Laparoscopic left-sided pancreatectomy (LLP) is an increasingly used surgical technique for the treatment of benign and malignant lesions of the left side of the pancreas. The results of LLP as a treatment for primary pancreatic lesions of the head and tail of the pancreas were evaluated.
MethodsFrom November 2011 to November 2017, 18 patients underwent surgery for primary lesions of the pancreas by means of a laparoscopic distal pancreatectomy. An intra-abdominal drain tube was used in all cases, and the recommendations of the International Study Group for Pancreatic Fistula (ISGPF) were followed.
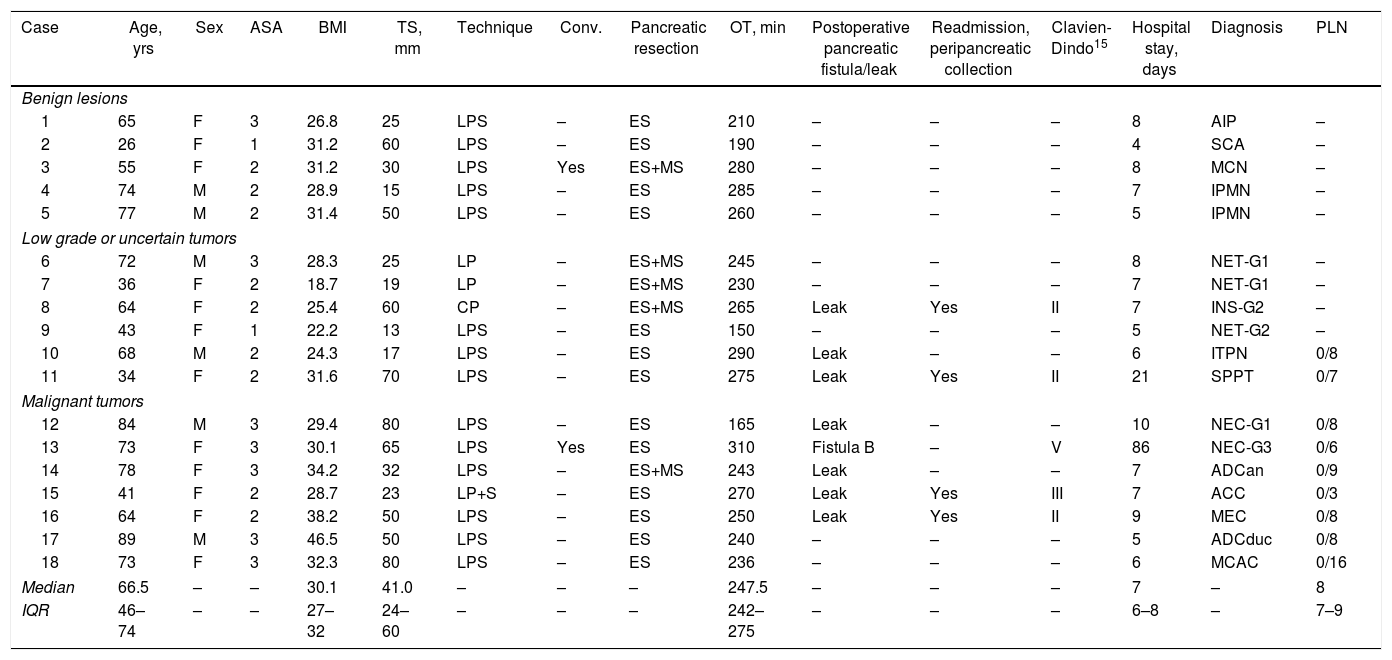
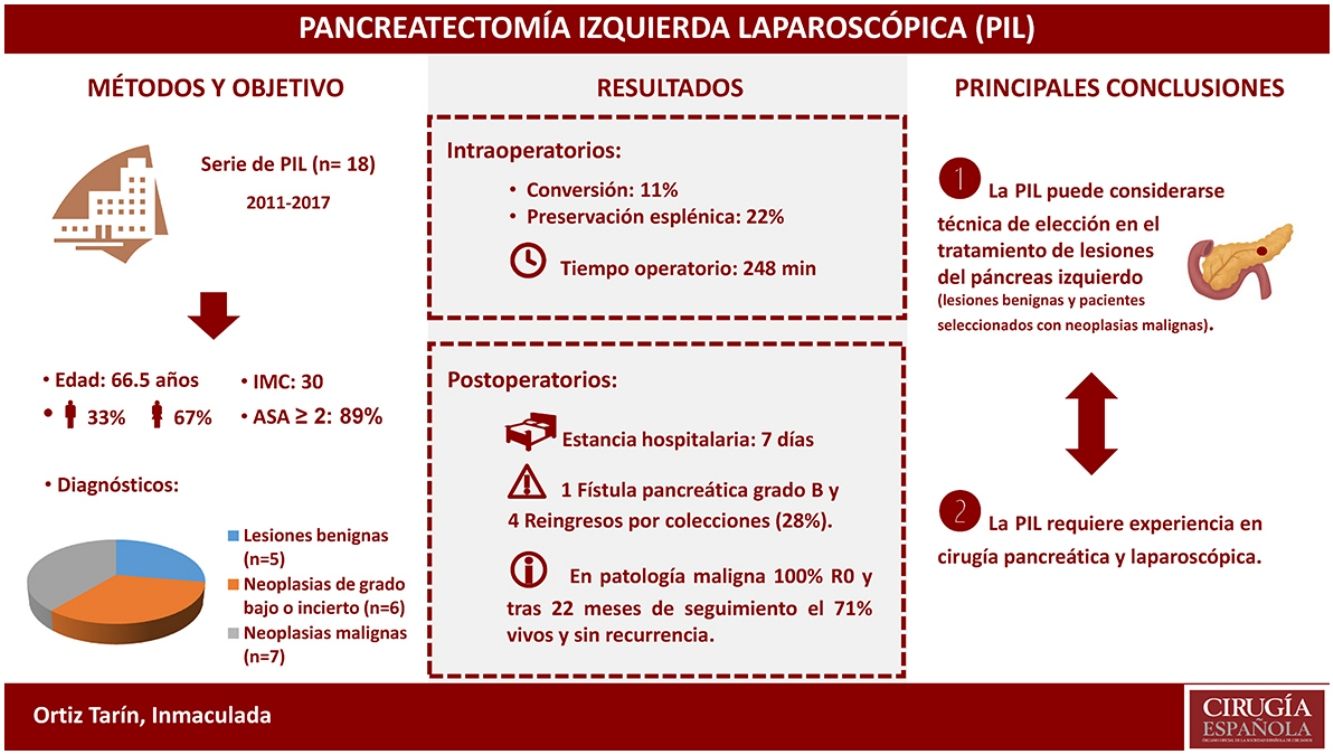
ResultsThe mean age was 66.5 years (IQR 46–74). Among the 18 left pancreatectomies performed, four were with splenic preservation, and one was a central pancreatectomy. There were two conversions. The median surgical time was 247.5 minutes (IQR 242–275). The median postoperative hospital stay was 7 days (IQR 6–8). After 90 days, complications were detected in five patients: three grade II, one grade III and one grade V according to the modified Clavien-Dindo classification. There was one grade B pancreatic fistula, and four patients had to be readmitted to hospital because of peripancreatic collections. The anatomic pathology diagnosis was malignant neoplasm in 38.9% of cases, all of them with negative resection margins.
ConclusionsLLP can be considered the technique of choice in the treatment of primary benign pancreatic lesions and an alternative to the open approach in selected patients diagnosed with malignant pancreatic lesions.
