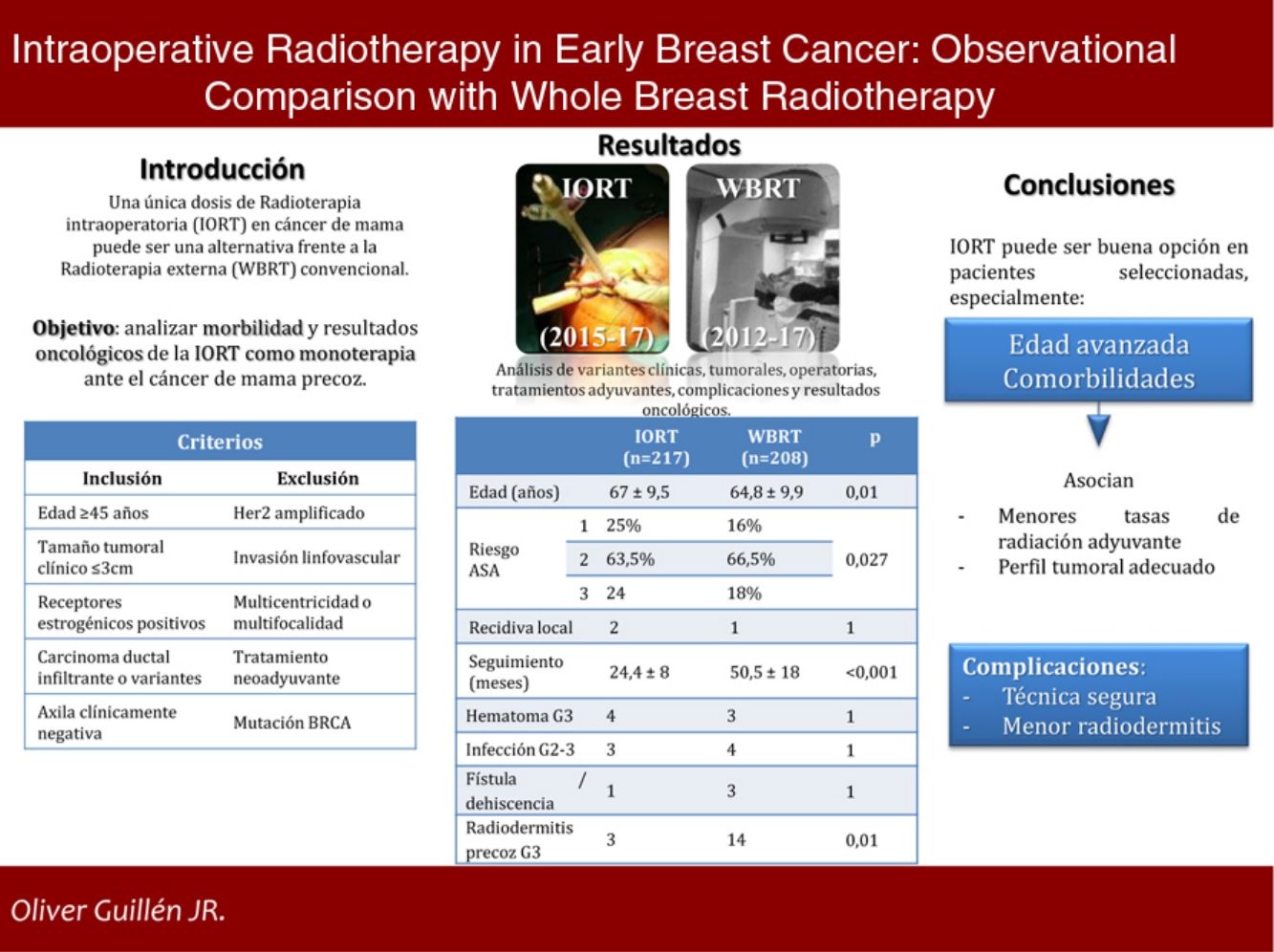
In early breast cancer (EBC), a single dose of intraoperative radiotherapy (IORT) might be an option to standard whole breast radiotherapy (WBRT). However, there is no consensus about its use and clinical results.
AimTo analyse the morbidity and oncological outcomes of IORT as monotherapy in EBC.
MethodsA single centre observational analytic study was performed. A prospective IORT cohort (2015−17) and a retrospective WBRT cohort (2012−17) were selected following the same criteria: ≥ 45 y.o., invasive ductal carcinoma or variants, radiological tumour size ≤ 3cm, positive oestrogenic receptors, negative HER2, cN0; exclusion criteria: lymphovascular invasion, multicentricity/multifocality, BRCA mutation and neoadjuvant therapy. Clinical, histological, surgical, oncological characteristics and complications were collected.
