo de los receptores hormonales incrementan el riesgo para la afectación de los márgenes quirúrgicos en la cirugía conservadora de mama tras quimioterapia neoadyuvante.
Breast-conserving surgery with disease-free margins in breast cancer is equivalent to mastectomy in terms of local control and survival,1 while presenting the advantage of a better psychosocial result.2
Neoadjuvant chemotherapy (NCT) is able to increase the survival rates of breast-conserving surgery without a significant increase in the percentages of local recurrence.3–5 Furthermore, complete pathologic response to treatment improves patient prognosis.6
The state of the resection margins after conservative surgery is one of the most important predictive factors for the risk of locoregional recurrence in breast cancer.7,8 Certain tumor characteristics can increase the risk for reintervention as a consequence of involved surgical margins.
The objective of this study was to identify preoperative clinical-pathological risk factors for the involvement of surgical margins after conservative surgery in patients with breast cancer treated with NCT.
MethodsStudy PopulationBetween October 2006 and June 2013, 91 consecutive patients with histopathological diagnosis of invasive breast cancer were treated with NCT at a single hospital. The initial diagnosis was based on mammogram and ultrasound studies, and histopathological confirmation was established by ultrasound-guided fine-needle aspiration of the lesions observed and by stereotaxis in the case of microcalcifications.
The criteria for neoadjuvant treatment were: clinical presentation in stage IIB-III, unfavorable tumor-to-breast volume ratio, or molecular profile with a high probability for complete pathologic response. Patients with distant metastasis at the time of diagnosis were excluded from the study. We conducted a retrospective review of the clinical and pathological data of the series. The study was approved by the Research Ethics Committee of our Healthcare Area (n 2015/059).
Immunohistochemistry StudyBased on the results from the initial diagnostic biopsy, the tumors were classified into 5 subtypes according to immunohistochemistry characteristics: luminal A, luminal B/HER2−, luminal B/HER2+, HER2+ and triple negative. The HER2 tumors with a score of 3+ were considered positive. If the score was 2+, the fluorescent in situ hybridization technique was used to determine whether there was amplification of the HER2 gene and to confirm or disprove positivity. The samples that did not express HER2 or had a score 1+ were considered HER2−. The cut-off point for ki-67 was set at 14% to determine whether the cell proliferation rate was high (≥14%) or low (<14%).
Axillary Lymph Node Status Prior to Neoadjuvant ChemotherapyIn all patients, axillary ultrasound was done before NCT. Selective biopsy of the sentinel lymph node was used before NCT to stage the axilla in women without clinical-radiological suspicion of axillary involvement. In patients with axillae suspicious of lymph node involvement, biopsies or ultrasound-guided FNA were used to confirm tumor infiltration, except in those cases with massive axillary lymph node involvement seen during magnetic resonance imaging (MRI).
Neoadjuvant Chemotherapy ProtocolThe therapeutic protocols were selected by the Oncology Department of the Breast Pathology Unit. Initial patient evaluation included complete medical history, physical examination, complete blood work-up, chest X-ray, thoracic and abdominal computed tomography, and bone scintigraphy. A titanium clip was placed at the tumor site in all patients before initiating chemotherapy in order to be able to identify the primary tumor area during surgery. Tumor response was monitored by MRI at the start and end of systemic treatment. All patients with HER2+ tumors had trastuzumab included in their preoperative therapeutic regimen.
Breast-conserving SurgeryTumorectomy was indicated in patients with a favorable ratio between the residual tumor volume and the breast volume. In those patients in whom severe deformity was expected, an oncoplastic pattern was indicated adapted to the breast type and tumor location. In subclinical residual lesions, a harpoon was used to mark the area of the clip. Conservative surgery was ruled out in patients with edema or cutaneous involvement, diffuse microcalcifications, multicentric residual disease or contraindication for treatment with radiotherapy. The indication for conservative surgery was considered correct when the surgical margins were disease free. If margins were positive, a second surgery was used to either extend the surgical margins or to carry out mastectomy.