La pancreatectomía izquierda laparoscópica (PIL) es una técnica quirúrgica cada vez más utilizada para el tratamiento de lesiones benignas y malignas del páncreas izquierdo. Analizamos los resultados de nuestra serie de PIL para el tratamiento de las lesiones primarias de cuerpo y cola pancreáticos.
MétodosDesde noviembre de 2011 a noviembre de 2017 se han intervenido 18 pacientes por lesiones primarias del páncreas realizándose una pancreatectomía distal laparoscópica. En todos los casos se dejó un drenaje intraabdominal y se siguieron las recomendaciones del International Study Group for Pancreatic Fistula (ISGPF).
ResultadosLa mediana de edad fue de 66,5 años (RIQ 46-74). De las 18 pancreatectomías izquierdas, cuatro se realizaron con preservación esplénica, una de ellas una pancreatectomía central. Hubo dos conversiones. La mediana del tiempo operatorio fue de 247,5 min (RIQ 242-275). La mediana de estancia hospitalaria fue de 7 días (RIQ 6-8). A los 90 días se detectaron complicaciones en cinco pacientes: tres grado II, una grado III y una grado V según la clasificación modificada de Clavien-Dindo. Hubo una fístula pancreática grado B y cuatro pacientes reingresaron por colecciones peripancreáticas. La anatomía patológica evidenció malignidad en el 38,9% de los casos, presentando todos ellos márgenes negativos.
ConclusionesLa PIL puede ser considerada técnica de elección para el tratamiento de las lesiones pancreáticas benignas y una alternativa al abordaje abierto para pacientes seleccionados diagnosticados de neoplasias malignas, siempre que la realicen cirujanos con experiencia en cirugía pancreática y laparoscópica avanzada.
