National information on the oncological results of gastric cancer surgery is scarce, so foreign figures are used, which may completely differ from local ones. The aim of our study is to analyse these results in the patients operated on in our centre.
MethodsSurvival results of 134 patients that underwent gastric cancer surgery with curative intent from 2004 to June 2016 were analysed.
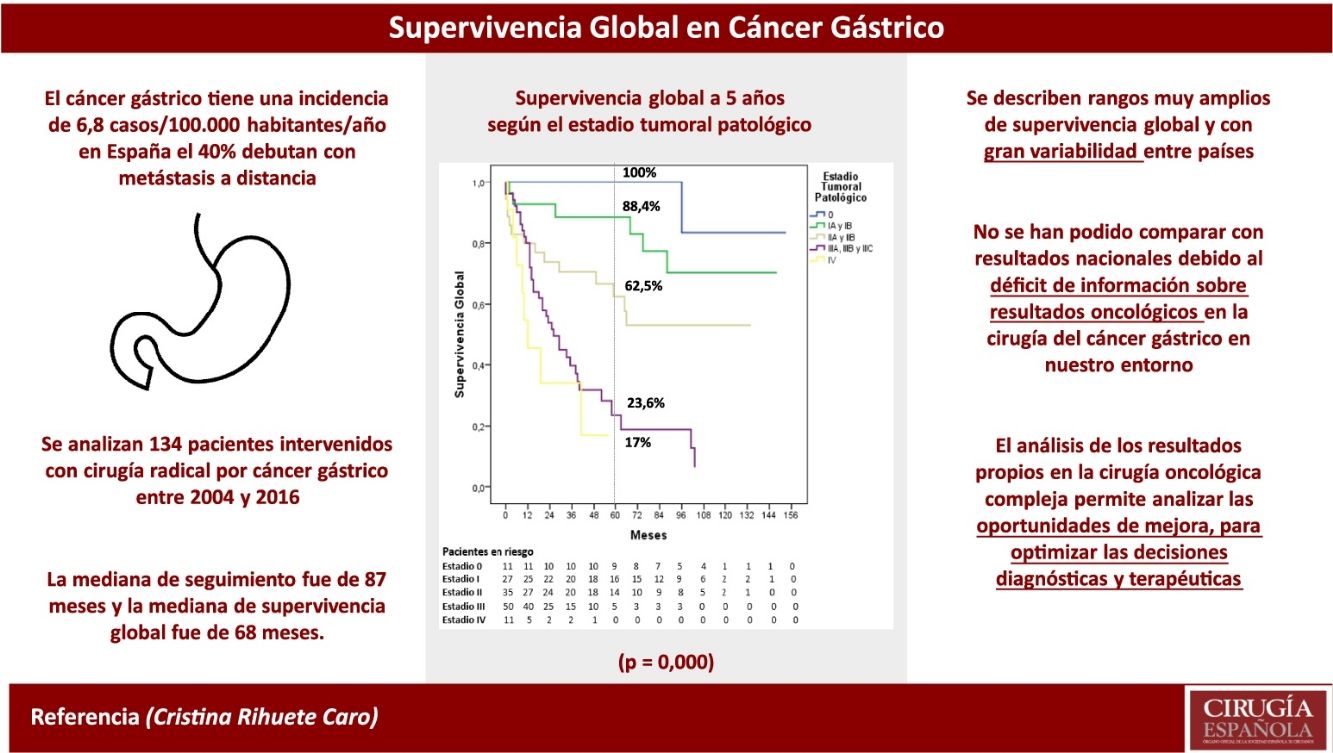
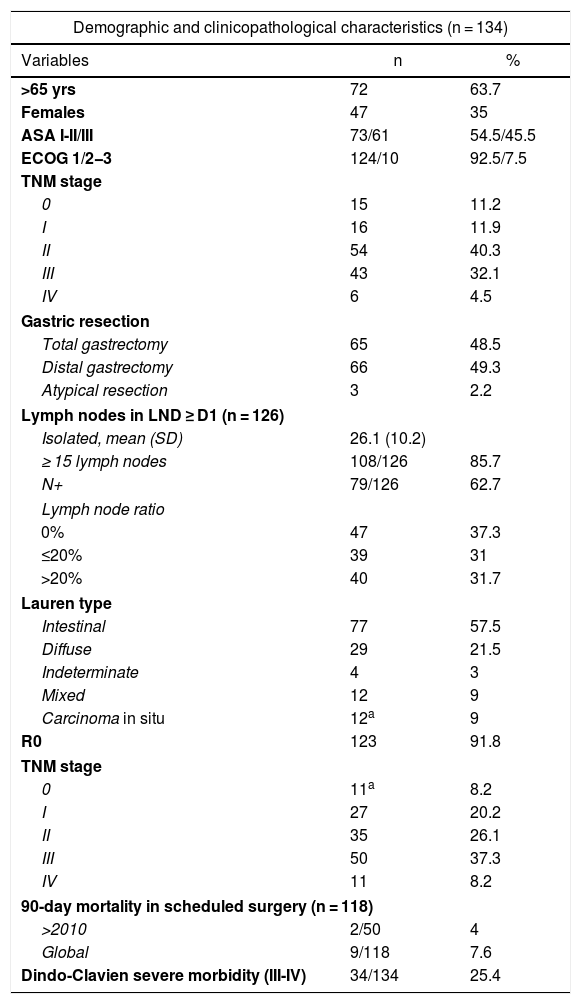
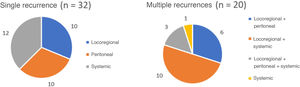
ResultsA percentage of 76.8 of the patients (103/134) presented in advanced clinical stages (≥ii). Staging laparoscopy was performed in 67% of them (69/103), an extensive lymphadenectomy (≥LD1+) was carried out in 89.3% of patients (92/103), and 76.7% (79/103) received perioperative chemotherapy. The distribution by pathological stage 0, i, ii, iii and iv was 8.2, 20.2, 26.1, 37.3, and 8.2%, respectively. Median follow-up was 87 months. Median OS was 68 months and one-, 3- and 5-year OS were 81.2, 62, and 53.8%, respectively. The 5-year OS according to pathological staging was 100% for stage 0, 88.4% for stage i, 62.5% for stage ii, 23.6% for stage iii and 17% for stage iv.
ConclusionsOur survival rates are in the high ranges of western literature. These results could not be compared with national ones due to the lack of information regarding oncological outcomes in gastric cancer surgery in our closest environment.
