To evaluate the effectiveness of conversion surgery in a bariatric surgery unit with 25 years of experience.
MethodRetrospective observational study of patients with type II obesity or higher who were reoperated by means of conversion surgery due to weight regain, residual body mass index (BMI) >35 kg/m2 or <50% of excess weight loss. The demographic and anthropometric data, comorbidities and perioperative data were analyzed in 5 periods of time: initial, post-surgery1, pre-surgery2, post-surgery2 and current.
ResultsA total of 112 patients were included, with a mean age of 40.2 years, who initially underwent vertical banded gastroplasty (VBG) (32.1%), gastric banding (GB) (23.2%), Roux-en-Y gastric bypass (RYGB) (21.4%) and sleeve gastrectomy (SG) (23.2%). The conversion techniques, with a median time between the two surgeries of 70 months, included: RYGB, SG, one-anastomosis gastric bypass (OAGB), shortening of the common loop (SCL) and biliopancreatic diversion (BPD). There was a reduction of the initial weight from 144.2 ± 30.3 Kg to 101.5 ± 21.8 after surgery-1; from 115.6 ± 24.0–91.5 ± 19.0 after surgery-2. The weight at present is 94.7 ± 16.4, with a median follow-up of 27.5 months. Similar results were seen with the BMI. The improvement of comorbidities mainly occurred after the first intervention.
ConclusionsConversion surgery causes a weight reduction that does not exceed the loss achieved after the first surgery; however, it does manage to stabilize weight over time. The perioperative morbidity rate is acceptable and would justify its application, despite the limited impact on comorbidities.
Valorar la eficacia de la cirugía de conversión en una unidad de cirugía bariátrica con 25 años de experiencia.
MétodoEstudio observacional retrospectivo de pacientes con obesidad tipo II o superior reintervenidos mediante cirugía de conversión por reganancia de peso, Índice de masa corporal (IMC) residual >35 kg/m2 o pérdida <50% del exceso de peso. Se analizaron los datos demográficos y antropométricos, comorbilidades y datos perioperatorios en 5 periodos de tiempo: inicial, post-cirugía1, pre-cirugía2, post-cirugía2 y actualidad.
ResultadosSe incluyeron un total de 112 pacientes con una media de edad de 40,2 años, intervenidos inicialmente mediante Gastroplastia vertical anillada (GVA) (32.1%), Banda gástrica ajustable (BGA) (23.2%), Bypass gástrico en Y de Roux (BGYR) (21.4%), y Gastrectomía Vertical (GV) (23.2%). Las técnicas de conversión, realizadas tras una mediana de 70 meses, incluyeron: BGYR (58,9%), GV (18%), Bypass gástrico de una anastomosis (BAGUA) (116%), Acortamiento de asa común (AAC) (241%) y Derivación bilio-pancreática (DBP) (36%). Hubo una reducción del peso inicial de 144,2 ± 30,3 Kg a 101,5 ± 21,8 tras la cirugía-1; de 115,6 ± 24,0 a 91,5 ± 19,0 tras la cirugía-2. El peso en la actualidad es de 94,7 ± 16,4 tras una mediana de seguimiento de 27,5 meses. Un grado de reducción similar ocurrió con el IMC. La mejoría de las comorbilidades se produjo sobre todo tras la primera intervención.
ConclusionesLa cirugía de conversión provoca una reducción de peso que no supera a la pérdida alcanzada tras la primera cirugía, pero a diferencia de ésta, logra estabilizar el peso a lo largo del tiempo. La tasa de morbilidad perioperatoria es aceptable y justificaría su aplicación, a pesar de que el impacto en las comorbilidades sea limitado.