The introduction of the laparoscopic approach in pancreatic surgical pathologies has been one of the latest implementations of minimally invasive surgery. The increase in the diagnosis of incidental pancreatic lesions due to the greater number of radiological studies that are currently carried out has generated growing interest in learning these minimally invasive techniques.1 Both left pancreatic resection and pancreaticoduodenectomy1,4 are possible and safe by laparoscopy, although the latter is technically more demanding since it involves a reconstructive phase.
Left laparoscopic pancreatectomy (LLP) is widely accepted for the treatment of cystic, benign or premalignant lesions, such as mucinous cystic neoplasms and intraductal papillary mucinous neoplasms, as well as neuroendocrine tumors located in the left pancreas.5,6 It is also considered indicated in the treatment of malignant lesions that require pancreatic resection with adequate margins and extended lymphadenectomy.2,6,7 Several groups have already demonstrated its safety when performed by experienced laparoscopic surgeons.8,11 However, in many hospitals of our country, LLP is still not considered the approach of choice and is used exclusively for selected cases.12
The objective of this study is to analyze the results of our series of patients treated with LLP as treatment for primary lesions of the body and tail of the pancreas in order to verify its effectiveness and safety, and to add our experience to the series published.
MethodsFor this study, we used data from the prospective database of patients treated with pancreatic resection by the surgery service at the Hospital Doctor Peset. From November 2011 to November 2017, 25 left pancreatectomies were performed due to primary pancreatic lesions in the body and tail: 18 (72%) were laparoscopic and 7 (28%) open. Patients who were selected for the laparoscopic approach had no previous supramesocolic surgeries, tumors that were not excessively voluminous and no invasion of other organs. The final study population included 18 patients who underwent laparoscopic surgery.
Left pancreatectomy is any type of pancreatic resection requiring the complete division of the gland to the left of the gastroduodenal artery. This term includes distal pancreatectomy and central pancreatectomy.12 Division of the pancreatic parenchyma was done with a linear endostapler, using a staple depth of 3.8mm. In central pancreatectomy, an end-to-end pancreaticojejunal anastomosis of the remaining left pancreas was also performed due to intussusception over the Roux-en Y. In all cases, a closed drain tube was placed close to the resection line of the pancreatic parenchyma. The administration of subcutaneous octreotide at 100μg/8h13 was initiated during the intervention. Amylase levels in the drained fluid were analyzed on the third postoperative day. If the amylase level was less than three times the hyperamylasemia level, the drain tube and administration of octreotide were withdrawn.
We followed the recommendations of the International Study Group for Pancreatic Fistula (ISGPF) for the diagnosis and classification of postoperative pancreatic fistula.14 The modified Clavien-Dindo classification was used to grade the postoperative complications, which were analyzed after 90 days. Hospital stay was calculated from the day of the intervention until discharge to home, and readmissions were those occurring in the first 30 postoperative days.
In case of preoperative suspicion of malignant neoplasm of the pancreas, an en bloc pancreatosplenectomy with standard lymphadenectomy was planned in accordance with the RAMPS technique (radical antegrade modular pancreatosplenectomy), previously described by Strasberg et al.16
For the descriptive statistical analysis, the Excel program was used. Discrete variables are expressed as frequencies and percentages. The continuous variables are expressed as median and interquartile ranges (IQR).
ResultsDuring the study period, 18 patients underwent LLP due to a primary pancreatic lesion located in the left pancreas. The series included 12 women and 6 men, with a median age of 66.5 years (IQR 46–74), body mass index (BMI) of 30.1 (IQR 27–32) and, according to the American Association of Anesthesiology (ASA), 88.9% of the patients were classified as ASA≥2. Table 1 shows the epidemiological and clinical characteristics of these patients.
Demographic, Operative and Postoperative Data of the Patients Treated With Laparoscopica.