No hay apenas información nacional sobre los resultados oncológicos de la cirugía del cáncer gástrico, por lo que se utilizan cifras foráneas, que pueden ser absolutamente distintas de las locales. El objetivo de nuestro trabajo es analizar estos resultados en los pacientes intervenidos en nuestro centro.
MétodosSe analizan los resultados de supervivencia de 134 pacientes intervenidos por cáncer gástrico con intención curativa desde 2004 hasta junio de 2016.
ResultadosEl 76,8% de los pacientes (103/134) tenían estadios clínicos avanzados (≥ii), se realizó laparoscopia de estadificación en el 67% de los mismos (69/103), linfadenectomía extensa (≥LD1+) en el 89,3% (92/103), y recibieron QT perioperatoria el 76,7% (79/103). La distribución final por estadios patológicos 0, i, ii, iii y iv fue del 8,2; 20,2; 26,1; 37,3 y 8,2%, respectivamente. La mediana de seguimiento fue de 87 meses. La mediana de SG fue de 68 meses y la SG a uno, 3 y 5 años fue del 81,2, 62 y 53,8%, respectivamente. La SG a 5 años según el estadio patológico fue del 100% para el estadio 0, del 88,4% para el estadio i, del 62,5% para el estadio ii, del 23,6% para el estadio iii y del 17% para el estadio iv.
ConclusionesNuestras tasas de supervivencia se encuentran en los rangos altos de la literatura occidental. No se han podido comparar con resultados nacionales debido al déficit de información sobre resultados oncológicos en la cirugía del cáncer gástrico en nuestro entorno más cercano.