Excess weight and obesity are a growing public health problem around the world.1 It is a chronic disease whose only proven effective treatment for maintained long-term weight loss is bariatric surgery, which is also able to control the comorbidities associated with obesity, improve quality of life and increase survival.2
In recent decades, the number of bariatric interventions has increased worldwide, as have the number of reoperations, which have become part of the standard clinical practice of bariatric surgeons. It has been reported that some 20%-25% of patients will require another bariatric procedure due to weight regain, insufficient weight loss or complications related with the procedure.3 According to the classic Reinhold criteria,4 failed bariatric surgery is defined as a weight loss of less than 50% of the patient’s excess weight, or, despite weight loss, if the body mass index (BMI) continues to be >35 kg/m2. After the failure of an initial bariatric technique, the ideal revision surgery procedure has not been defined, although the most frequently performed is the Roux-en-Y gastric bypass (RYGB).5,6
Re-operations have an associated increased risk of complications and perioperative morbidity (20%-28% vs 8%-28% in the initial surgery),7 since the anatomy is distorted by the first intervention and there are intra-abdominal adhesions. The long-term results are also inconsistent (less weight loss and less remission of comorbidities compared to the initial intervention). Therefore, the risk/benefit ratio is high.7 According to some authors, this type of more complex operations must be carried out by expert bariatric surgeons of high-volume units that have adequate resources to deal with any associated problems or complications that may appear during the process.8
The objective of the present study is to assess the overall results and effectiveness of conversion surgery after the failure of a primary bariatric procedure conducted in a bariatric surgery unit with 25 years of experience.
MethodsWe performed a retrospective review of all patients treated by our unit who had undergone bariatric surgery between December 1994 and January 2019, extracting data from the prospective database of our unit. The study was approved by the Ethics Committee of the Hospital and was exempted from obtaining informed consent as it was a retrospective study conducted with anonymized data.
Inclusion CriteriaPatients who had undergone conversion surgery due to:
- 1
Weight regain (gain of more than 10% of the minimum weight reached)
- 2
Loss <50 % of excess weight.
- 3
Maintained BMI > 35 kg/m2 despite an initial weight loss.4
- 1
Patients treated for reasons other than insufficient weight loss (excessive weight loss, gastroesophageal reflux resistant to medical treatment, nutritional problems, anastomotic stenosis, etc).
- 2
Patients with follow-up of less than 2 years after conversion surgery.
Study variables included: demographic and anthropometric data (age, sex, weight, height, BMI, percent excess BMI loss [%EBMIL], percent excess weight loss [%EWL], percent total weight loss [%TWL]); comorbidities such as type 2 diabetes mellitus (DM2), hypertension (HTN), dyslipidemia (DL), obstructive sleep apnea syndrome (OSAS); and surgical factors (open or laparoscopic approach, type of surgery, conversion rate, time between interventions, surgical time, hospital stay, morbidity and mortality in the first 30 postoperative days). All were evaluated in 5 time periods: initial (prior to the first surgery), postoperative1, preoperative2 (prior to conversion surgery), postoperative2 and current (last visit).
The preoperative study of the patients before conversion surgery always included upper digestive endoscopy and abdominal ultrasound, as well as upper gastrointestinal series on many occasions, to rule out gastric reservoir fistulae, check the size of the reservoir, confirm the location of the gastric band, etc. Each case was evaluated in a multidisciplinary committee of bariatric surgeons, endocrinologists and nutritionists, who analyzed any dietary deviations and personal/social factors. All patients underwent a hypocaloric diet <800 kcal/day (Optifast©, Nestlé Healthcare Nutrition, NJ, USA) 4 weeks prior to surgery and were monitored by the Nutrition Unit.
In general, the decision to conduct a second surgical intervention was made when the previously-mentioned circumstances appeared (weight regain, insufficient weight loss, continued BMI of severe obesity) and when the patient, after understanding the possibilities for weight loss and complications, accepted said possibility. These circumstances made the period of time between the two interventions very variable.
Conversion surgery after adjustable gastric band (AGB) was usually performed in one stage, removing the band and subsequently performing a RYGB or one-anastomosis gastric bypass (OAGB). Our group is in favor of trying to resolve the case in a single intervention if local conditions allow. In two cases, the conversion procedure was performed in a second stage one year after the withdrawal of the band.
Surgery after sleeve gastrectomy (SG) was performed in most cases by dividing the gastric tube at an appropriate height according to the conversion procedure, which was longer in the case of converting to OAGB. When the gastric tube was dilated, the reservoir was adjusted as if it were an initial surgery.