| Case | Age, yrs | Sex | ASA | BMI | TS, mm | Technique | Conv. | Pancreatic resection | OT, min | Postoperative pancreatic fistula/leak | Readmission, peripancreatic collection | Clavien-Dindo15 | Hospital stay, days | Diagnosis | PLN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benign lesions | |||||||||||||||
| 1 | 65 | F | 3 | 26.8 | 25 | LPS | – | ES | 210 | – | – | – | 8 | AIP | – |
| 2 | 26 | F | 1 | 31.2 | 60 | LPS | – | ES | 190 | – | – | – | 4 | SCA | – |
| 3 | 55 | F | 2 | 31.2 | 30 | LPS | Yes | ES+MS | 280 | – | – | – | 8 | MCN | – |
| 4 | 74 | M | 2 | 28.9 | 15 | LPS | – | ES | 285 | – | – | – | 7 | IPMN | – |
| 5 | 77 | M | 2 | 31.4 | 50 | LPS | – | ES | 260 | – | – | – | 5 | IPMN | – |
| Low grade or uncertain tumors | |||||||||||||||
| 6 | 72 | M | 3 | 28.3 | 25 | LP | – | ES+MS | 245 | – | – | – | 8 | NET-G1 | – |
| 7 | 36 | F | 2 | 18.7 | 19 | LP | – | ES+MS | 230 | – | – | – | 7 | NET-G1 | – |
| 8 | 64 | F | 2 | 25.4 | 60 | CP | – | ES+MS | 265 | Leak | Yes | II | 7 | INS-G2 | – |
| 9 | 43 | F | 1 | 22.2 | 13 | LPS | – | ES | 150 | – | – | – | 5 | NET-G2 | – |
| 10 | 68 | M | 2 | 24.3 | 17 | LPS | – | ES | 290 | Leak | – | – | 6 | ITPN | 0/8 |
| 11 | 34 | F | 2 | 31.6 | 70 | LPS | – | ES | 275 | Leak | Yes | II | 21 | SPPT | 0/7 |
| Malignant tumors | |||||||||||||||
| 12 | 84 | M | 3 | 29.4 | 80 | LPS | – | ES | 165 | Leak | – | – | 10 | NEC-G1 | 0/8 |
| 13 | 73 | F | 3 | 30.1 | 65 | LPS | Yes | ES | 310 | Fistula B | – | V | 86 | NEC-G3 | 0/6 |
| 14 | 78 | F | 3 | 34.2 | 32 | LPS | – | ES+MS | 243 | Leak | – | – | 7 | ADCan | 0/9 |
| 15 | 41 | F | 2 | 28.7 | 23 | LP+S | – | ES | 270 | Leak | Yes | III | 7 | ACC | 0/3 |
| 16 | 64 | F | 2 | 38.2 | 50 | LPS | – | ES | 250 | Leak | Yes | II | 9 | MEC | 0/8 |
| 17 | 89 | M | 3 | 46.5 | 50 | LPS | – | ES | 240 | – | – | – | 5 | ADCduc | 0/8 |
| 18 | 73 | F | 3 | 32.3 | 80 | LPS | – | ES | 236 | – | – | – | 6 | MCAC | 0/16 |
| Median | 66.5 | – | – | 30.1 | 41.0 | – | – | – | 247.5 | – | – | – | 7 | – | 8 |
| IQR | 46–74 | – | – | 27–32 | 24–60 | – | – | – | 242–275 | – | – | – | 6–8 | – | 7–9 |
ADCan: anaplastic adenocarcinoma; ADCduc: ductal adenocarcinoma; PLN: positive lymph nodes/total resected nodes (only for malignant neoplasms); ASA: American Society of Anesthesiologists; MCAC: mucinous cystadenocarcinoma; SCA: serous cystadenoma; ACC: acinar cell carcinoma; MEC: mucoepidermoid carcinoma; NEC: neuroendocrine carcinoma; Conv.: conversion to open surgery; ES: endostapler; ES+MS: endostapler and continuous manual suture to reinforce pancreatic margin; LPS: left pancreatosplenectomy; FistulaB: grade B pancreatic fistula; Leak: biochemical leak; G1, G2, G3: low, intermediate, high histologic grade according to the World Health Organization (WHO) Classification for neuroendocrine tumors 2010–2017; BMI: body mass index; INS: insulinoma; F: female; MCN: mucinous cystic neoplasm; ITPN: intraductal tubulopapillary neoplasm; AIP: autoimmune pancreatitis; CP: central pancreatectomy; LP: left pancreatectomy with spleen and splenic vessel preservation; PI+S: left pancreatectomy with spleen preservation and splenectomy in a second operation; IQR: interquartile range; IPMN: intraductal papillary mucinous neoplasm; NET: non-functioning neuroendocrine tumor; OT: operative time; SPPT: solid pseudopapillary tumor; TS: tumor size; M: male.