ResultsA total of 425 cases were selected: 217 in IORT cohort and 208 in WBRT cohort. Average age in IORT and WBRT groups was 67±9.5 and 64.8±9.9 y.o. respectively (p=0.01). ASA 3 risk score patients were 17.7% in IORT and 24 cases in WBRT (p=0.027). There were no differences in histological results or tumoral stage. Average follow up was 24.4±8 months in IORT and 50.5±18 months in WBRT (p<0.001). No differences were detected in local recurrence, metastases or mortality. Complications that required reintervention or hospitalization were similar in both groups. A total of 3 and 14 cases developed early severe dermatitis in IORT and WBRT groups respectively (p=0.01).
ConclusionIORT as monotherapy in selected patients with EBC stands for an alternative option versus WBRT. It seems especially useful in advanced-age patients with severe comorbidities. IORT associates lesser early severe dermatitis.
Una única dosis de radioterapia intraoperatoria (IORT) en cáncer de mama precoz (EBC) puede ser una opción frente a la radioterapia externa estándar (WBRT). Sin embargo, no existe consenso sobre su uso y resultados.
ObjetivoAnalizar la morbilidad y resultados oncológicos de la IORT como monoterapia en el tratamiento del EBC.
MétodosSe realiza un estudio analítico observacional unicéntrico, comparando una cohorte prospectiva IORT (2015-17) con una cohorte retrospectiva WBRT (2012-17). Los criterios de selección aplicados son: ≥ 45 años de edad, carcinoma ductal infiltrante o variantes, tamaño tumoral radiológico ≤ 3cm, receptores estrogénicos positivos, HER2 negativo, cN0; criterios de exclusión: invasión linfovascular, multicentricidad/multifocalidad, mutaciones BRCA y tratamiento neoadyuvante.
Se valoran características clínicas, tumorales, quirúrgicas, oncológicas y complicaciones.
ResultadosSe estudiaron 425 casos: 217 tratados con IORT y 208 con WBRT. La edad media en IORT y WBRT fue 67±9,5 y 64,8±9,9 años, respectivamente (p=0,01). El riesgo ASA 3 en IORT fue 17,7%, frente a 24 casos de WBRT (p=0,027). No hubo diferencias en resultados anatomopatológicos o estadificación. El seguimiento medio de IORT fue 24,4±8 meses, frente a 50,5±18 meses de WBRT (p<0,001). No se hallaron diferencias significativas en recidiva local, metástasis o mortalidad. Las complicaciones que precisaron reintervención u hospitalización resultaron equiparables. La radiodermitis precoz grave se presentó en tres casos IORT frente a 14 casos WBRT (p=0,01).
ConclusionesLa IORT como monoterapia en pacientes seleccionadas con EBC representa una opción alternativa frente a WBRT, especialmente en aquellas con edad avanzada y comorbilidades. Se asocia, además, con menos radiodermitis precoz grave.
The standard treatment for early breast cancer (EBC) is breast-conserving surgery (BCS) followed by adjuvant external whole breast radiotherapy (WBRT) for 3 to 5 weeks.1
15%–30% of patients in initial stages do not complete adjuvant treatment (especially those who live far from radiotherapy centers or are elderly), opting for more radical surgeries.2
Up to 90% of breast cancer recurrences are located in the vicinity of the primary tumor.3 Hence, a new paradigm has emerged: accelerated partial breast irradiation (APBI), which focuses therapy on the region with the highest risk of recurrence.
Intraoperative radiation therapy (IORT) is a form of APBI. It irradiates the area with the highest probability of tumor recurrence in a single session, in addition to reducing treatment compliance problems, radiation exposure of healthy tissue, and radiation-induced toxicity.4 It can be administered either as monotherapy when established criteria are met, or as a boost to complement WBRT therapy.
IORT is currently the subject of controversy. It has been evaluated in two highly relevant randomized clinical trials,5,6 in addition to various series. However, its use as monotherapy is currently being questioned by certain medical societies.7
Our objective is to analyze the morbidity and oncological outcomes of IORT as monotherapy in the treatment of early stage breast cancer.
MethodsWe conducted a single-center observational analytical study comparing an IORT cohort and a WBRT cohort. The IORT cohort was a prospective series of patients diagnosed with EBC treated with BCS and IORT as monotherapy from 2015 to 2017, who met the selection criteria established in the Multidisciplinary Tumor Committee and accepted the treatment. The WBRT cohort was retrospective: we selected patients with EBC treated with BCS and WBRT from 2012 to 2017, applying selection criteria that were subsequently established to administer IORT. The objective was to evaluate the two types of treatment with the same patient profile.