A primary objective of any healthcare care system should be to guarantee the quality of care in oncological-surgical processes, and the survival (SV) rate of cancer patients is a key parameter to determine the effectiveness of a healthcare system.1 The EUROCARE-52 observational retrospective study used data from 107 registries with more than 10 million patients between 1999 and 2007 and showed that there are still important differences in cancer SV rates among European countries. The European Registry of Cancer Care (EURECCA) project was created to evaluate these differences and try to establish norms aimed at improving quality and results.3
Gastric cancer (GC) is relatively rare (6.8 cases/100 000 inhabitants/year in Spain) and highly lethal (40% present with distant metastasis), Therefore, multidisciplinary management for clinical staging and therapeutic decision-making takes on greater importance. The EURECCA gastroesophageal cancer project initially selected 7 European countries with national registries (Denmark, France, Ireland, Netherlands, Poland, Sweden and the United Kingdom) and determined relevant and common data to be able to compare results between countries.4 Subsequently, a prospective observational study was conducted with the participation of 5 countries (United Kingdom, Netherlands, France, Spain and Ireland), including a total of 4668 patients diagnosed with squamous cell carcinoma or gastroesophageal adenocarcinoma treated surgically with curative intent during a 12-month period,5 demonstrating that it is feasible to implement a uniform registry to record the results of gastroesophageal cancer treatment. However, long-term SV data were not published.
After a thorough review of the literature, we were only able to find a small number of national series, mainly the esophagogastric tumour registry of the Valencian Community published in 20176 and the publication of the Spanish EURECCA group for esophagogastric cancer from 2019,7 which do not include SV data by stages. Therefore, there are no national oncological results for reference, and foreign data are normally used, which may not represent the local reality. As there is no global initiative in our country to determine cancer SV, the objective of this retrospective study is to analyse the SV of patients who have been treated surgically for GC in our hospital, which could be used as a reference to compare results in the absence of national multicentre registries.
MethodsWe conducted a retrospective study with data from a prospective database of all patients who underwent surgery for GC from 2004 to June 2016, with a follow-up of at least one year. Data were collected to analyse the demographic, diagnostic, surgical, postoperative, pathological, complementary perioperative and follow-up treatment variables. The study was approved by the Ethics and Clinical Research Committee of our hospital, which is a 450-bed hospital serving a population of approximately 300 000 inhabitants, equipped with the maximum infrastructure for cancer treatment.
Patients were diagnosed and staged by upper gastrointestinal endoscopy with biopsy and thoracic-abdominal-pelvic CT. MRI and PET were only performed if there were doubts about the existence of distant disease. We indicated staging laparoscopy in c ≥ T3 and/or N + tumours. The 7th Edition (2009) of the TNM classification was used, and patients operated on in the period prior to 2009 were reclassified with it. Although this classification considers Siewert II oesophageal tumours, some of these cases have been treated as gastric tumours, resulting from the ongoing controversy that even continues in the 8th Edition (2018) of the TNM. We indicated neoadjuvant treatment when the clinical staging was ≥ T3 and/or N+, and exclusive postoperative treatment when the indication was established after the pathological analysis. During the study period, the ECF regimen was generally used. Subtotal gastrectomy was performed on distal tumours and total gastrectomy on the rest, with extended D1+/D2 lymphadenectomy (LND). The non-inclusion of group 10 depended on the surgeon. In the latest edition of the Japanese classification, group 10 is no longer included in LND2 in total gastrectomy. In patients with early GC in the clinical staging, high morbidity or urgent surgery, more limited LND were conducted. Postoperative complications were recorded 90 days after surgery according to the Dindo-Clavien classification.
150 patients underwent surgery for GC during the study period. The surgery was performed on a scheduled basis in 126, 118 of which were macroscopically radical resections, so the rate of resection with curative intent in elective surgery was 93.7%. Radical surgery was also achieved in 16 of the 24 patients who underwent emergency surgery due to complications of the primary tumour, for a total of 134 patients with radical surgery. The cases of all patients were presented to the Multidisciplinary Tumour Committee, some preoperatively (scheduled surgery) and all postoperatively. The SV analysis was carried out for the 134 patients treated with macroscopically radical resection.