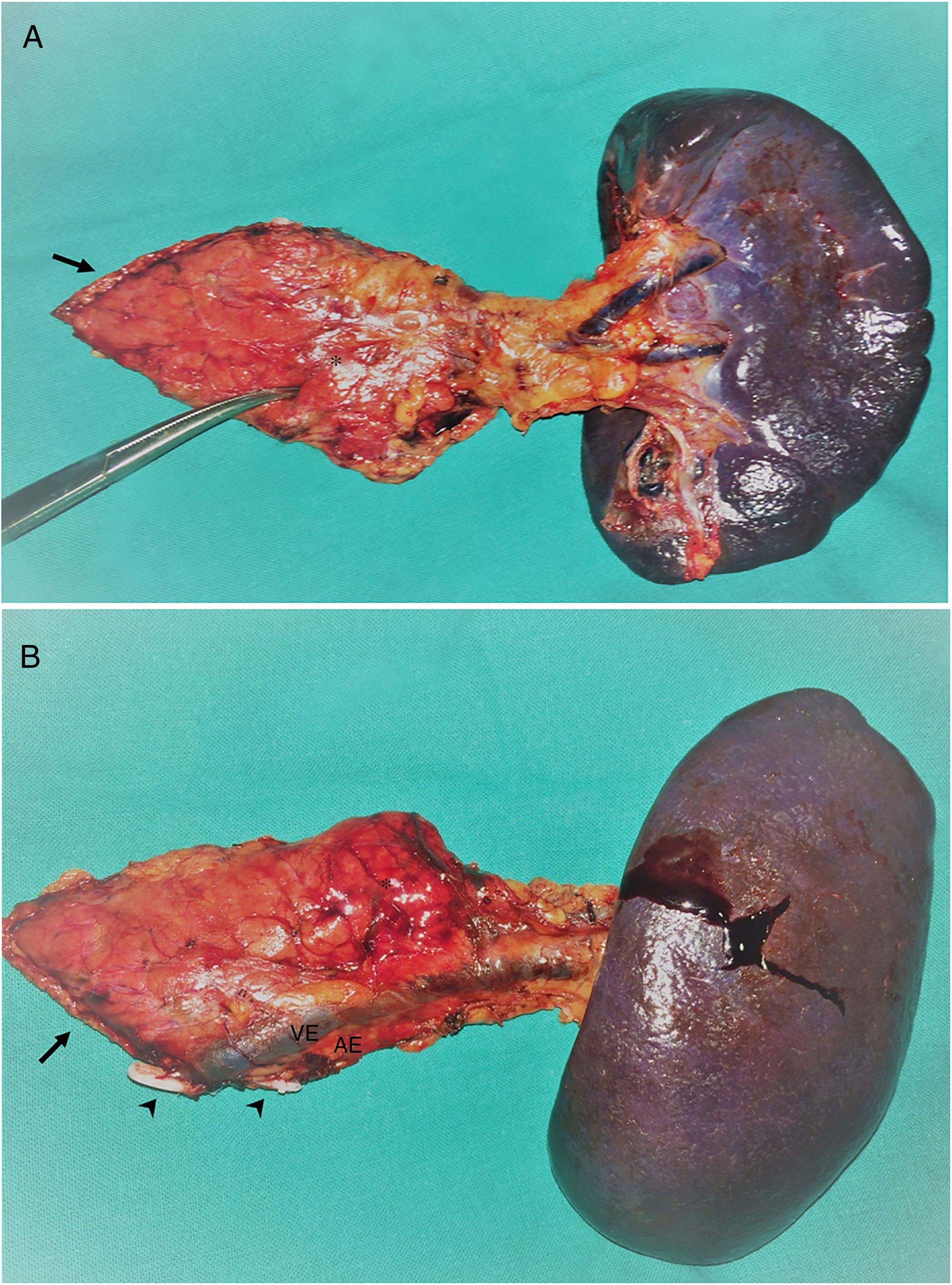
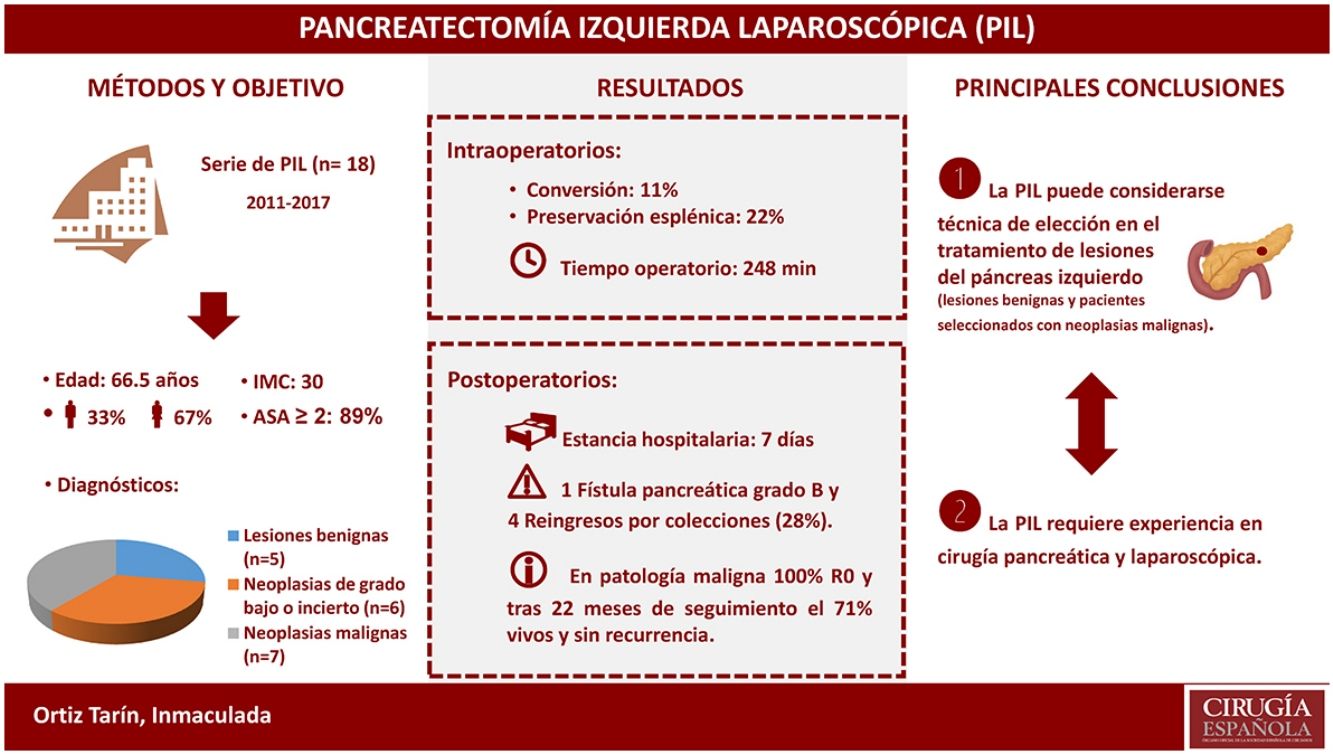
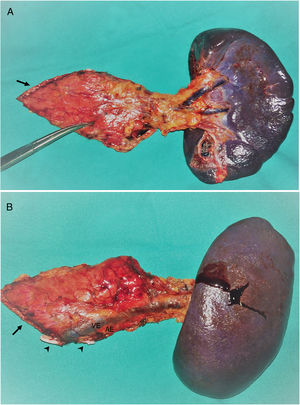
A total of 17 (94.4%) distal pancreatectomies and one (5.6%) central pancreatectomy were performed, four of which (22.2%) were completed with spleen and splenic vessel preservation. Fig. 1 shows a left pancreatectomy piece. The median operative time was 247.5min (IQR 242–275). In three (16.7%) patients, another surgery was also associated: one cholecystectomy, one myomectomy and one cholecystectomy with atypical gastrectomy, the latter due to tumor invasion. In two (11.1%) patients, conversion to open surgery was necessary: one due to technical difficulties in the caudal dissection of the pancreas, and another due to intraoperative hemorrhage that was uncontrollable by laparoscopy. Two (11.1%) patients required blood transfusion in the perioperative period.