In our unit, RYGB is performed with a small gastric reservoir measuring 20cc, a gastrojejunal anastomosis measuring 10–12 mm in diameter 80 cm from the Treitz, and a 200-cm intestinal loop. In these cases, the conversion has always been aimed at shortening the common channel (SCC) to 100–150 cm, in most cases due to lengthening of the biliopancreatic loop.
Among the cases treated with SCC, in three patients it was done as a second revision surgery in patients with vertical banded gastroplasty (VBG) and previous conversion to RYGB.
All patients initiated tolerance to liquids the day after surgery, while early walking was initiated the afternoon after surgery. Hospital discharge was offered to patients with adequate oral tolerance, normal hemodynamic parameters and adequate pain control. In recent years, the use of fast-track protocols has been incorporated in the management of patients by our unit, but their use had not been established at the time of the primary surgeries. By having excluded patients with a follow-up of less than 2 years, most of the patients in the series were not included in these protocols. Weight-adjusted thromboprophylaxis was administered daily with 40–60 mg of subcutaneous low-molecular-weight heparin in all patients for 10 days after the intervention, which was only expanded to 30 days in the 3 patients with elevated risk of deep vein thrombosis. The surgical time did not usually exceed 2 and a half hours. As it is an intervention for a benign pathology, patients also begin walking early. With this guideline, we have had no cases of lethal thromboembolism in our unit, and only 2 cases of venous thrombosis with clinical repercussions. Supplementation with vitamins, iron, calcium or other elements was done in accordance with follow-up lab work results.
The patients followed a liquid diet for 10–14 days after surgery, reintroducing semi-solid foods starting the third week. Close patient follow-up was conducted in the surgery consultations the first postoperative year in all cases, and thereafter depending on the patient evolution, although an annual follow-up appointment was usually maintained for 5 years. At the same time, the patients were continuously monitored by the Nutrition Unit of the Endocrinology Service, whose duration was practically indefinite. During the office visits, patients were weighed and examined, lab work was done and the evolution of comorbidities was registered. The patient medical records were accessible electronically by both Services.
The associated comorbidities were assessed at each visit. Complete remission of comorbidities was defined as follows: DM2, fasting blood glucose <100 mg/dL and HbA1c ≤ 6, with no medication for a minimum of 1 year9; HTN, blood pressure <120/80 mmHg with no medication;10 dyslipidemia, cLDL < 100 mg/dL; triglycerides <150 mg/dL; total cholesterol <200 mg/dL and cHDL >60 mg/dL10; OSAS: normal polysomnography (<5 events/hour).
Statistical AnalysisContinuous variables are expressed as mean and standard deviation or median and range. Qualitative variables are described as absolute or relative frequencies. For the comparisons, the Student’s t-test was used for continuous variables and the chi-squared test for categorical variables.
The %EBMIL, %EWL and %TWL were calculated with the following formulas10,11: %EBMIL = (initial BMI ― current BMI/initial BMI ― 25) × 100; %EWL = (initial weight ― final weight)/(initial weight ― ideal weight), with the ideal weight defined by a BMI 25 kg/m2; %TWL = (initial weight ― current weight/initial weight) × 100. In the case of conversion surgery, the initial weight was the weight prior to the second intervention.
SPSS version 22 (SPSS, Inc, Chicago, IL, USA) was used for the data analysis.
ResultsBetween December 1994 and January 2017, a total of 1468 patients underwent bariatric surgery, performed by our unit; 112 of these patients required later conversion surgery (7.6% of the total bariatric surgery) and are included in this review. Considering that there were 19 AGB reoperations in patients not initially done at our hospital, reoperation was performed in 6.8% of the total primary interventions, according to the inclusion and exclusion criteria described previously.
The sample consisted of 80 women (71.4%) and 32 men (28.6%). The median follow-up after the last operation was 27.5 months [0–56]. Twenty patients were lost during follow-up (17.8%). These cases were patients who did not belong to our NHS hospital area but were referred to our unit because bariatric surgery was not offered at their corresponding hospital. Subsequently, they did not continue follow-up at our hospital and were monitored by the endocrinology unit at their corresponding hospital. In any case, these patients were followed by our service for more than 2 years after the second surgery. The median time elapsed between the first and the second surgeries was 70 months [14–271].
