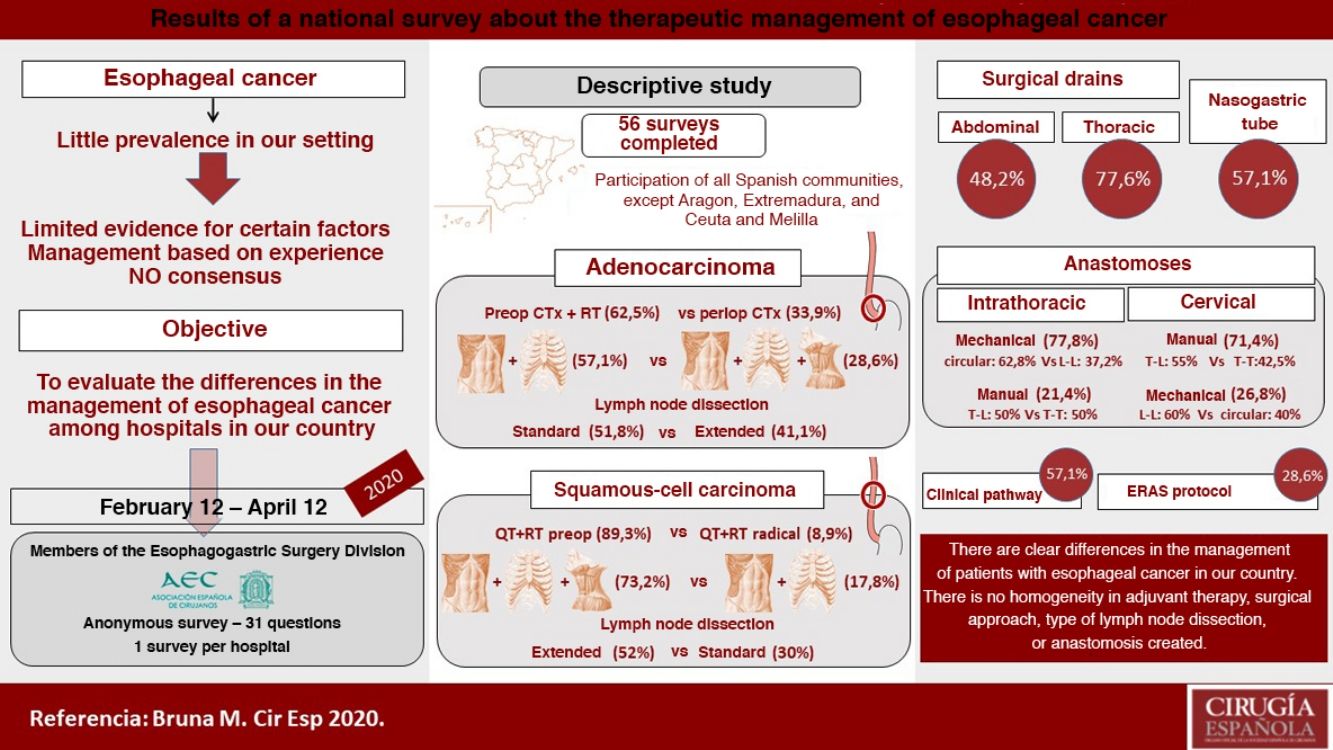
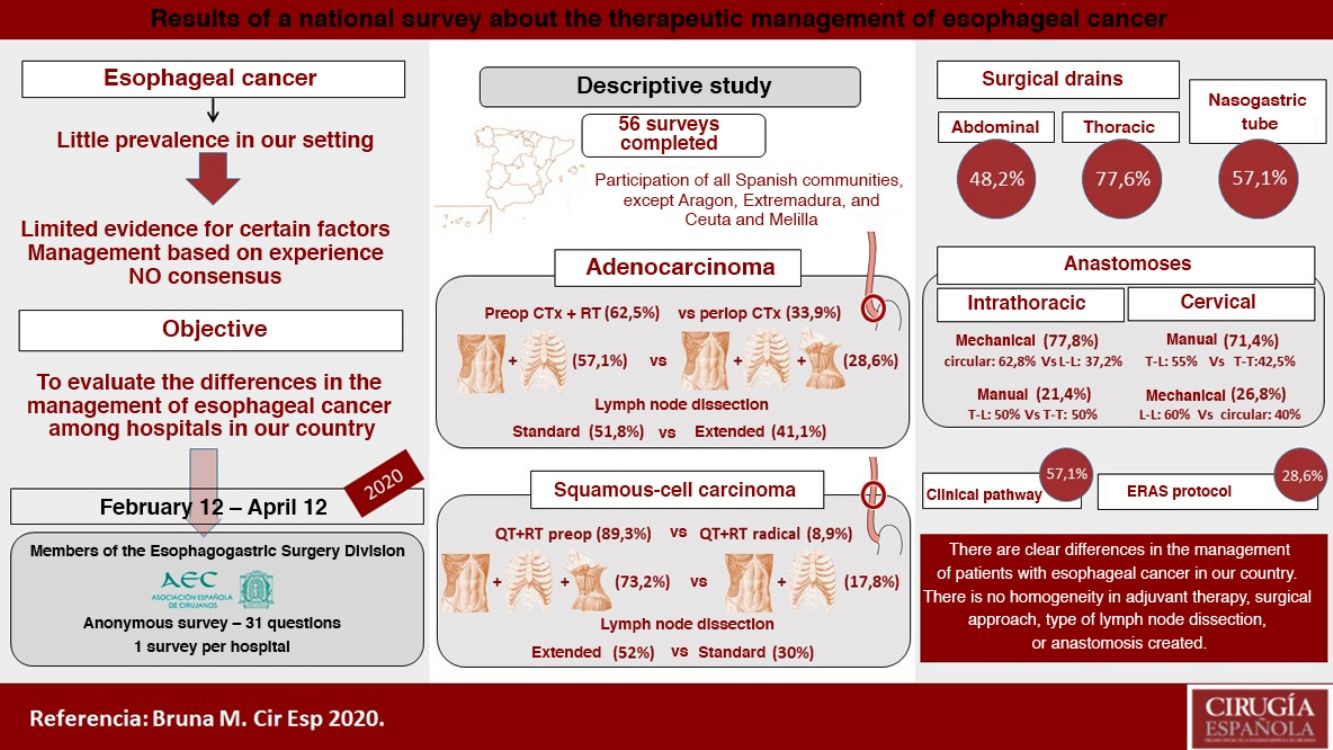
There are numerous controversial aspects in the perioperative and surgical management of patients with esophageal cancer. The aim of this study is to evaluate the differences between the hospitals of our country in the adjuvant and surgical treatment of these patients. We conducted a descriptive study of 56 surveys answered from February to April 2020, evaluating hospital characteristics, number of procedures, management of distal adenocarcinoma and squamous cell carcinoma of the middle third of the esophagus, type of anastomosis, use of nasogastric tube and drains, and clinical follow-up. The median number of annual esophagectomies per hospital was 10, and only 7.1% performed more than 20. In distal adenocarcinoma, 62.5% use preoperative chemoradiotherapy, an abdominal and transthoracic approach (57.1%), and an infracarinal lymphadenectomy (51.8%) or extended to right paratracheal lymph nodes (41.1%). In squamous cell carcinoma of the middle third of the esophagus, 89.3% use preoperative chemoradiotherapy, surgery in three fields (73.2%) and extended mediastinal lymphadenectomy (52%). Intrathoracic anastomosis is performed mechanically in 77.8% and cervical anastomosis preferably manually (71.4%). Pleural and abdominal drains are usually placed by 77.6% and 48.2%, respectively, while the nasogastric tube is normally used by 57.1%. A clinical pathway is followed by 57.1%, and 28.6% use a specific enhanced recovery after surgery protocol. Thus, in the management of esophageal cancer, there are some clear differences between hospitals in our country regarding adjuvant treatment, surgical approach, type of lymphadenectomy and anastomosis performed.
En la actualidad existen numerosos puntos de controversia en el manejo perioperatorio y quirúrgico de los pacientes con cáncer de esófago. El objetivo de este trabajo es describir las posibles diferencias en el tratamiento coadyuvante y quirúrgico de estos pacientes entre los hospitales de nuestro país mediante un estudio descriptivo de las encuestas respondidas entre febrero y abril de 2020. Se evaluaron las características de cada centro, el número de procedimientos, el manejo del adenocarcinoma de tercio distal y del carcinoma escamoso de tercio medio, el tipo de anastomosis, el empleo de sonda nasogástrica y drenajes y el seguimiento de una vía clínica. La mediana de esofaguectomías anuales por centro es de 10, realizando solamente el 7,1% más de 20. En el adenocarcinoma