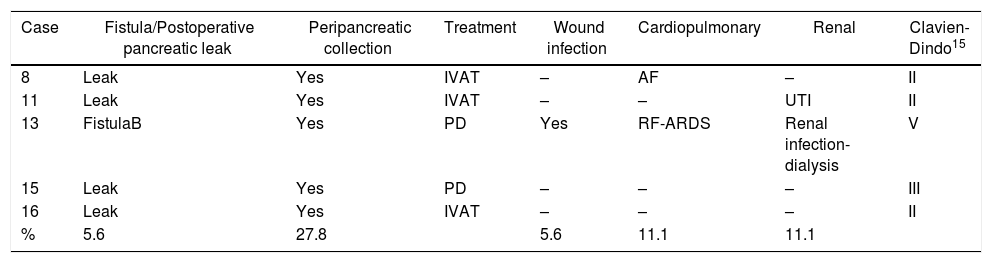
During the postoperative period, one (5.6%) pancreatic fistula was detected, requiring percutaneous drainage (grade B). This patient died 86 days after surgery due to an episode of respiratory failure. Seven (38.9%) biochemical leaks were also detected, which, according to the latest review of the ISGPF,14 were not initially considered pancreatic fistulae. Four (22.2%) of these patients were readmitted during the first month after discharge due to peripancreatic collections, only one of which required percutaneous drainage. Thus, the percentage of fistulae was 27.8%. None of the patients required surgical reoperation during the postoperative period. Complications were detected after 90 days in five (31.3%) patients of the study: three Clavien-Dindo grade II, one grade III and one grade V, which are shown in Table 2. The median hospital stay was 7 days (IQR 6–8).