Initial Bariatric SurgeryThe average age of the patients at the time of the first bariatric procedure was 40 ± 9 years. The average weight was 144.2 ± 30.3 kg, and the average BMI was 52.0 ± 8.6 kg/m2.
As for surgery-related data, 47.3% of the interventions were carried out laparoscopically and 52.7% with an open approach. The type of intervention included: VBG 32.1% (the most frequent); AGB 23.2%; RYGB 21.4%; and 23.2% SG. The mean surgical time was 130.20 ± 47 min and the hospital stay was 6.8 ± 6.9 days.
The overall morbidity rate was 8%, the main complications being: dehiscence of the anastomosis or staple line (3.6%), surgical wound infection (2.7%), surgical wound hematoma (0.9%) and upper gastrointestinal bleeding (0.9%). The reoperation rate was 3.6%. Surgical revision was carried out in 4 patients: 2 patients with staple-line leaks, one patient with partial dehiscence of the gastrojejunal anastomosis, and one with enterocutaneous fistula and peritoneal abscess. The mortality rate was 0.
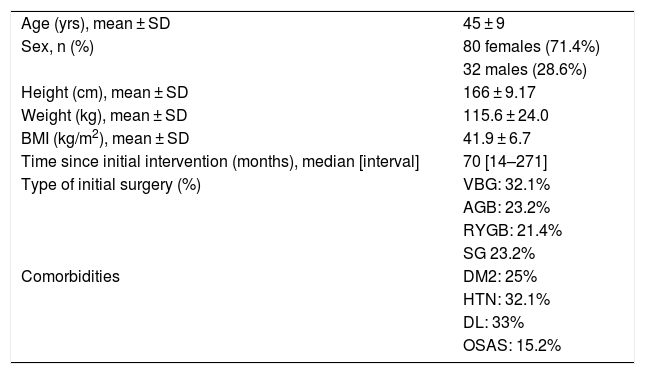
Conversion Bariatric SurgeryMean patient age at the time of the second surgery was 45 ± 9. The average weight was 115.6 ± 24.0 kg, and the mean BMI was 41.9 ± 6.7 kg/m2 (Table 1).
Baseline Characteristics Prior to Conversion Surgery.
| Age (yrs), mean ± SD | 45 ± 9 |
| Sex, n (%) | 80 females (71.4%) |
| 32 males (28.6%) | |
| Height (cm), mean ± SD | 166 ± 9.17 |
| Weight (kg), mean ± SD | 115.6 ± 24.0 |
| BMI (kg/m2), mean ± SD | 41.9 ± 6.7 |
| Time since initial intervention (months), median [interval] | 70 [14–271] |
| Type of initial surgery (%) | VBG: 32.1% |
| AGB: 23.2% | |
| RYGB: 21.4% | |
| SG 23.2% | |
| Comorbidities | DM2: 25% |
| HTN: 32.1% | |
| DL: 33% | |
| OSAS: 15.2% |
AGB: adjustable gastric band; RYGB: Roux-en-Y gastric bypass; SD: standard deviation; DL: dyslipidemia; DM2: diabetes mellitus, type 2; SG: sleeve gastrectomy; VBG: vertical banded gastroplasty; HTN: hypertension; BMI: body mass index; OSAS: obstructive sleep apnea syndrome.
The second bariatric procedure was carried out laparoscopically in 50%, while in the other 50% it was performed openly. In general, the surgery was open when the first technique had been open, and vice versa. However, the most recent cases were all laparoscopic, regardless of the initial route. The conversion to RYGB was the most frequently practiced procedure (58.9%), generally after initial VBG, AGB and SG procedures; this was followed by SCC (24.1%), performed after failed RYGB; OAGB (11.6%), conducted after VBG, SG and AGB; biliopancreatic diversion (DBP) (3.6%), used after VBG; and SG (1.78%), performed in 2 cases after AGB. At our hospital, 77% of VBG, 26% of SG and 2.2% of RYGB were converted due to failure of the initial technique. The AGB technique was only performed at our hospital in one case, which was converted to RYGB 2 years later. The mean surgical time was 135.8 ± 47.7 min, and the hospital stay was 4.9 ± 3.3 days. There were no conversions to laparotomy in laparoscopic cases.