distal el 62,5% emplea quimiorradioterapia preoperatoria, un abordaje abdominal y transtorácico (57,1%) y una linfadenectomía infracarinal (51,8%) o extendida (41,1%). En el carcinoma escamoso de tercio medio el 89,3% emplea quimiorradioterapia preoperatoria, una cirugía en 3 campos (73,2%) y una linfadenectomía mediastínica ampliada (52%). La anastomosis intratorácica se realiza de forma mecánica en el 77,8% y la cervical preferentemente de forma manual (71,4%). Los drenajes pleurales y abdominales son colocados habitualmente por el 77,6 y el 48,2%, respectivamente, mientras que la sonda nasogástrica es empleada normalmente por el 57,1%. El 57,1% siguen una vía clínica y el 28,6% un protocolo de recuperación intensificada específico. Por tanto, en el manejo del cáncer de esófago, existen claras diferencias entre los hospitales de nuestro país con relación al tratamiento coadyuvante, abordaje quirúrgico, tipo de linfadenectomía y anastomosis practicadas.
Esophageal cancer is a disease that is not very prevalent in our setting compared to other regions of the world. Despite optimized multidisciplinary management, the associated postoperative morbidity is usually significant, and 5-year survival rates are not very encouraging. In order to increase the survival of these patients, new chemotherapy and radiotherapy regimens, improvements in perioperative management, and less invasive surgical techniques have been developed.
Nonetheless, there is no global, clearly established consensus on the treatment of esophageal cancer. There are controversial points and important differences between groups of experts regarding the management and surgical strategy to be used in these patients.1,2 Thus, the measures applied in clinical practice attempt to rely on the experience of each hospital and the limited evidence available for certain points.
The objective of this study is to evaluate the trends and possible differences in the adjuvant and surgical treatment of patients with esophageal cancer among the hospitals in our country.
MethodsWe conducted a descriptive study of data from the surveys answered between February 12 and April 12, 2020 by Spanish surgeons in relation to the adjuvant and surgical treatment of patients with esophageal cancer. The 574 members of the Spanish Association of Surgeons (Asociación Española de Cirujanos, AEC) Esophagogastric Surgery Division were invited by email to anonymously complete the online survey for this study, urging them to fill out only one survey for each work center.