Statistical analysisTo calculate SV and the impact of each of the variables, the Kaplan-Meier method was used, and the Log-Rank method was used to compare SV curves. For the calculation of overall survival (OS), the follow-up period was defined as the period from the date treatment was started (either the date of surgery or the start date of neoadjuvant chemotherapy [CTx]), until the date of death of the patient or the last office visit before the study was closed. Median follow-up was estimated using reverse Kaplan-Meier, which is recommended for calculating follow-up times in oncology clinical trials due to the high mortality of these patients.8 A Cox multiple regression model was performed to estimate the possible relationship of OS with different variables recorded, calculating the hazard ratios for the relevant variables according to clinical criteria.
ResultsThe demographic and clinicopathological characteristics of the 134 patients are shown in Table 1. There are 20 cases whose clinical stage was early GC (stages 0-IA) who underwent surgery after endoscopic resection was ruled out. The majority of patients (76.9%, or 103/134) were diagnosed in advanced clinical stages (≥II). Staging laparoscopy was performed in 67% of these patients (69/103), extensive LND (≥LND1+) in 89.3% (92/103), and 76.7% (79/103) were administered perioperative CTx (pre- and/or postoperative), which was ECF in most (68.4%) and similar regimens in the remainder. The 6 cases with preoperatively identified metastatic disease were 4 patients with para-aortic lymphadenopathies and 2 patients with solitary liver metastases who responded to neoadjuvant treatment. In addition, after the histological analysis there were another 5 pM1 patients (one with positive cytology and 4 with microscopic foci in the omentum or gallbladder). Surgery was performed electively in 88% of patients (118/134), and the approach was mostly open (86.6%). The median (IQR) duration of the intervention was 215 (28) min, and 35% of the procedures lasted more than 4 h. The 90-day mortality rate dropped to 4% as of 2010, when the Esophago-Gastric Surgery Unit was consolidated. No complications were observed in 47 patients (35%) within 90 days. Meanwhile, mild complications (grades I-II) were observed in 40 patients (29.9%; i 0.8% and ii 29.1%), and severe complications (grades III-V) were seen in 34 (35.1%; IIIa 6.7%; IIIb 4.5%; IVa 7.5%; IVb 6.7% and V 9.7%). Hospital stay exceeded 2 weeks in 46.3% of cases, and the readmission rate was 8.2%. The most frequent anatomical location was antral (46.3%), and the series included 19 UEG tumours (5 Siewert II and 14 Siewert III) treated as GC.
Demographic and clinicopathological characteristics of the patients with gastric cancer treated with radical intent.
| Demographic and clinicopathological characteristics (n = 134) | ||
|---|---|---|
| Variables | n | % |
| >65 yrs | 72 | 63.7 |
| Females | 47 | 35 |
| ASA I-II/III | 73/61 | 54.5/45.5 |
| ECOG 1/2−3 | 124/10 | 92.5/7.5 |
| TNM stage | ||
| 0 | 15 | 11.2 |
| I | 16 | 11.9 |
| II | 54 | 40.3 |
| III | 43 | 32.1 |
| IV | 6 | 4.5 |
| Gastric resection | ||
| Total gastrectomy | 65 | 48.5 |
| Distal gastrectomy | 66 | 49.3 |
| Atypical resection | 3 | 2.2 |
| Lymph nodes in LND ≥ D1 (n = 126) | ||
| Isolated, mean (SD) | 26.1 (10.2) | |
| ≥ 15 lymph nodes | 108/126 | 85.7 |
| N+ | 79/126 | 62.7 |
| Lymph node ratio | ||
| 0% | 47 | 37.3 |
| ≤20% | 39 | 31 |
| >20% | 40 | 31.7 |
| Lauren type | ||
| Intestinal | 77 | 57.5 |
| Diffuse | 29 | 21.5 |
| Indeterminate | 4 | 3 |
| Mixed | 12 | 9 |
| Carcinoma in situ | 12a | 9 |
| R0 | 123 | 91.8 |
| TNM stage | ||
| 0 | 11a | 8.2 |
| I | 27 | 20.2 |
| II | 35 | 26.1 |
| III | 50 | 37.3 |
| IV | 11 | 8.2 |
| 90-day mortality in scheduled surgery (n = 118) | ||
| >2010 | 2/50 | 4 |
| Global | 9/118 | 7.6 |
| Dindo-Clavien severe morbidity (III-IV) | 34/134 | 25.4 |
ASA: American Society of Anesthesiologists; SD: standard deviation; ECOG: Eastern Cooperative Oncology Group; BMI: body mass index; LND: lymph node dissection.