Selection criteriaSeveral international organizations have established ‘appropriateness’ criteria for administering APBI.8,9 The selection criteria established at our hospital for the administration of IORT as monotherapy are very similar: age ≥ 45 years, histology of invasive ductal carcinoma (IDC) or variants with good prognosis, radiological tumor size ≤ 3cm, positive estrogen receptors (ER), negative HER2, no lymphovascular invasion (LVI), no multicentricity or multifocality, clinically negative axilla, no BRCA mutations, and no neoadjuvant treatment.
All patients were clinically evaluated by mammography, ultrasound and biopsy. Bilateral breast MRI was performed in cases with dense glandular pattern (category D). Bone scintigraphy was performed in cT2 tumors.
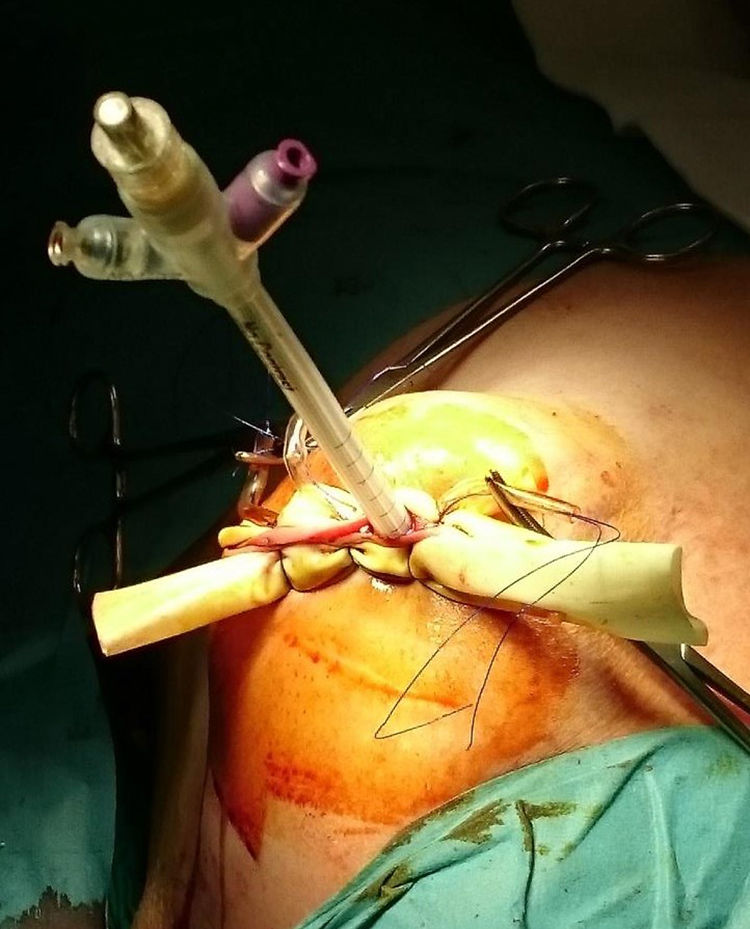
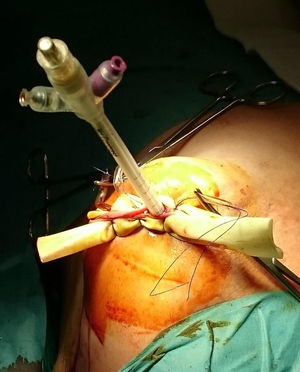
ProcedureIn the IORT cohort, patients were treated with BCS, selective sentinel lymph node biopsy (SLNB) and IORT. We used Axxent® Xoft, Inc., Sunnyvale, CA, a high-dose, low-energy electronic device (50kV peak energy) with an integrated X-ray tube in a flexible multilumen catheter to deliver radiation.10 After completing BCS with intraoperative confirmation of no margin involvement, the applicator balloon was inserted and adapted to the cavity, administering radiation (Fig. 1).
In the WBRT cohort, patients underwent BCS and SLNB. They received WBRT, both conventional and hypofractionated therapies.
In both cohorts, the axillary study was performed with the OSNA® technique (one-step nucleic acid amplification) in CK19+ tumors, and with HE (hematoxylin-eosin) in CK19- lesions. Starting in 2016, the ACOSOG (American College of Surgeons Oncology Group) Z0011 criteria11 were implemented.
Variables under studyThe following variables were collected in both groups: clinical (age, ASA risk [American Society of Anesthesiologists]), tumor (histology, histological grade, immunohistochemical [IHC] study, stage), operative (type of procedure, time of IORT, duration of surgery, axillary approach), adjuvant treatments, major complications and oncological results.