The survey consisted of 31 questions and evaluated the following factors:
- 1
Characteristics of the hospitals surveyed:
- ‐
Region
- ‐
Characteristics of the work center
- ‐
Volume of procedures performed annually
- 2
Management of patients with adenocarcinoma of the distal third of the esophagus and with squamous-cell carcinoma of the middle third, both locally advanced and non-metastatic:
- ‐
Adjuvant treatment
- ‐
Surgical approach: abdominal, thoracic and/or cervical by open, minimally invasive or mixed surgery
- ‐
Type of lymph node dissection (standard: subcarinal; extended: including right paratracheal nodes; or total mediastinal: also including bilateral recurrent lymph nodes and left paratracheal nodes)
- ‐
Type of reconstruction and pull-up route
- 3
Intrathoracic and cervical anastomosis technique
- 4
Use of drains and nasogastric (NG) tube
- 5
Follow-up of the clinical pathway or perioperative care protocol
The statistical study was carried out using IBM® SPSS® Statistics program version 20, and the results are presented as number of cases and percentage or as median and interquartile range (IQR).
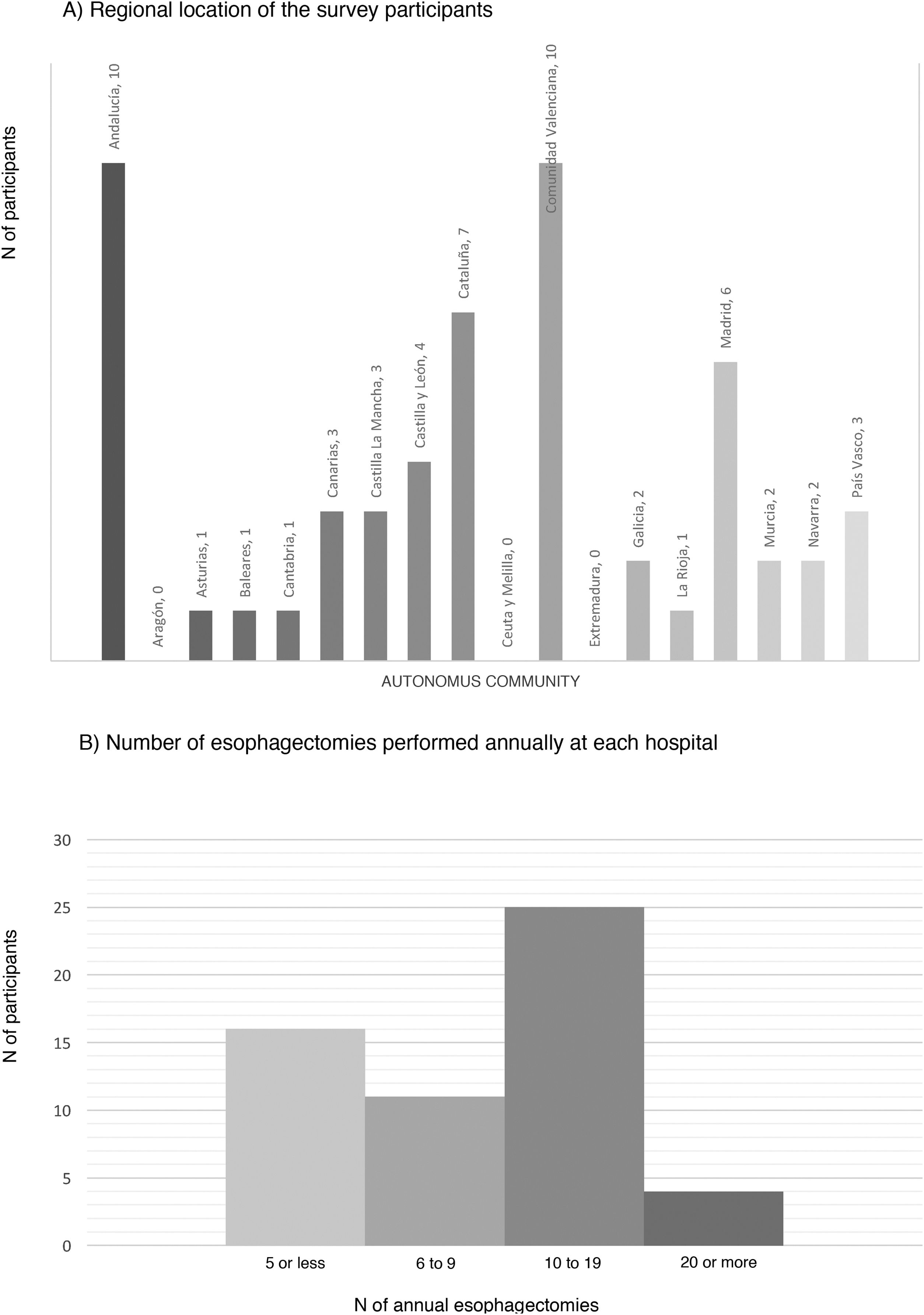
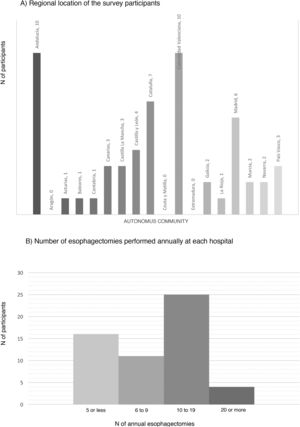
ResultsCharacteristics of the participating hospitalsFifty-six surveys were answered, with representation from almost the entire national territory, except the communities of Aragón, Extremadura, and Ceuta and Melilla. The regions with the highest participation were Andalusia (10), the Valencian Community (10), Catalonia (7) and the Community of Madrid (6) (Fig. 1). In terms of hospital size, 71.4% work in a hospital with more than 500 beds, 14.3% with 300–500 beds, and 14.3% with less than 300 beds. Only 14.3% dedicate more than 80% of their activity exclusively to esophageal disease. The median number of members of the esophagogastric surgery units was 3 (IQR: 2–4).