Eleven patients presented complications in the first 30 days of the postoperative period (9.8%). The most frequent complications included wound seroma (1.8%), surgical wound infection (3.6%), anastomotic dehiscence (2.7%), surgical wound hematoma (0.9%) and gastrointestinal bleeding (0.9%). The reoperation rate was 2.7% (3/112 patients), all due to small anastomotic leaks. There was no mortality.
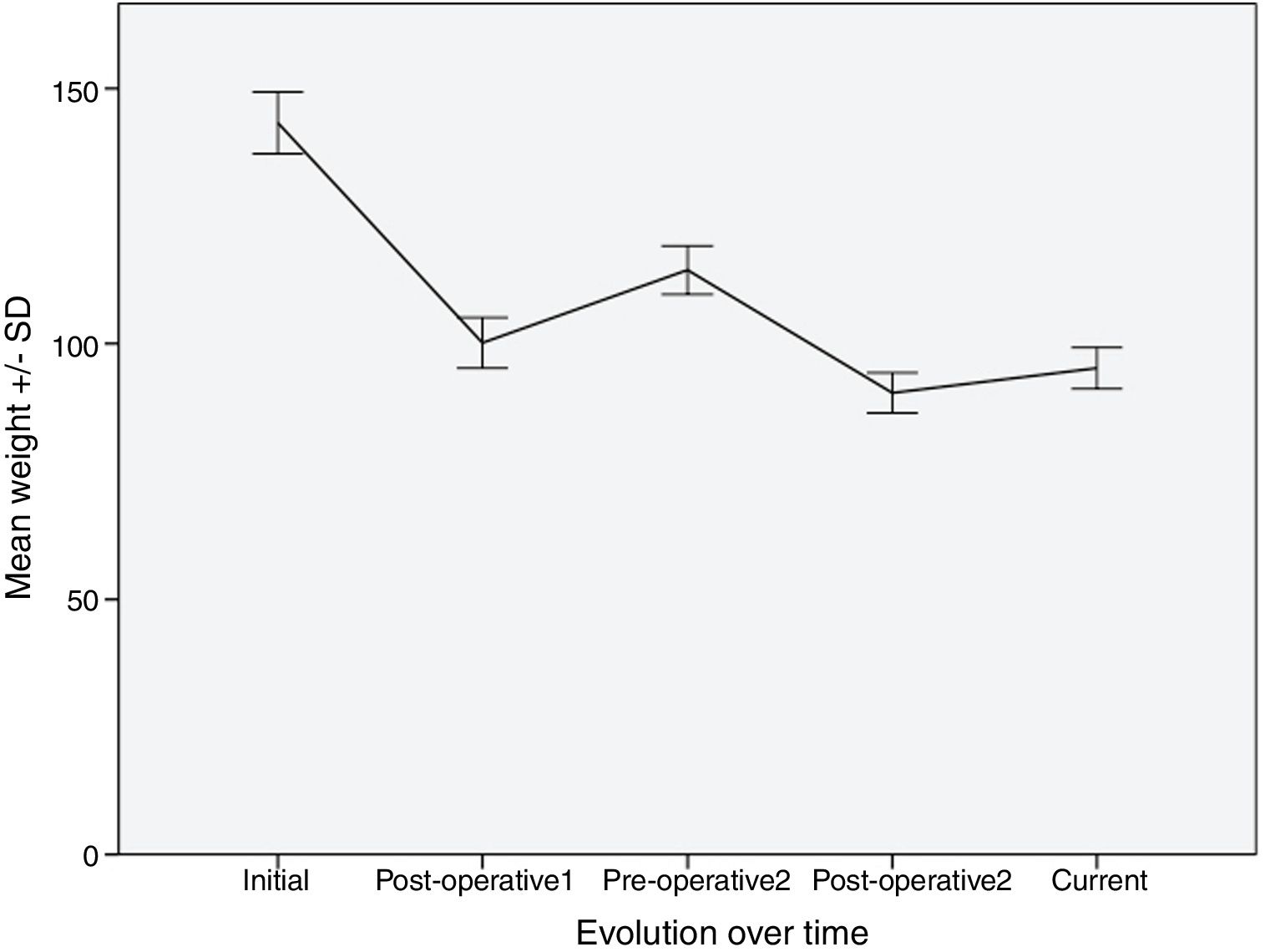
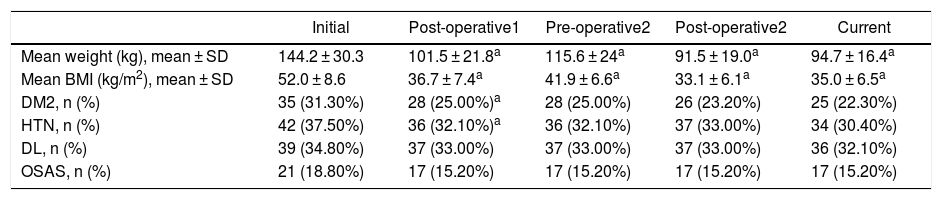
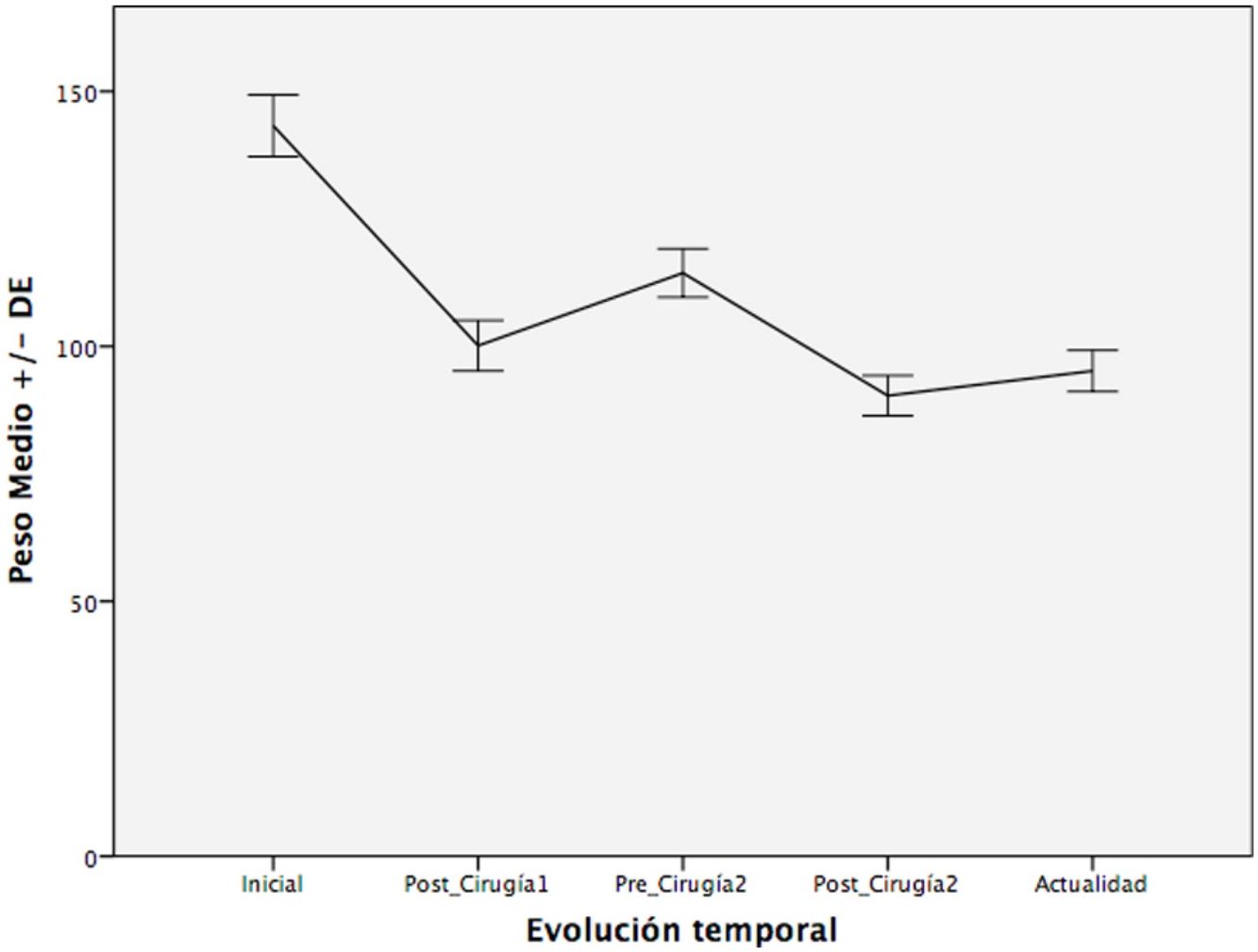
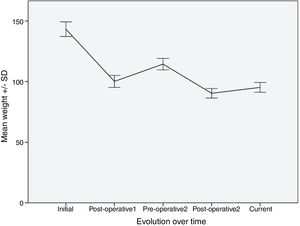
Evolution of Weight and BMITable 2 shows the variation in the average weight of the patients in the different periods. The greatest weight loss occurred after the first intervention, and the loss achieved with conversion surgery was less. However, this weight loss was better maintained over time. The same happened with BMI.
Evolution of Mean Weight, BMI and Comorbidities in the Different Periods.
| Initial | Post-operative1 | Pre-operative2 | Post-operative2 | Current | |