Local recurrence, regional recurrence, and distant metastasis were diagnosed by radiological tests, biopsies, or both.
Major complications were defined as grades 3 and 4 radiodermatitis (RTOG/EORTC criteria12), infection that required intravenous antibiotic therapy (grade 2) or surgical drainage (grade 3) and hematoma that required surgical drainage (grade 3) (CTCAE criteria13), in addition to a fistula that required surgical repair (defined as a delay in the healing of a portion of the surgical wound).
The different degrees of early radiodermatitis were evaluated in both groups, and late-onset in the IORT group. Some data are not available in the retrospective WBRT cohort, including late radiodermatitis, so the number of data collected in the tables may not coincide with the total number of cases.
Statistical analysisIn the descriptive study, the results were expressed as means and standard deviation for continuous variables, and the number of cases and their frequencies for categorical variables. The variables were compared using Student’s t test in the case of continuous variables, and the chi-squared test for categorical variables. Yates’ correction was applied in the chi-squared test when at least the value of one expected frequency was less than 5. The results were considered significant when P<.05. All statistical calculations have been carried out using R statistical software, version 3.1.3 (www.https://www.r-project.org/).
Results215 patients were studied in the IORT cohort, two of whom presented bilateral cancer, and 208 controls were included in the WBRT cohort.
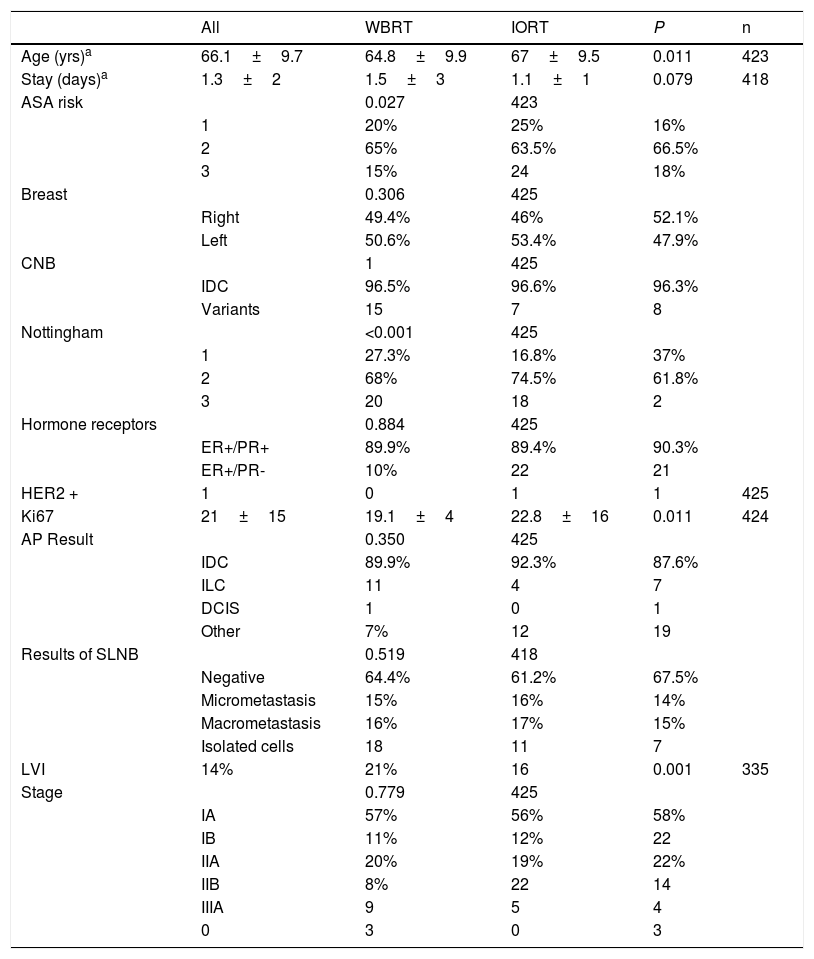
The mean ages in the IORT and WBRT groups were 67±9.5 and 64.8±9.9, respectively (P=.01). The ASA 3 risk in the IORT group was 18%, compared to 24 cases in the WBRT group (P=.027) (Table 1).