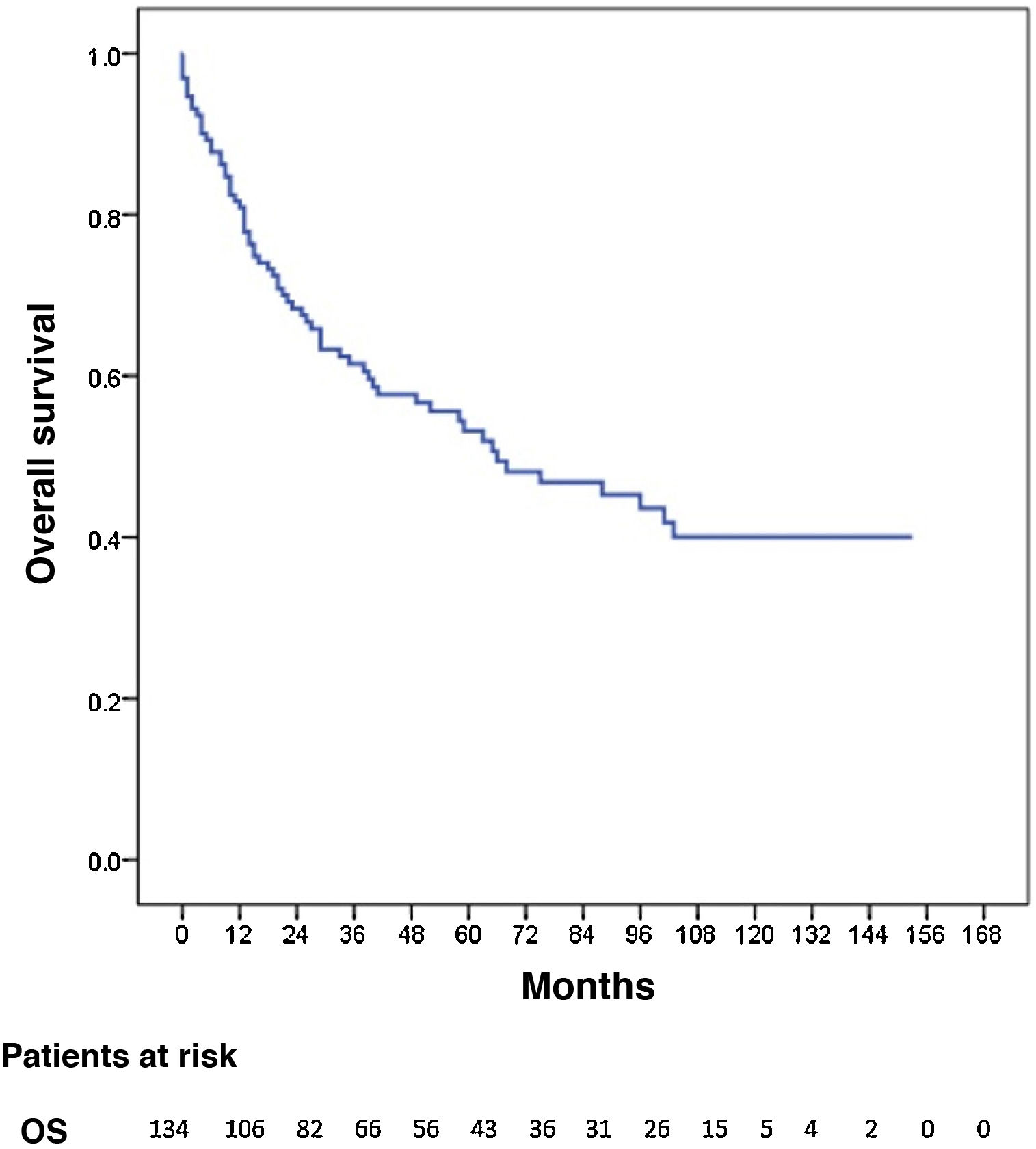
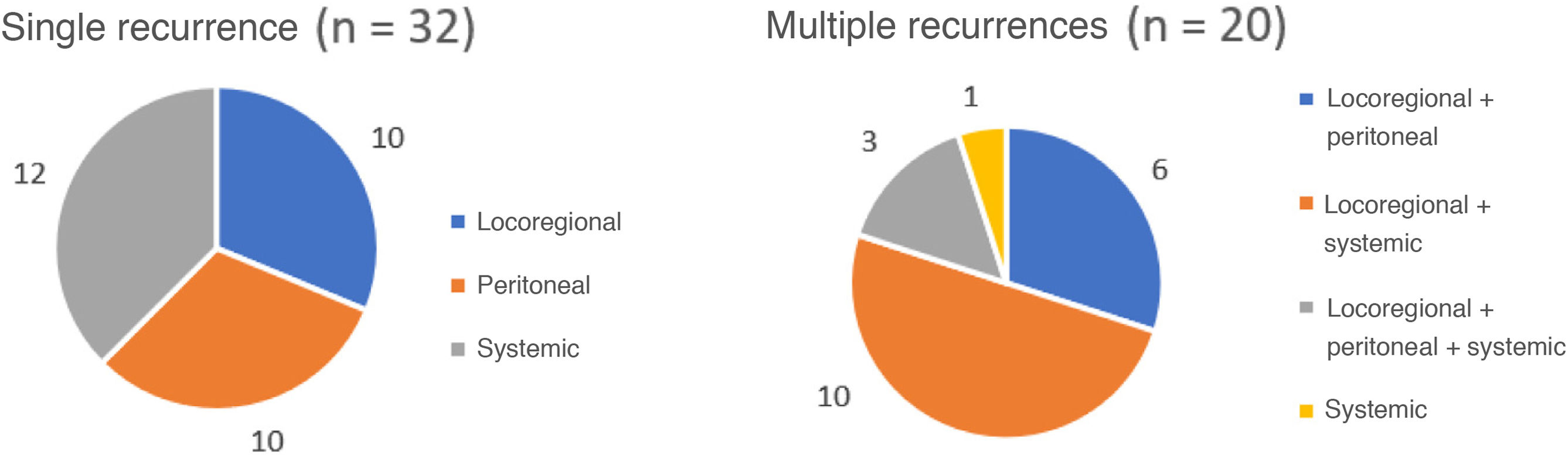
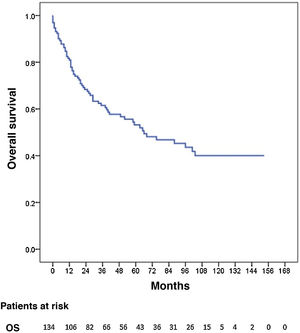
Three patients were lost to follow-up (2.2%). The median follow-up was 87 months (reverse Kaplan-Meier), and median OS was 68 months. OS after 1, 3, and 5 years was 81.2%, 62% and 53.8%, respectively (Fig. 1). Median disease-free OS was 62 months, and median 5-year survival was 50.3%. The recurrence rate after one, 3 and 5 years was 28.9%, 40.4%, and 42.7%, respectively. The recurrence patterns are shown in Fig. 2.