The median number of esophagectomies performed annually at each hospital was 10 (IQR: 5–15); 24 medical centers performed between 10 and 19, and only 4 centers (7.1%) performed more than 20 esophagectomies per year (Fig. 1).
Management of non-metastatic locally advanced adenocarcinoma of the distal third of the esophagusIn this type of tumors, 62.5% opted for a preoperative chemoradiotherapy regimen and 33.9% for perioperative chemotherapy.
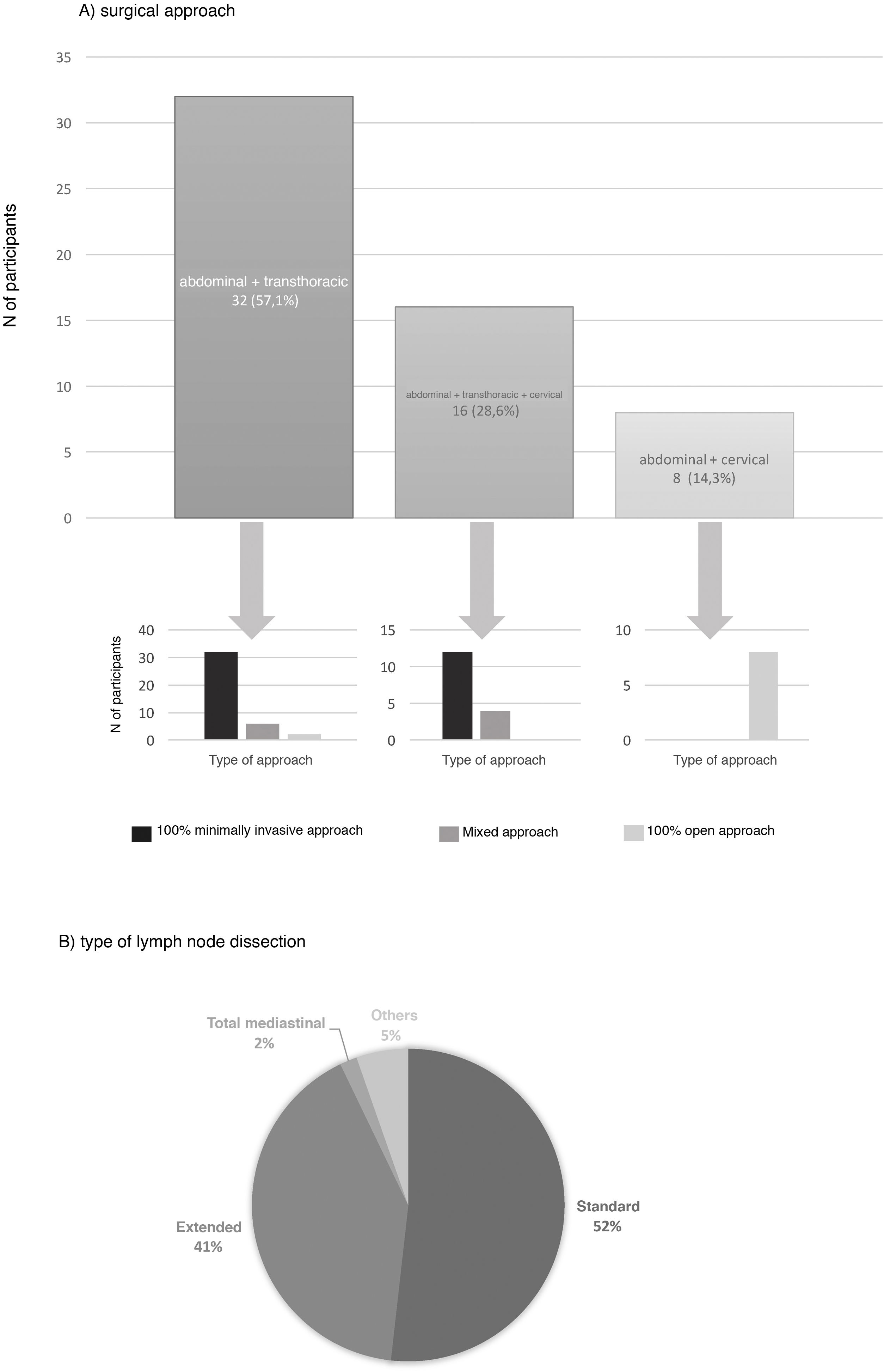
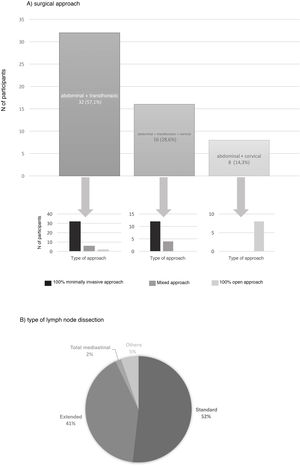
The preferred surgical approach in these cases was the abdominal and transthoracic approach (57.1%), and 75% of these were performed with a minimally invasive approach in both fields (Fig. 2). The approach chosen was 3-field (abdominal, transthoracic and cervical) in 28.3%, and in 75% of these the abdominal and thoracic approaches were by minimally invasive surgery (Fig. 2). The transhiatal abdominal and cervical approach was chosen by 14.3%, and laparotomy was the access route used in all of them (Fig. 2).