Tumor and patients characteristics (age, comorbidities).
| All | WBRT | IORT | P | n | |
|---|---|---|---|---|---|
| Age (yrs)a | 66.1±9.7 | 64.8±9.9 | 67±9.5 | 0.011 | 423 |
| Stay (days)a | 1.3±2 | 1.5±3 | 1.1±1 | 0.079 | 418 |
| ASA risk | 0.027 | 423 | |||
| 1 | 20% | 25% | 16% | ||
| 2 | 65% | 63.5% | 66.5% | ||
| 3 | 15% | 24 | 18% | ||
| Breast | 0.306 | 425 | |||
| Right | 49.4% | 46% | 52.1% | ||
| Left | 50.6% | 53.4% | 47.9% | ||
| CNB | 1 | 425 | |||
| IDC | 96.5% | 96.6% | 96.3% | ||
| Variants | 15 | 7 | 8 | ||
| Nottingham | <0.001 | 425 | |||
| 1 | 27.3% | 16.8% | 37% | ||
| 2 | 68% | 74.5% | 61.8% | ||
| 3 | 20 | 18 | 2 | ||
| Hormone receptors | 0.884 | 425 | |||
| ER+/PR+ | 89.9% | 89.4% | 90.3% | ||
| ER+/PR- | 10% | 22 | 21 | ||
| HER2 + | 1 | 0 | 1 | 1 | 425 |
| Ki67 | 21±15 | 19.1±4 | 22.8±16 | 0.011 | 424 |
| AP Result | 0.350 | 425 | |||
| IDC | 89.9% | 92.3% | 87.6% | ||
| ILC | 11 | 4 | 7 | ||
| DCIS | 1 | 0 | 1 | ||
| Other | 7% | 12 | 19 | ||
| Results of SLNB | 0.519 | 418 | |||
| Negative | 64.4% | 61.2% | 67.5% | ||
| Micrometastasis | 15% | 16% | 14% | ||
| Macrometastasis | 16% | 17% | 15% | ||
| Isolated cells | 18 | 11 | 7 | ||
| LVI | 14% | 21% | 16 | 0.001 | 335 |
| Stage | 0.779 | 425 | |||
| IA | 57% | 56% | 58% | ||
| IB | 11% | 12% | 22 | ||
| IIA | 20% | 19% | 22% | ||
| IIB | 8% | 22 | 14 | ||
| IIIA | 9 | 5 | 4 | ||
| 0 | 3 | 0 | 3 |
n: number of patients for whom information was available.
Mean ± standard deviation. ASA: American Society of Anesthesiologists. CNB: core needle biopsy. IDC: invasive ductal carcinoma. ILC: invasive lobular carcinoma. DCIS: ductal carcinoma in situ. ER: estrogen receptors. PR: progesterone receptors. SLNB: sentinel lymph node biopsy. LVI: lymphovascular invasion. BCS: breast-conserving surgery. ALND: axillary lymph node dissection.
Hormone-sensitive tumors were defined as lesions that had positive hormone receptors in ≥ 1% of cells. All cases showed positive estrogen receptors.
No significant differences were identified in baseline biopsy histology, laterality, lymph node involvement, or tumor staging. Although the initial biopsies were entirely IDC or its variants, in a small number of cases the definitive result was invasive lobular carcinoma (ILC) and ductal carcinoma in situ (DCIS); however, the data were comparable and no significant differences were found between the two groups. Papillary, mucinous, and tubular tumor subtypes were included in the ‘other’ category. Definitive staging revealed 46 patients with stages IIB and IIIA. These were pT2 tumors with pN1-2 lymph node involvement. In the IORT cohort, three cases were included as stage 0 because one case of DCIS and two cases of encapsulated papillary carcinoma were obtained in the final result (Table 1).
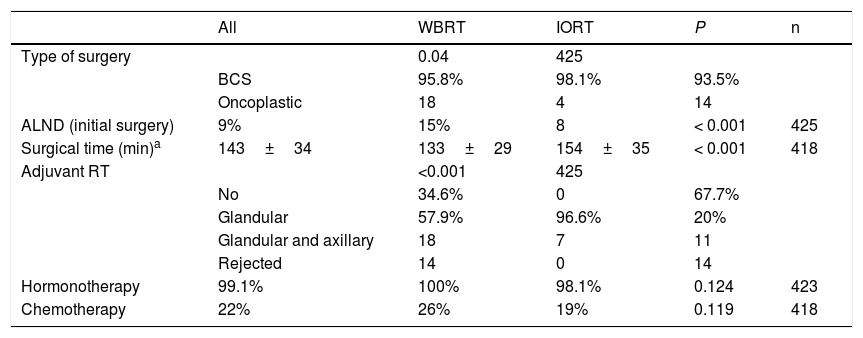
In terms of surgical data, a higher number of oncoplastic surgeries was found in the IORT group (IORT: 14 cases vs. WBRT: 4 cases; P=.04) (Table 2), with no margin involvement in the specimens extracted using these techniques in the two cohorts. The number of axillary lymph node dissections (ALND) in the initial intervention was higher in the WBRT group. In general, surgical times were longer in the IORT group (IORT: 154±35min vs WBRT: 133±29min; P<.001) (Table 2).