Intraoperative Tumor EvaluationThe intraoperative pathological study of the surgical specimen was only done in patients with persistent lesion after NCT and consisted of a macroscopic analysis of the sample to determine the distance of the tumor from the surgical resection edges. Radiological intraoperative evaluations were routinely used in the samples marked with harpoons to identify the clip, residual calcifications or radiological abnormalities.
Histopathologic AnalysisThe samples for the histopathological exam were prepared by making a series of 5mm slices of the surgical specimen, affixed in 10% neutral buffered formalin, to try to identify any lesion that corresponded with invasive carcinoma. If the tumor lesion was evident, it was completely included for morphological study with hematoxylin–eosin stain. When there was no evident tumor lesion, the marking clip was identified; the section containing the clip and the adjacent tissue sections were included in the histological study. The margins were considered negative when there was microscopic absence of invasive carcinoma on the surgical edges.
Statistical AnalysisA descriptive analysis was completed of the variables included in the study. The quantitative variables were expressed as means and standard deviation, and the qualitative variables were expressed as absolute value and percentage with the estimation of their 95% confidence interval. The association of qualitative variables was estimated by means of the Chi-squared or Fisher's exact tests, as necessary. The comparison of quantitative variables was done using the non-parametric Mann–Whitney U test. A bivariate and multivariate analysis was conducted using logistic regression models of a series of clinical-pathological parameters to predict the involvement of the surgical margins after breast-conserving surgery. The multivariate analysis included the variables that were significantly associated with the state of the surgical margins after the bivariate analysis. The follow-up time and disease-free survival (DFS) of each patient were determined by the difference between the date of surgery and date of recurrence, death or end of study. DFS was analyzed by estimating Kaplan–Meier curves and their comparison by means of the log-rank test.
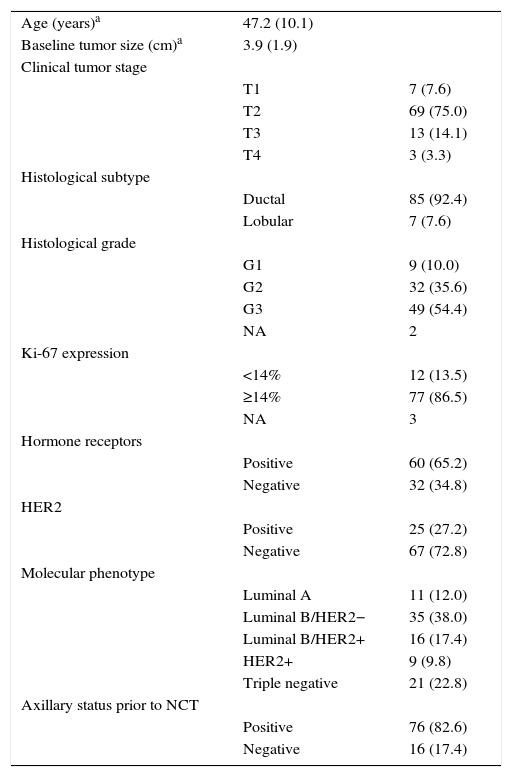
ResultsClinical-pathological Characteristics of the PatientsThe baseline clinical-pathological characteristics of the study patients are shown in Table 1. A total of 91 women with invasive breast cancer received NCT and, afterwards, excision of the primary tumor was indicated. A total of 92 tumors were analyzed, as one patient was diagnosed with bilateral breast cancer.4 Mean patient age at the time of diagnosis was 47.2 years (range: 31–75). Mean baseline tumor size (BTS), defined by MRI, was 3.9cm. The majority of the tumors were T2 (75%) and T3 (54.4%). The biopsies revealed 85 cases of ductal carcinoma (92.4%) and 7 cases of lobular carcinoma (7.6%). Hormone receptors (HR) were negative in 32.6% of the cases, and there was no evidence of overexpression of the HER2 gene in 72.8% of the tumors.