The type of lymph node dissection chosen by most of the teams (51.8%) to treat these patients was standard infracarinal lymph node dissection, while 41.1% of those surveyed also included the right paratracheal nodes (Fig. 2). Reconstruction of the tract was performed by all groups with a transmediastinal gastroplasty.
Management of locally advanced non-metastatic squamous cell carcinoma of the middle third of the esophagusIn this case, 89.3% of those surveyed opted for preoperative chemoradiotherapy, and only 8.9% preferred a radical-intent chemoradiotherapy scheme.
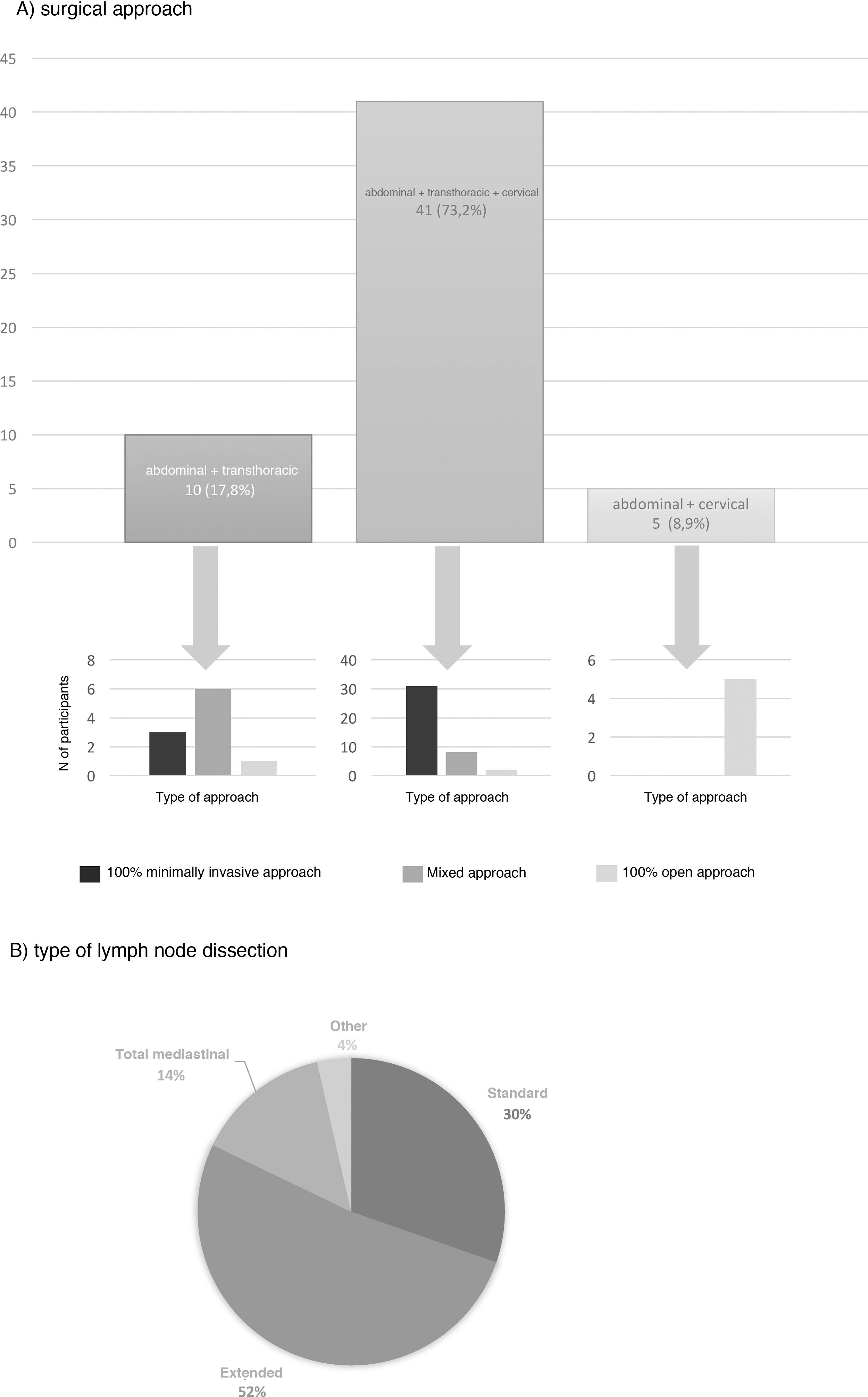
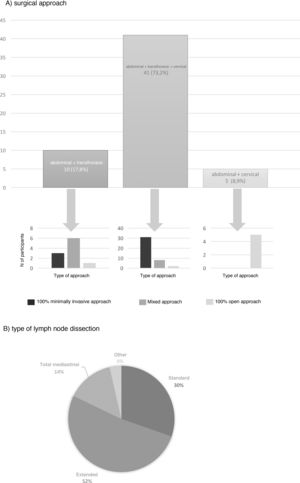
The most frequent surgical approach (73.2%) was 3-field surgery, and 75.6% of these were performed with a totally minimally invasive approach (Fig. 3). 17.8% chose an abdominal and transthoracic approach, and 60% performed the abdominal approach laparoscopically and the thoracic part by thoracotomy; only 30% performed both approaches by minimally invasive surgery (Fig. 3). The transhiatal and cervical abdominal approach was chosen by 8.9%, and laparotomy was the access of choice for all of these (Fig. 3).