Surgical characteristics and adjuvant therapies.
| All | WBRT | IORT | P | n | |
|---|---|---|---|---|---|
| Type of surgery | 0.04 | 425 | |||
| BCS | 95.8% | 98.1% | 93.5% | ||
| Oncoplastic | 18 | 4 | 14 | ||
| ALND (initial surgery) | 9% | 15% | 8 | < 0.001 | 425 |
| Surgical time (min)a | 143±34 | 133±29 | 154±35 | < 0.001 | 418 |
| Adjuvant RT | <0.001 | 425 | |||
| No | 34.6% | 0 | 67.7% | ||
| Glandular | 57.9% | 96.6% | 20% | ||
| Glandular and axillary | 18 | 7 | 11 | ||
| Rejected | 14 | 0 | 14 | ||
| Hormonotherapy | 99.1% | 100% | 98.1% | 0.124 | 423 |
| Chemotherapy | 22% | 26% | 19% | 0.119 | 418 |
n: number of patients for whom information is available.
Margins in the definitive anatomic pathology study (AP) were involved in seven cases in the IORT cohort and 10 in the WBRT cohort. All of them were reoperated for re-excision, except for one case in the IORT cohort that required modified radical mastectomy (MRM) due to a definitive AP result of ILC (Table 3). This patient was excluded from the complications results for having no breast tissue (Tables 3 and 4).
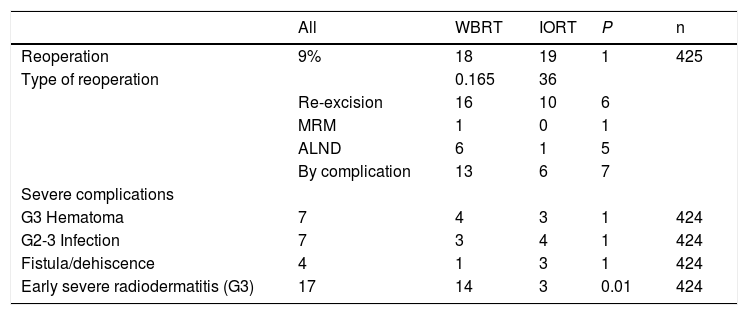
Reoperations and complications.
| All | WBRT | IORT | P | n | |
|---|---|---|---|---|---|
| Reoperation | 9% | 18 | 19 | 1 | 425 |
| Type of reoperation | 0.165 | 36 | |||
| Re-excision | 16 | 10 | 6 | ||
| MRM | 1 | 0 | 1 | ||
| ALND | 6 | 1 | 5 | ||
| By complication | 13 | 6 | 7 | ||
| Severe complications | |||||
| G3 Hematoma | 7 | 4 | 3 | 1 | 424 |
| G2-3 Infection | 7 | 3 | 4 | 1 | 424 |
| Fistula/dehiscence | 4 | 1 | 3 | 1 | 424 |
| Early severe radiodermatitis (G3) | 17 | 14 | 3 | 0.01 | 424 |
n: number of patients for whom information is available. MRM: modified radical mastectomy. G3 Hematoma: requires urgent surgery. G2 Infection: requires intravenous antibiotic treatment. G3 Infection: requires urgent surgery. G3 Early Radiodermatitis: confluent, moist desquamation; significant edema.
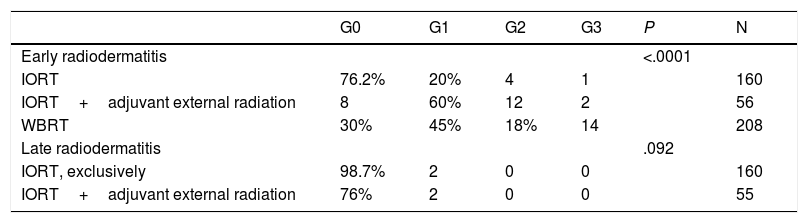
Radiodermatitis (RTOG/EORTC grades).
| G0 | G1 | G2 | G3 | P | N | |
|---|---|---|---|---|---|---|
| Early radiodermatitis | <.0001 | |||||
| IORT | 76.2% | 20% | 4 | 1 | 160 | |
| IORT+adjuvant external radiation | 8 | 60% | 12 | 2 | 56 | |
| WBRT | 30% | 45% | 18% | 14 | 208 | |
| Late radiodermatitis | .092 | |||||
| IORT, exclusively | 98.7% | 2 | 0 | 0 | 160 | |
| IORT+adjuvant external radiation | 76% | 2 | 0 | 0 | 55 |
n: number of patients for whom information is available. RTOG/EORTC: Acute and Late Radiation Morbidity Scoring Scheme.12 G: grade.