|---|---|---|---|---|---|
| Mean weight (kg), mean ± SD | 144.2 ± 30.3 | 101.5 ± 21.8a | 115.6 ± 24a | 91.5 ± 19.0a | 94.7 ± 16.4a |
| Mean BMI (kg/m2), mean ± SD | 52.0 ± 8.6 | 36.7 ± 7.4a | 41.9 ± 6.6a | 33.1 ± 6.1a | 35.0 ± 6.5a |
| DM2, n (%) | 35 (31.30%) | 28 (25.00%)a | 28 (25.00%) | 26 (23.20%) | 25 (22.30%) |
| HTN, n (%) | 42 (37.50%) | 36 (32.10%)a | 36 (32.10%) | 37 (33.00%) | 34 (30.40%) |
| DL, n (%) | 39 (34.80%) | 37 (33.00%) | 37 (33.00%) | 37 (33.00%) | 36 (32.10%) |
| OSAS, n (%) | 21 (18.80%) | 17 (15.20%) | 17 (15.20%) | 17 (15.20%) | 17 (15.20%) |
SD: standard deviation; DL: dyslipidemia; DM2: diabetes mellitus, type 2; HTN: hypertension; BMI: body mass index; OSAS: obstructive sleep apnea syndrome.
After the first operation, the patients lost an average of 29.6% of the total weight. They regained an average of 13.9% of this weight, with a median interval of 57.5 months. After the second intervention, they lost 20.8% of the weight, recovering only 3.5% of the weight lost after a median follow-up of 27.5 months.
Fig. 1 shows a graph of the weight loss of patients over the different periods.
The evolution of the percentage of excess weight loss was as follows: between the starting point and the postoperative period of the first intervention, the percentage of patients with losses > 50% of excess weight was 52.5%; in the second period (from before the second intervention until after it) this was 38%, follows by 27.4% in the last period (from before the second intervention until today).
With the percentage of excess BMI loss, we found a similar situation, with 60.2% of patients who manage to reduce excess BMI by 50% in the first period, 50% in the second and 44.2% in the third period.
Evolution of Comorbidities Associated With ObesityDespite the restrictive criteria used to define remissions, there was a reduction in comorbidities after the first intervention, mainly in the case of DM2 and HTN, with no significant reductions in the following time periods (Table 2).