Extended mediastinal lymphadenectomy was performed by 52%, using standard lymph node dissection for 30% and total mediastinal lymphadenectomy for 14% of the participants (Fig. 3). The reconstruction of the tract was also carried out by all groups using a gastric pull-up via the transmediastinal route.
In hospitals that perform a minimum of 10 esophagectomies per year, the percentage of totally minimally invasive surgery was 76.4% in the Ivor Lewis technique for distal adenocarcinoma, and 80.9% in the McKeown technique in middle-third squamous disease, compared to 73.3% and 68.4%, respectively, of the groups that perform fewer than 10 procedures per year.
Also, more than half of the respondents performed the same surgical approach (53.6%) and extension of the lymph node dissection (66.1%) regardless of the type or location of the tumor treated.
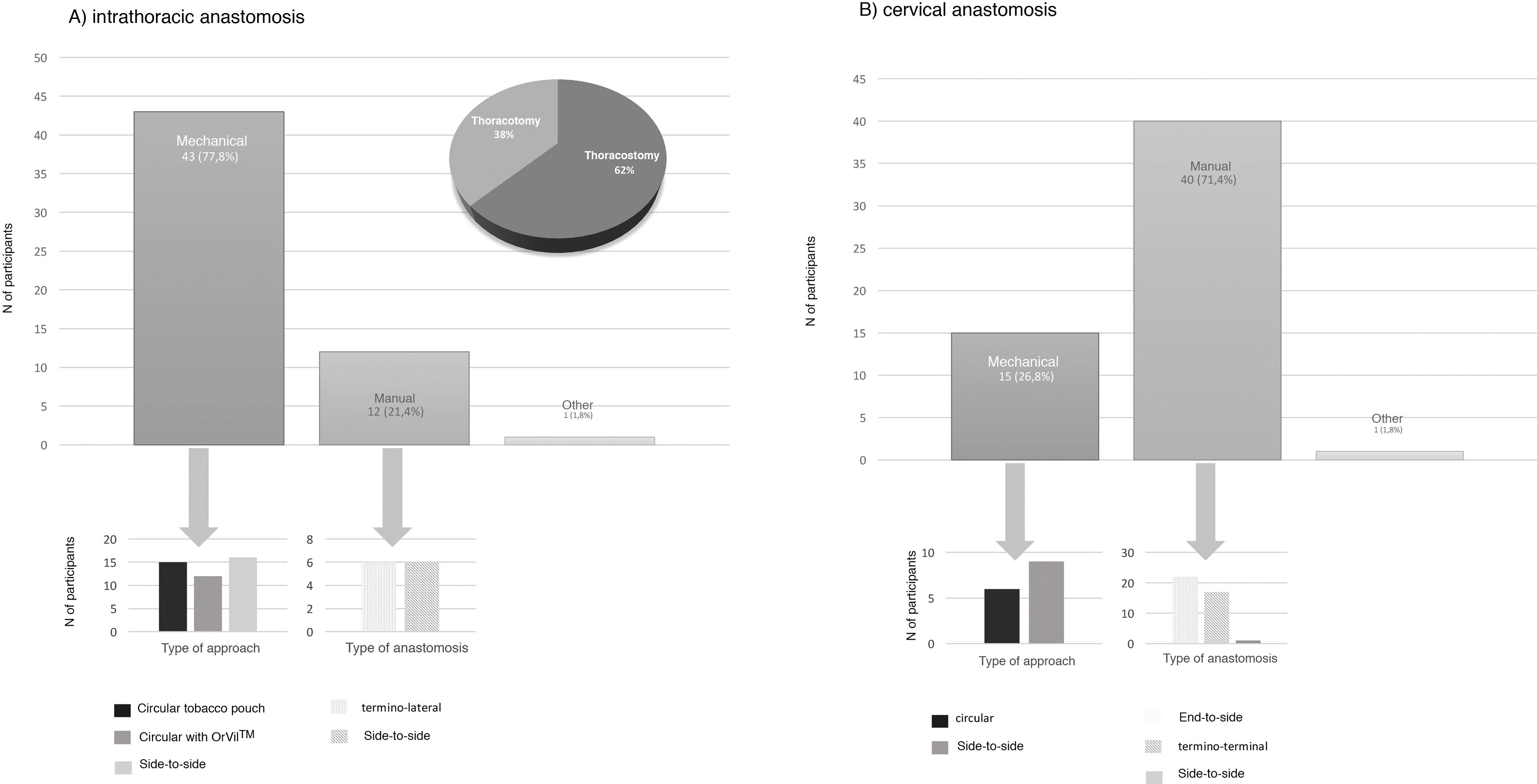
Type of anastomosisThe intrathoracic anastomosis was created thoracoscopically in 62% of cases. Of these, 77.8% were mechanical anastomoses: 37.2% side-to-side, 34.9% tobacco pouch, and 27.9% circular with EEA™ OrVil™ (Fig. 4). This anastomosis was created manually by 21.4% of those surveyed, half of them performing an end-to-side anastomosis and the other half an end-to-end (Fig. 4).
Regarding cervical anastomosis, 71.4% opted for a manual anastomosis. Of these, 55% performed end-to-side and 42.5% end-to-end (Fig. 4). This anastomosis was created mechanically by 26.8% (60% side-to-side and 40% circular) (Fig. 4).