Early RTOG (acute radiodermatitis): G0: no changes. G1: faint or mild erythema, epilation, dry desquamation, reduced sweating. G2: bright erythema, moist and patchy desquamation, moderate edema. G3: confluent moist desquamation, significant edema.
Late RTOG (six months after the end of treatment): G0: absent. G1: mild atrophy, changes in pigmentation, hair loss, slight hardness, and loss of subcutaneous fat. G2: patchy atrophy, moderate telangiectasis, total hair loss, moderate fibrosis, mild contracture < 10%. G3: severe atrophy, notable telangiectasis, severe hardness, loss of subcutaneous tissue, contracture > 10%.
In the IORT cohort, 26% of patients received adjuvant WBRT, due to definitive AP findings of: pN+, ILC and LVI. There were no significant differences in adjuvant hormone therapy (HT) or chemotherapy (CT) treatments, which were administered according to OncotypeDx criteria, staging, pN+, and histological grade criteria.
No differences were found in the number and types of reoperation, including those due to complications (Table 3). There were no cases of RTOG (Radiation Therapy Oncology Group) grade 4 radiation dermatitis. Global acute radiodermatitis was greater in the WBRT cohort (Table 4). Table 4 shows the different degrees of acute radiodermatitis, differentiating those treated exclusively with IORT from those treated with IORT requiring adjuvant WBRT and controls. When RTOG grade 3 severe early radiodermatitis was analyzed exclusively, it was found to be lower in the IORT cohort compared to the WBRT cohort (grade 3 severe radiodermatitis: 3 cases IORT vs 14 cases WBRT; P=.01).
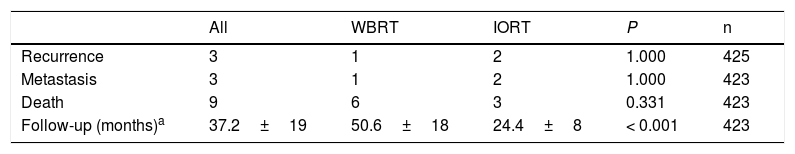
With a mean follow-up of 24.4±8 months in the IORT cohort and 50.5±18 months in the WBRT cohort (P<.001), the results for local recurrence, metastasis, and mortality showed no differences. No deaths attributable to breast cancer were identified (Table 5).
Oncological results and mortality.
| All | WBRT | IORT | P | n | |
|---|---|---|---|---|---|
| Recurrence | 3 | 1 | 2 | 1.000 | 425 |
| Metastasis | 3 | 1 | 2 | 1.000 | 423 |
| Death | 9 | 6 | 3 | 0.331 | 423 |
| Follow-up (months)a | 37.2±19 | 50.6±18 | 24.4±8 | < 0.001 | 423 |
n: number of patients for whom information is available.
The causes of death in the IORT cohort were: respiratory sepsis, squamous cell carcinoma of the metastatic trigone, and suspected autolysis. In the WBRT cohort, deaths were due to: metastatic pancreatic neoplasm in two patients, pneumonia aggravated by idiopathic pulmonary fibrosis, hepatic encephalopathy secondary to metastatic colorectal carcinoma, alcoholic cirrhosis with edematous-ascitic decompensation, and urinary sepsis.
DiscussionIn standard EBC treatment, BCS is accompanied by WBRT.14 In these cases, local recurrence was estimated at 1%, reaching a 20-year cumulative rate of 14%, compared to 39% if no WBRT had been applied. This reduction has been observed even in low-risk elderly patients who had considered omitting it.15,16
The elderly population has lower rates of adjuvant radiation.17 Only 79% and 41% of patients ≥ 70 years and ≥ 80 years of age received WBRT after BCS, respectively.18 This is even more true of patients living in rural areas, who tend to be older and transportation can be a barrier that negatively affects the adjuvant radiation rate.17 It is especially in these cases where IORT as monotherapy is presented as a good therapeutic alternative.19
Other brachytherapy techniques (interstitial, three-dimensional, modulated intensity) have been evaluated in different clinical trials versus WBRT with follow-up periods of more than five years, which have been more widely accepted by some medical societies. However, IORT is currently a subject for debate.7
As in other series,17,20 the IORT cohort was older than the WBRT cohort, as were the comorbidities assessed using the ASA scale. These two characteristics, as in the general population, have influenced the adjuvant radiation of the IORT cohort. Some 6.5% of patients proposed this treatment have not received it due to the patient’s own decision, comorbidities, or social situation.