Preoperative Clinical-pathological Characteristics of the 91 Patients in the Study (92 Tumors).
| Age (years)a | 47.2 (10.1) | |
| Baseline tumor size (cm)a | 3.9 (1.9) | |
| Clinical tumor stage | ||
| T1 | 7 (7.6) | |
| T2 | 69 (75.0) | |
| T3 | 13 (14.1) | |
| T4 | 3 (3.3) | |
| Histological subtype | ||
| Ductal | 85 (92.4) | |
| Lobular | 7 (7.6) | |
| Histological grade | ||
| G1 | 9 (10.0) | |
| G2 | 32 (35.6) | |
| G3 | 49 (54.4) | |
| NA | 2 | |
| Ki-67 expression | ||
| <14% | 12 (13.5) | |
| ≥14% | 77 (86.5) | |
| NA | 3 | |
| Hormone receptors | ||
| Positive | 60 (65.2) | |
| Negative | 32 (34.8) | |
| HER2 | ||
| Positive | 25 (27.2) | |
| Negative | 67 (72.8) | |
| Molecular phenotype | ||
| Luminal A | 11 (12.0) | |
| Luminal B/HER2− | 35 (38.0) | |
| Luminal B/HER2+ | 16 (17.4) | |
| HER2+ | 9 (9.8) | |
| Triple negative | 21 (22.8) | |
| Axillary status prior to NCT | ||
| Positive | 76 (82.6) | |
| Negative | 16 (17.4) | |
NA: not available; NCT: neoadjuvant chemotherapy.
Out of the 92 tumors included in the study, 76 (82.6%) presented axillary lymph node disease at the time of diagnosis. Histopathologic confirmation was obtained in 62 (81.6%) of these cases (46 cases by core needle biopsy or fine-needle aspiration, and 16 cases by SLNB done before NCT), while in 14 cases (18.4%), MRI revealed massive axillary lymph node involvement. In 16 tumors (17.4%), no axillary clinical-radiological involvement was detected at the time of diagnosis.
The distribution of the different primary systemic therapies was as follows: 60 patients (65.9%) received a regimen combining anthracycline and taxane; 25 patients (27.5%) received a regime that included trastuzumab, in addition to the combination of anthracycline and taxane; 6 patients (6.6%) received a regime containing taxane (nab-paclitaxel). Preoperative MRI verified a reduction in tumor size after NCT compared to the initial MRI in 88% of cases (81/92). We observed complete tumor remission in 38 cases (41.3%), partial remission in 43 cases (46.7%), stable disease in 10 cases (10.9%) and progressive disease in one single case (1.1%).
After NCT, 21 cases (21.8%) were treated with mastectomy (one patient with bilateral mastectomy) and breast-conserving surgery was attempted in 71 cases (77.2%). From this last group, 16 patients (22.5%) were reoperated on due to the involvement of the surgical margins; extended breast-conserving surgery was possible in 13 cases, and mastectomy was performed in 3 patients. The final percentages of breast preservation and mastectomy were 73.9 and 26.1%, respectively. The pathology study revealed the presence of invasive residual tumor in 3 of the 16 cases reoperated (18.7%).
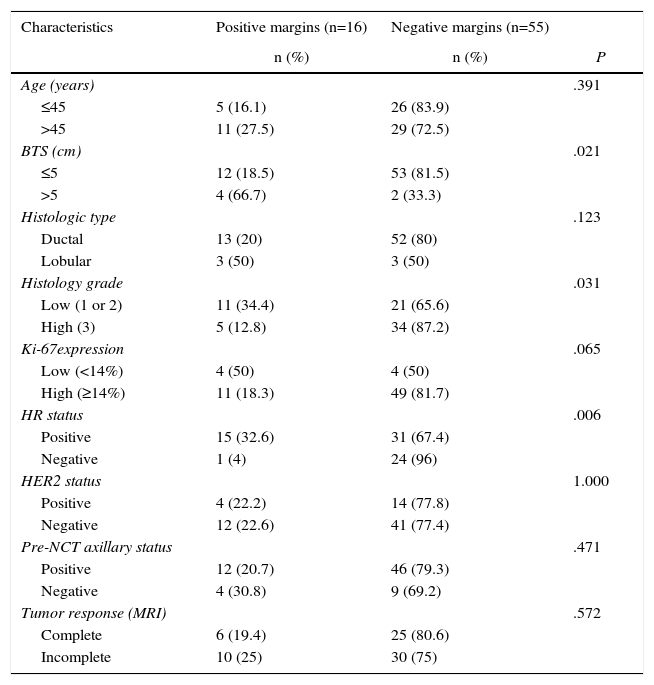
Bivariate and Multivariate Analyses for Predicting the State of the Surgical MarginsTable 2 compares the characteristics of the 71 patients initially treated by conservative surgery after NCT versus the state of the surgical margins. In the bivariate analysis, significant differences were observed in BTS variables, tumor histology grade and HR status. Lesions with a BTS higher than 5cm had a greater incidence of positive margins compared to the tumors with a baseline size equal to or less than 5cm (66.7 vs 18.5%; P=.021). The incidence of positive margins was also greater in low-grade tumors than in high-grade tumors (34.4 vs 12.8%; P=.031). As for HR status, involvement of the margins was observed in 32.6% of the tumors with positive HR, versus 4% of the tumors with negative HR (P=.006). No significant differences were found regarding the state of the margins for the variables age, histology type, ki-67 and status of the HER2 gene.