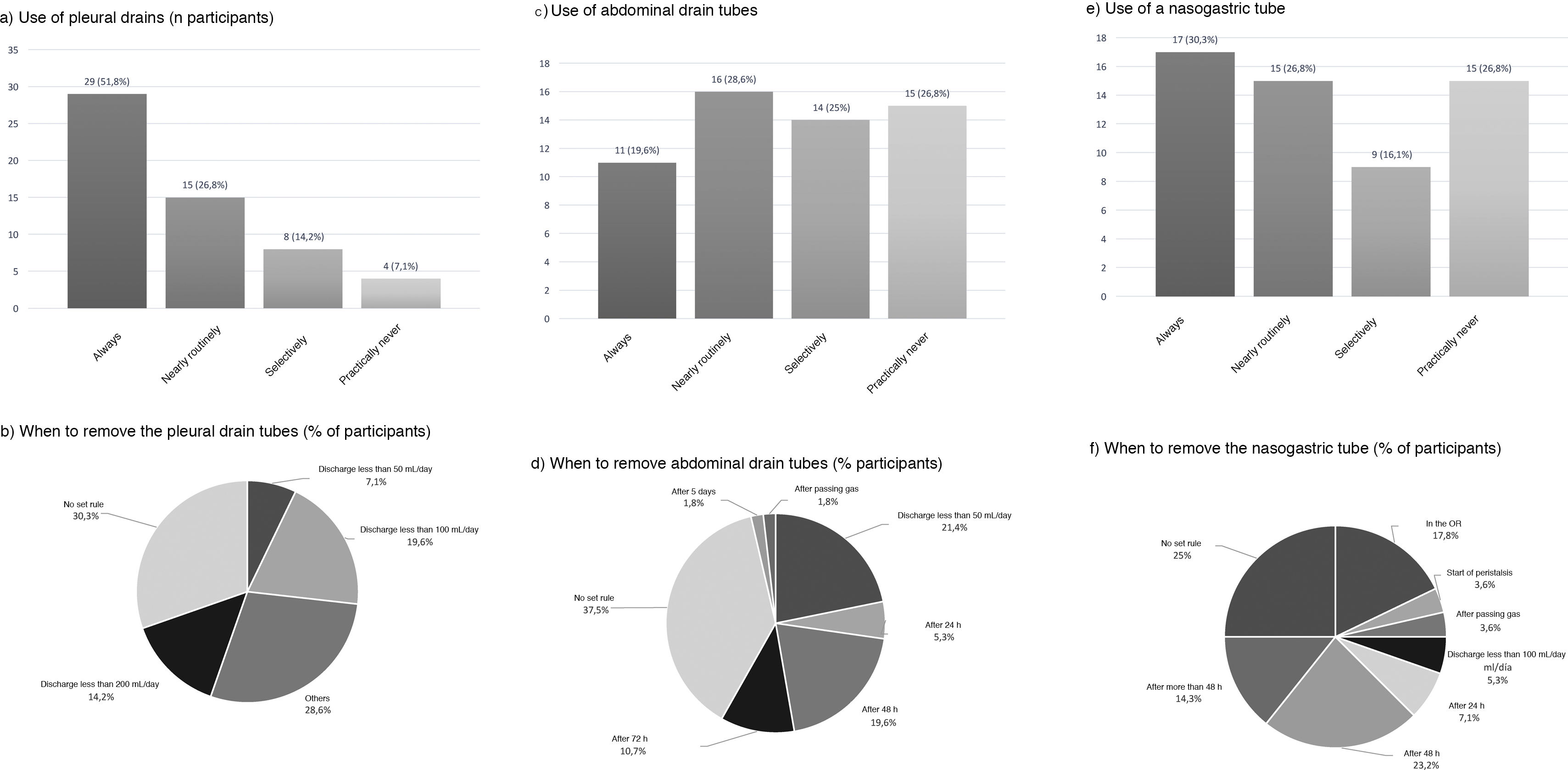
Use of drains and nasogastric tubePleural drains were always or routinely placed by 77.6% of those surveyed, and 7.1% practically never used them. 57.1% placed a single drain, and 39.3% placed 2. More than one-third removed them based on the daily discharge (less than 200 mL: 14.2%; less than 100 mL: 19.6%; and less than 50 mL: 7.1%) and 30.3% follow no set norm (Fig. 5).
Abdominal drains were always or routinely placed by 48.2%, and 26.8% practically never used them (Fig. 5). For their removal, 37.5% did so with no set rule, 21.4% when the discharge was less than 50 mL per day, and 19.6% removed them 48 h after surgery (Fig. 5).
An NG tube was always or routinely used in 57.1% and practically never used in 26.8% (Fig. 5). There was no clear trend regarding the time to remove the NG tube (25% with no set norm); 17.8% removed it in the operating room, and 23.2% 48 h after surgery (Fig. 5).
Clinical pathway57.1% used a clinical pathway for the care and management of these patients, and only 28.6% used a specific enhanced recovery protocol. 65.5% of the groups that performed more than 10 esophagectomies per year followed a clinical pathway, and 31% followed an ERAS protocol, compared to 48.1% and 25.9%, respectively, of the hospitals that performed fewer than 10 esophagectomies per year.
DiscussionIn our country, the therapeutic management of patients with esophageal cancer presents differences among the groups participating in this survey, and there is no uniform criteria for factors such as adjuvant treatment, surgical approach, extension of lymph node dissection, type of anastomosis, and use of tubes or drains. A similar conclusion was noted by van Rijswijk et al1 in an international survey on esophageal cancer, where a clear variability of criteria was noted among the 50 expert surgeons who participated.