Postoperative Complications of Patients After Laparoscopica.
| Case | Fistula/Postoperative pancreatic leak | Peripancreatic collection | Treatment | Wound infection | Cardiopulmonary | Renal | Clavien-Dindo15 |
|---|---|---|---|---|---|---|---|
| 8 | Leak | Yes | IVAT | – | AF | – | II |
| 11 | Leak | Yes | IVAT | – | – | UTI | II |
| 13 | FistulaB | Yes | PD | Yes | RF-ARDS | Renal infection-dialysis | V |
| 15 | Leak | Yes | PD | – | – | – | III |
| 16 | Leak | Yes | IVAT | – | – | – | II |
| % | 5.6 | 27.8 | 5.6 | 11.1 | 11.1 |
IVAT: intravenous antibiotic therapy; PD: percutaneous drainage; AF: paroxysmal atrial fibrillation; FistulaB: grade B pancreatic fistula; Leak: biochemical leak; RF: respiratory failure; ARDS: adult respiratory distress syndrome; %: percentage of patients of total patients treated.
The size of the lesions was variable, with a median of 41mm (IQR 24–60). The definitive diagnoses are shown in Table 1. In seven (38.9%) patients, the definitive diagnosis was malignancy. One patient with suspected preoperative diagnosis of a solid pseudopapillary tumor underwent spleen-preserving LLP, and, with a definitive diagnosis of acinar cell carcinoma of the pancreas, a laparoscopic radical splenectomy was performed in a second surgery. Pancreatic resection margins were free of disease in all cases. The median number of lymph nodes obtained in patients with a diagnosis of malignancy was 8 (IQR 7–9). After a median follow-up of 22 months (IQR 17–41), 71.4% of the patients with malignant neoplasm of the left pancreas continue to be disease-free.
DiscussionAlthough LLP is currently accepted for the treatment of benign lesions located in the left pancreas and its safety has been proven for malignant lesions,2,10 its use has not yet been standardized in many hospitals because it is a very demanding technique that requires experience in pancreatic surgery and advanced laparoscopic surgery. Furthermore, the small number of patients requiring this treatment makes it difficult to acquire experience.
Reports on the complications, clinically relevant pancreatic fistula and mortality for resections of the left pancreas provide rates that vary widely: 25%–70%, 6%–40% and 0%–7%, respectively.5,12,17,22 The European DISPACT study,22 which includes 450 patients undergoing left pancreatectomy using a conventional approach, published a total rate of postoperative complications of 70%, including 45% of patients with at least one serious complication, 6% wound infection and a 90-day mortality rate of 3%. A retrospective multicenter study20 with 127 patients operated on at European medical centers with laparoscopic experience reported a pancreas-related rate of complications of 31%, including 17% clinically relevant pancreatic fistulae, a conversion rate of 14%, 6.3% reoperations and a mortality rate of 0%. In our country, Fernández-Cruz et al.5 published in 2007 an extensive LLP series with a conversion rate of 7%, postoperative morbidity rates of 25.2, 16.7 and 40%, and pancreatic fistula rates of 7.7, 10 and 35% in the left pancreatectomy group with spleen preservation, left pancreatosplenectomy and enucleation, respectively, with 0% mortality. Most of these series mix different types of pancreatic lesions as well as different surgical techniques. Furthermore, the results obtained are closely related to the definition and classification of the complications used, thoroughness of the data collection, as well as the experience of the surgical teams. Regardless, to date the results of retrospective studies comparing the open and laparoscopic approaches17,19,23 and published meta-analyses2,3,24,25 have not found significant differences in terms of operative time, rate of pancreatic fistula in left pancreatectomy, mortality or even oncological radicality. Advantages, however, have been found in favor of the laparoscopic approach in terms of reduction of blood loss, postoperative stay, total complications and faster postoperative recovery. Recently, in a study of patients over the age of 70 and fragile patients treated with LLP, Konstantinidis et al.26 have found fewer Clavien-Dindo grade IV complications and less mortality than in patients treated by open surgery, with statistically significant results. This highlights the importance of the laparoscopic approach, especially in patients with a reduced physiological reserve.