Comparison of the Characteristics of the Study Patients Who Had Initially Undergone Conservative Surgery With the State of the Surgical Margins.
| Characteristics | Positive margins (n=16) | Negative margins (n=55) | |
|---|---|---|---|
| n (%) | n (%) | P | |
| Age (years) | .391 | ||
| ≤45 | 5 (16.1) | 26 (83.9) | |
| >45 | 11 (27.5) | 29 (72.5) | |
| BTS (cm) | .021 | ||
| ≤5 | 12 (18.5) | 53 (81.5) | |
| >5 | 4 (66.7) | 2 (33.3) | |
| Histologic type | .123 | ||
| Ductal | 13 (20) | 52 (80) | |
| Lobular | 3 (50) | 3 (50) | |
| Histology grade | .031 | ||
| Low (1 or 2) | 11 (34.4) | 21 (65.6) | |
| High (3) | 5 (12.8) | 34 (87.2) | |
| Ki-67expression | .065 | ||
| Low (<14%) | 4 (50) | 4 (50) | |
| High (≥14%) | 11 (18.3) | 49 (81.7) | |
| HR status | .006 | ||
| Positive | 15 (32.6) | 31 (67.4) | |
| Negative | 1 (4) | 24 (96) | |
| HER2 status | 1.000 | ||
| Positive | 4 (22.2) | 14 (77.8) | |
| Negative | 12 (22.6) | 41 (77.4) | |
| Pre-NCT axillary status | .471 | ||
| Positive | 12 (20.7) | 46 (79.3) | |
| Negative | 4 (30.8) | 9 (69.2) | |
| Tumor response (MRI) | .572 | ||
| Complete | 6 (19.4) | 25 (80.6) | |
| Incomplete | 10 (25) | 30 (75) |
HER2: human epidermal growth factor receptor 2; NCT: neoadjuvant chemotherapy; HR: hormone receptors; MRI: magnetic resonance imaging; BTS: baseline tumor size.
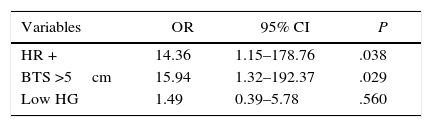
The multivariate analysis revealed that the only independent variables associated with positive resection margins were HR status and BTS (Table 3). The risk for involvement of the surgical margins was 14 times greater in the tumors with positive HR than in the tumors with negative HR (P=.038), and this risk is 16 times greater in tumors with an initial size greater than 5cm than in the tumors with an initial size equal to or less than 5cm (P=.029).
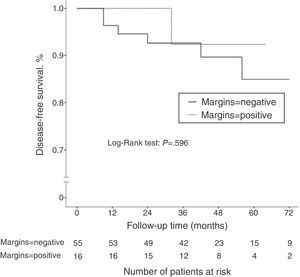
Disease-free SurvivalAfter a mean follow-up of 45.2 months, 13 patients (14.3%) presented disease recurrence (locoregional or systemic). DFS after 2 and 5 years was 88.9 and 83%, respectively. In the group of patients who initially underwent conservative surgery (n=71), 7 patients presented disease recurrence (9.8%): 2 ipsilateral breast tumor recurrence (IBTR) (2.8%), and 5 distant metastases (7%). When we compared the DFS according to the state of the surgical margins, no statistically significant differences were observed (P=.596) (Fig. 1). The incidence of IBTR was 1.8% in women with negative margins and 6.3% in women with positive margins (P=.402).