In order to apply IORT, it is essential to follow selection criteria.5,6 In the ELIOT study, recurrence in high-risk patients was 11.3% versus 1.5% in low-risk patients.6 Criteria have been established for selecting ‘appropriate’ or ‘low-risk’ patients for treatment with APBI,9,21 very similar to those established at our hospital.
One of the problems of IORT is not being able to have the definitive AP result at the time of applying the therapy. This is why some patients have been treated with ILC, mutated HER2 or LVI. Core-needle biopsy was used in all patients, but there may be discrepancies with the final result.22,23 In the TARGIT-A5 study, the prepathology group (therapy during surgery) had a recurrence of 2.1% (1.1%–4.2%), with an adjuvant therapy rate of 22%. This is a figure that is slightly lower than 26% of this series, in which we find the axillary involvement as the main cause of adjuvant radiation. Axillary ultrasound in EBC has a low sensitivity for detecting lymph node metastases preoperatively.24 In these cases, the treatment can be complemented with WBRT and IORT used as an overprint of the surgical bed.
In this series, the follow-up of the IORT cohort (24.4±8 months) prevents reaching definitive conclusions on the oncological results, even though it exceeds the average time of recurrence of other studies (19.4 months)25 and has not shown statistically significant differences in local recurrence, metastasis, or mortality versus the WBRT cohort. Since a retrospective cohort has been used, follow-up was longer in the control cohort versus the IORT cohort.
The device used in our hospital is Xoft®Axxent®. It uses low-energy X-rays, such as Intrabeam®, but has different technical differences and, so far, the results of the RCT26 have not been published. Recurrence rates of 3.4% have been described with a median follow-up of 50 (range 12–81) months.19 The average irradiation times of the Xoft® Axxent® device (11.8±8min in this series) were shorter than the Intrabeam times (20−45min).5
As for the procedures, the number of oncoplastic surgeries in this study was higher in the IORT group. This was probably due to greater surgical experience acquired and the benefits of combining both techniques, which have enhanced their use. The loss of references to the tumor bed after oncoplastic surgery can be a real challenge when administering external radiation therapy (RT). However, IORT avoids these problems. Regarding axillary management, more ALND (axillary lymph node dissection) during the initial intervention were performed in the WBRT cohort. This is due to the application of ACOSOG Z001111 starting in 2016. However, surgical times were longer in the IORT cohort, most likely due to the need for therapy time and multidisciplinary coordination.
In this series, we found no differences in reoperations, including those due to complications. Some studies report lower rates of RTOG 3–4 radiodermatitis in the IORT group compared to WBRT.5 The analysis of radiodermatitis in this study confirmed increased early toxicity of any grade in patients treated with WBRT. Only four cases of late-onset G1 radiodermatitis were reported in the IORT cohort.
This study has limitations. It is an observational analysis that has found differences in age, ASA and LVI risk. However, these differences in age and ASA risk may be a reflection of the lower rate of WBRT in elderly patients and comorbidities.
It is accepted that variability exists among adjuvant treatments. The aim of the study is to compare radiotherapy treatments, so there must be differences between the two groups. However, for the other adjuvant treatments (HT and CT), the data were comparable. The follow-up of the IORT cohort was limited, in addition to the fact that the number of events that occurred (oncological and mortality) was low, which makes it impossible to draw definitive conclusions about these results.
In conclusion, IORT as monotherapy in selected patients with EBC is an alternative option to WBRT. It has great advantages thanks to its administration in a single dose, so it can be especially useful in elderly patients or those with comorbidities.
It is also a safe technique in terms of complications. In fact, there is less severe early radiodermatitis than with conventional treatment.
FundingThis study was funded by the Mutual Médica Foundation (research access prize, 2018). However, the entity has not participated in the design or composition of the study.
Authorization data confidentialityThis study has been evaluated and approved by the Aragon Research Ethics Committee (CEICA). All established protocols were followed for the access, collection and publication of the data from patient files.
Conflict of interestThe authors have no conflict of interests to declare.
The authors would like to thank the Breast Unit at the HU Miguel Servet (Zaragoza) for their work and collaboration with the preparation of this manuscript, especially Dr Cecilia Escuin (Oncological Radiotherapy Unit) for her work with patient follow-up and data collection.
Please cite this article as: Oliver Guillén JR, Hernando Almudi E, Molina Osorio G, Ibañez Carreras R, Font Gómez JA, Vicente Gómez I, et al. Radioterapia intraoperatoria en cáncer de mama precoz: análisis observacional frente a radioterapia externa. Cir Esp. 2021;99:132–139.