DiscussionConversion surgery involves a complex situation, as these patients have experienced the failure of a previous intervention for the control of their obesity and associated comorbidities. Conversion also entails a higher risk of postoperative complications, and long-term results are inconsistent.7 In our study, patients achieved less weight loss after conversion surgery compared to the initial procedure, and the morbidity rate was somewhat higher (9.8% vs. the initial 8%) with the longer surgical time. However, the hospital stay (4.9 ± 3.3 vs 6.8 ± 6.8 days) and the reoperation rate are lower (2.67% vs. 3.57%). The few differences in surgical time and morbidity between the primary surgery and revision surgery can be explained by the increased surgeon experience; the reoperations are later in time, and, in the interim, several hundred more operations had been performed by the same surgical team. Also the duration of the stay was shorter in the case of reoperations for the same reason: in the time elapsed, fast-track protocols have been implemented, which have greatly shortened the stays in our unit (the current average stay of our bariatric patients is <48 h).
The fact that smaller losses are achieved with conversion surgery is something already described in the literature,3,12,13 especially with the RYGB as a conversion procedure. However, after the conversion procedure there was a tendency towards smaller increases in BMI and a slower decrease in %EBMIL, with a more stable weight maintained over time. This fact has already been observed by other authors, such as Dardamanis et al.6 in their recently published study, where they compare primary vs. revision RYGB and demonstrate that starting at 18 months after the intervention the patients in the review group presented greater weight stabilization.
Conversion surgery presents the greatest difficulties for bariatric surgeons, and technically it is highly demanding. The risk of postoperative complications, as in any reintervention, is higher due to the ‘hostile’ surgical field caused by adhesions and fibrosis resulting from the previous surgery. This also increases the chances of conversion to laparotomy in laparoscopic cases.
There are studies in which the morbidity rate after conversion surgery is higher,3,13–16 while other groups conclude that revision surgery is safe, reporting postoperative complication rates equivalent to those of primary surgery.12,17,18 In our study, the morbidity rate was somewhat higher in conversion surgery (9.8% vs. 8%); however, in accordance with the quality standards established by the Spanish Association of Surgeons (AEC) and the Spanish Society of Obesity Surgery19 in 2017, this is still within the established ranges (accepted general morbidity rate <10%). In addition, none of the laparoscopic cases during conversion surgery had to be converted to laparotomy, a fact already described by other groups,12 even in a third bariatric procedure.20 Carried out by expert surgeons in specialized high-volume units, it does not imply a greater risk of complications or a compromised laparoscopic approach.21
Anastomotic dehiscence is one of the main sources of complications of bariatric surgery. Our rate in conversion surgery (2.7%) is similar to that reported by other studies: 2.1%6 and 3.3%.22
The limitations of our study include its retrospective and descriptive nature. In addition, different types of bariatric procedures are included, with varying effects on patient weight and comorbidities, which can make interpreting the results difficult. However, we believe that our data provide an overview of the long-term results of conversion surgery, while serving as a starting point for new study hypotheses for prospective and randomized clinical trials that seek to determine which procedure is most appropriate for conversion surgery in patients with obesity when all other therapeutic options, including bariatric surgery, have failed.
It is interesting to emphasize the fact that weight loss after conversion surgery achieves a very small percentage of loss compared to the minimum weight achieved after the primary surgery, although in general it manages to halt the evolution towards weight regain. Therefore, patients should consider the risk-benefit balance before the second surgery.
In conclusion, we can affirm that conversion surgery represents a significant percentage of surgical cases in all bariatric surgery units. Conversion surgery is a complex and demanding surgery with inferior results in weight loss compared to the initial bariatric surgery, although the losses achieved after this second surgery allow for stabilization and long-term weight maintenance. The improvement in comorbidities occurs after the first surgical intervention, with no significant benefits observed in successive surgeries. The morbidity rate is acceptable as long as it is carried out in experienced bariatric units and in patients requiring another procedure for adequate control of their disease after other measures have failed.
FundingThis study has received no funding.
Conflict of InterestsThe authors of this article have no conflict of interests with its content.
Please cite this article as: Mora Oliver I, Cassinello Fernández N, Alfonso Ballester R, Cuenca Ramírez MD, Ortega Serrano J. Cirugía bariátrica de conversión por fallo de la técnica inicial: 25 años de experiencia en una Unidad especializada de Cirugía de la Obesidad en España. Cir Esp. 2019;97:568–574.