DiscussionNCT has the capability to increase breast preservation percentages in patients with operable breast cancer who are initially candidates for mastectomy. Conversion rates to conservative surgery have been reported to range between 23 and 46%.3,9–11
The use of a series of clinical-pathological characteristics as predictive factors for locoregional recurrence can improve the risk stratification for recurrence in patients treated with NCT and breast-conserving surgery.12 The presence of positive margins increases the incidence of IBTR, with no significant impact on overall survival.13,14
The evaluation of surgical margins is more complicated after NCT, which is due to the variability in tumor regression patterns.15 Nonetheless, a greater incidence of positive margins has not been observed in conservative surgery after NCT compared to primary conservative surgery.16 The incidence of positive margins in our series (22.5%) is similar to the 21% reported in other studies for patients treated with NCT.16,17
Oncoplastic resection and segmentation can decrease the risk for reintervention. Losken et al.18 demonstrated a lower incidence of positive margins in oncoplastic surgery compared to classical breast-conserving surgery (12.2 vs 20.6%). Our better understanding of the characteristics of each breast region provides for improved oncologic safety and esthetic results in conservative surgical planning.19
Risk factors associated with a higher incidence of positive margins include: the lobular histological type, positive HR status and a large BTS.16,20–22 In the context of NCT, there are also studies that report a greater incidence of tumor involvement of the margins in lobular carcinomas versus ductal carcinomas.23,24
In our study patients initially treated with breast-conserving surgery, a significant association was observed between the state of the surgical margins and BTS, tumor grade and HR status. The tumors with positive HR, low-grade tumors and lesions with a BTS>5cm presented higher rates of margin involvement. In spite of observing a greater incidence of positive margins in the lobular carcinomas than in the ductal carcinomas (50 vs 20%), no significant association was observed with the histology type variable, which is possibly due to the small sample size of lobular carcinomas in our study.
In our logistic regression model, BTS and HR status were the only independent predictive factors of involvement of the surgical margins. The risk for presenting positive margins was 16 times greater in lesions with a BTS>5cm compared to the lesions with a BTS≤5cm and 14 times greater in luminal tumors compared to non-luminal tumors. It has been demonstrated that large lesions and luminal tumors have a poorer response to NCT.11,15 Furthermore, it is relatively frequent that luminal tumors present as diffuse lesions with a non-concentric tumor regression pattern after NCT. These circumstances make it complicated to perform resections with negative margins.
Finally, when we analyzed DFS according to the state of the surgical margins, no significant differences were found. The IBTR incidence of 2.8% is low (only 2 cases). Therefore, there were no significant differences when comparing the incidence of IBTR according to the state of the margins, even though this was greater in the group of women with positive margins compared to the group with negative margins (6.3 vs 1.8%). These 2 patients presented low-grade invasive ductal carcinoma with subtype luminal A and partial radiological response to NCT.
This study has several limitations. The current molecular classification of breast cancer sets the cut-off point for ki-67 expression at 20% to define high and low grades.25 The study did not include radiological parameters among the risk factors for margin involvement. Furthermore, only 7 cases with lobular carcinoma were registered.
In conclusion, this study shows that, although it is feasible to perform conservative surgery in the majority of patients with operable breast cancer treated with NCT, the probability to achieve negative margins is lower in luminal tumors with low histologic grade and an initial tumor size greater than 5cm.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Bouzón A, Acea B, García A, Iglesias Á, Mosquera J, Santiago P, et al. Factores de riesgo de afectación de los márgenes quirúrgicos en la cirugía conservadora del cáncer de mama tras quimioterapia neoadyuvante. Cir Esp. 2016;94:379–384.