Even though centralization and the performance of a greater number of procedures have had a favorable impact in terms of survival, safety and efficacy in esophageal cancer,3,4 the regionalization and creation of reference units are not established globally in our country. This explains how only 7% of those surveyed belong to hospitals where more than 20 esophagectomies are performed annually. For this reason, and similar to what happens in other countries, it is essential for scientific societies and health authorities of our country to endorse and support the centralization of the treatment for this disease with the creation of reference units.
In recent decades, improvements in adjuvant therapies, perioperative care and surgical advances have facilitated a reduction in complications, earlier functional recovery, and better survival rates in patients with esophageal cancer. In terms of adjuvant therapies, the most widely accepted are currently perioperative chemotherapy and preoperative chemoradiotherapy,5–7 while radical chemoradiotherapy treatment is generally reserved for proximal squamous-cell carcinomas or patients with high surgical risk.8 As in other surveys,1 and in accordance with what is normally used in countries such as France and Germany,9 the preferred scheme in our country for this type of tumors is preoperative chemoradiotherapy, although in the case of distal adenocarcinoma up to one-third of the groups use a perioperative chemotherapy regimen. This variation could be because the available evidence on the superiority of one scheme over another is still limited.10–12
Minimally invasive surgery presents better results compared to the open approach in terms of recovery and postoperative complications, without altering the oncological radicality of the procedure.13,14 In this study, more than 75% of the groups perform minimally invasive esophagectomy in the case of distal adenocarcinoma, but less than one-third of those who choose the thoracic and abdominal approach to treat mid-third squamous cancer do so using a completely minimally invasive technique. Perhaps the extent of the required lymphadenectomy or the technical difficulty in performing the intrathoracic anastomosis more cranially in these cases may justify this result, similar to the usual practice in other countries, such as Austria or France.9
In accordance with the results of other international surveys,1 most of the participants in the present study carry out an approach in 2 fields for the treatment of distal adenocarcinoma and in 3 stages for squamous-cell carcinoma of the middle third. It is striking that more than half of those surveyed perform the same approach and type of lymph node dissection regardless of the tumor type and location. The extent of the lymphadenectomy in esophageal cancer surgery continues to be controversial,15 and, although some studies have shown greater survival with more extensive dissections,16,17 there is no clear evidence to clarify lymph node involvement in this type of tumors18 and the convenience or not of a more extensive lymph node dissection. Clear differences have been described among international groups of experts in terms of the extension of the lymphadenectomy within the possible clinic scenario of esophageal cancer.1,19 These differences were much more significant when comparing Western versus Asian series,20 where proximal tumors are more prevalent and 3-field lymph node dissection is used more frequently.21
Many types of anastomoses have been described after esophagectomy,22 and all of them have shown comparable results.9 In our setting and similar to other international surveys, an intrathoracic anastomosis is usually created in distal adenocarcinoma and a cervical anastomosis in squamous-cell tumors of the middle third.1 In accordance with the European trend and unlike the North American,2 71.4% of those surveyed in this study carried out the cervical anastomosis manually, and almost 80% performed the mechanical intrathoracic anastomosis, a figure comparable to that of the rest of European and Asian surgeons.2
Despite the existing evidence in this regard, and the fact that some societies have published clinical guidelines for the application of multimodal rehabilitation protocols in esophagectomy procedures,23,24 less than one-third of the participants report using a clinical pathway with these measures, which is somewhat better than data published for the management of gastric cancer in our country.25
The use of abdominal drains, which is not routinely recommended in this type of surgery,24 is still a procedure that is always or nearly routinely carried out by about 40% of the groups surveyed. Similarly, the ERAS Society23 and other groups of experts24,26 recommend the limited use in number and time of pleural drains (safely removed with daily discharge not exceeding 450 mL, provided there is no leakage of pathological material, such as saliva, gastrointestinal fluid, or lymph). The use of a nasogastric tube is currently controversial and, although most experts recommend its use,23,24 current evidence seems to be leaning towards not using it systematically.27–30
Although some documents attempt to specify the recommended measures for the treatment of these patients with esophageal cancer,31–33 there is no well-established global consensus,19 and wide variability in their management is described. For this reason, it is essential to create an audited national registry and to conduct multicenter studies that take these differences into account and provide more information.
Obviously, this study provides data from anonymous surveys, so its results should be evaluated and considered based on the limited evidence from this type of study. Furthermore, due to the length of the questionnaire, some of the perioperative therapeutic management measures of patients with esophageal cancer could not be evaluated (such as cases with cervical tumors, early stages, nutritional strategies, treatment of complications, etc.).
Nevertheless, this paper provides indicative data on the management of this type of disease in Spanish hospitals and the lack of uniform consensus, showing clear differences in the management of patients with esophageal cancer in our country, with no homogeneity in adjuvant treatment, surgical approach, type of lymphadenectomy, or anastomosis performed.
Conflict of interestsThe authors have no conflict of interests to declare regarding this manuscript.
Ismael Díez del Val, Rafael López, Carla Bettonica, Purificación Parada, Mª José Palacios, Felipe Carlos Parreño, Mª Asunción Acosta, Peter Vorwald, Elisabet Vidaña.
Members of the Esofagogástric Surgery Division of the Spanish Asociation of Surgeons (Asociación Española de Cirujanos) are listed in Appendix A.
Please cite this article as: Bruna M, Mingol F, Vaqué FJ, Sección de Cirugía Esofagogástrica de la Asociación Española de Cirujanos. Resultados de una encuesta nacional sobre el manejo terapéutico del cáncer de esófago. Cir Esp. 2021;99:329–338.