In our series, five (27.8%) patients had a fistula or peripancreatic collection. After the division of the pancreas with the endostapler, depending on the quality of the stapling, the thickness and the consistency of the pancreas, the pancreatic resection margin was reinforced with a continuous manual suture in five of the 18 patients, only one of which presented a postoperative pancreatic fistula (POPF). For the purpose of preventing POPF, somatostatin analogs have been used,13,27 as well as different methods of closing the residual pancreatic stump. Two DISPACT studies22,28 and a Cochrane meta-analysis29 have demonstrated no differences between division of the pancreas either with an endostapler or with a scalpel followed by manual closure of the pancreatic remnant in terms of pancreatic fistula or postoperative mortality. Currently, the choice of the pancreatic stump closure technique depends on the preference of the surgeon and the anatomical characteristics of the pancreas. Notwithstanding, we agree with Poves et al.12 in that the pancreas should be divided at the neck, where the parenchyma is thinner and stapling is usually safer and more effective.
The ISGPS has recently revised the POPF criteria accepted in 2005.14 The new definition includes any volume of drained fluid with amylase levels greater than three times the normal upper limit of plasma amylase after the third postoperative day, and adds that this condition should be clinically relevant. Consequently, the old grade A fistula is renamed ‘biochemical leak’, since it has no clinical relevance and is no longer considered a true pancreatic fistula or a complication. In fact, many series had already excluded grade A fistulae from the analysis of their results.14 However, although they cannot be called fistulae, they also cannot be ignored. Even minimal leaks can lead to late-onset collections and readmissions, as in four of our patients. In a recently published article,10 the incidence of POPF was significantly reduced by placing a small low-pressure suction drain in the pancreatic resection bed instead of a multi-tube system. The criteria indicate that, despite the risk of abdominal collections, these are asymptomatic in most cases. Meanwhile, pancreatic fistula grades B and C are also more precisely defined.
LLP is a complex technique, with operative times longer than 3h in most series.10,12,21 Our operative time was 247.5min, which was greater than in some published studies17,18 but similar to others.12,21,28 Large tumors, spleen preservation or oncological resections increase the operative time, and in our series many pancreatic lesions were voluminous (median 41mm), while 38.9% were malignant and patients also had a higher BMI (median 30.1). However, conversion to open surgery was only necessary in two (12.5%) patients: in one due to technical difficulties and in another due to hemorrhage. Jusoh and Ammori1 indicted that the most common causes for conversion were hemorrhage, difficult exposure, intra-abdominal adhesions, retroperitoneal adhesions secondary to malignancy or chronic pancreatitis and failure to progress with the surgery. Visceral obesity hinders exposure, identification of the pancreas and dissection maneuvers,10 and it has also been considered a significant predictor of conversion in left pancreatectomy.30
In our series, we considered spleen-preserving surgery when the preoperative suspected diagnosis was benign, provided the anatomical conditions enabled it. This underlines the importance of determining the type of pancreatic lesion before the intervention to propose the most adequate excision. However, the advantages of splenic preservation must be weighed against the drawbacks of an incomplete resection in the case of a malignant tumor, as occurred in one of our patients who required radical splenectomy in a second operation.
There are currently no randomized studies comparing laparoscopic distal pancreatectomy with open surgery in patients with malignant pancreatic neoplasms, and recently the European DIPLOMA group has initiated the first clinical trial with these characteristics. However, several groups have indicated that optimal oncological radicality is possible and that the laparoscopic approach can be a safe alternative to open surgery in patients with adenocarcinoma.2,5,8,9,11 Data from retrospective studies suggest that the rate of negative margins and the number of lymph nodes resected in the laparoscopic and open approaches are similar,2,7,9 and although the data for recurrence and survival are limited, similar data are also shown.7,9 A retrospective multicenter matched cohort study obtained a higher percentage of R0 resections and a lower number of lymph nodes removed in the laparoscopic approach, with comparable mean survival.31 However, it is still a retrospective study, and the authors themselves point out possible biases and the need to carry out randomized studies. In the seven patients with malignant pathology operated on in our series, we obtained 100% negative resection margins and a median of eight isolated lymph nodes. After a median follow-up of 22 months (IQR 17–41), 71.4% of the patients with malignant neoplasm of the left pancreas were alive and recurrence-free.
In conclusion, LLP can be considered the technique of choice for the treatment of benign pancreatic lesions and an alternative to the open approach for selected patients diagnosed with malignant neoplasms, provided that the procedure is performed by surgeons with experience in advanced laparoscopic and pancreatic surgery. There is no doubt that there are still controversial aspects to be resolved in LLP, but it is clear that the pancreatic surgeon must be trained in this technique, which can be considered standard for left pancreatic resections.
FundingThis present study has received no specific funding from the public, commercial or nonprofit sectors.
Conflict of InterestNone.
Please cite this article as: Ortiz Tarín I, Domingo del Pozo C, Martínez Pérez A, Sebastián Tomás JC, Payá Llorente C, Martínez Blasco A, et al. Abordaje laparoscópico del páncreas izquierdo. Cir Esp. 2019;97:162–168.