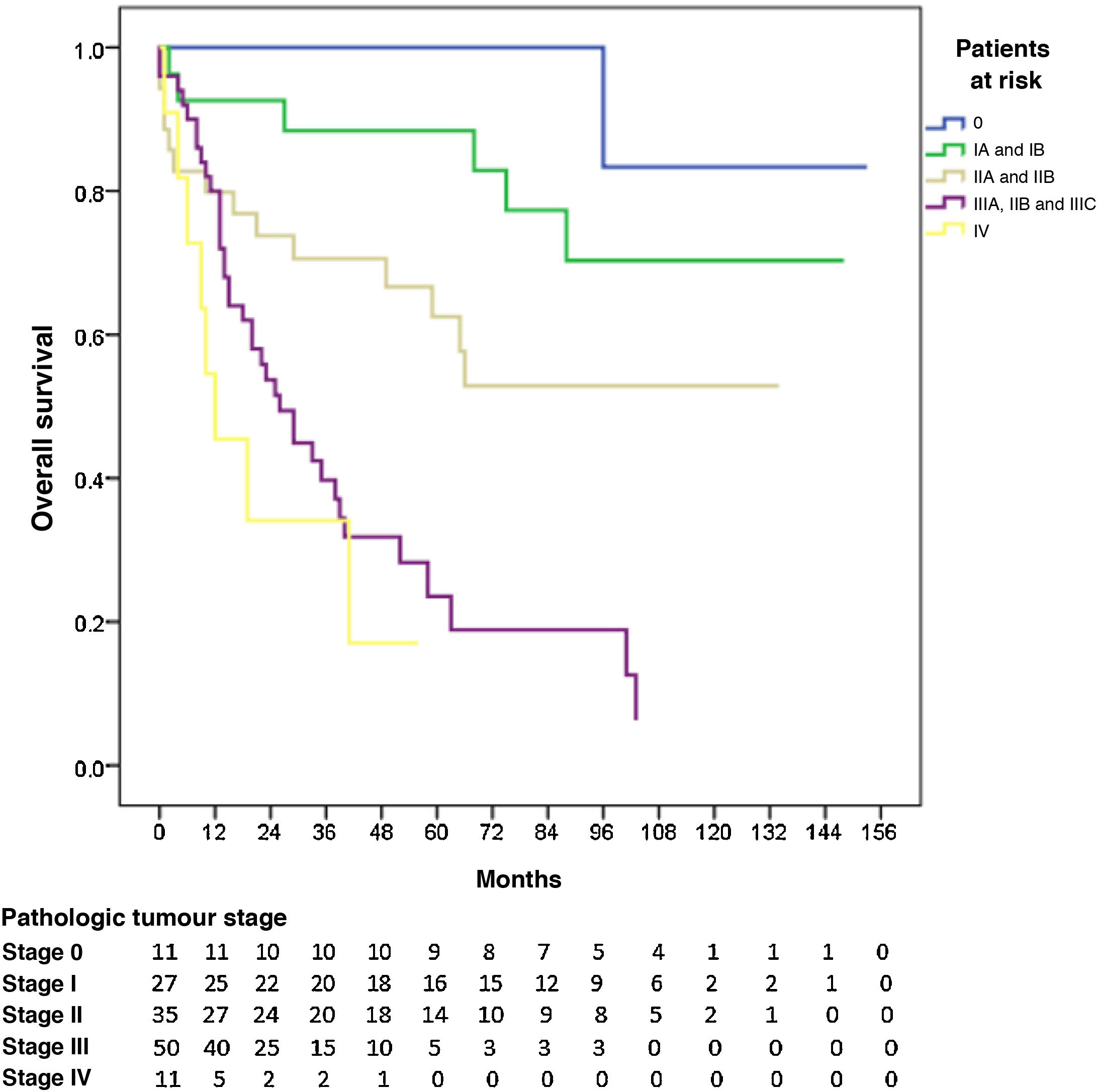
The 5-year OS by pathologic tumour stage was 100% for stage 0, 88.4% for stage I, 62.5% for stage II, 23.6% for stage III, and 17% for stage IV (P = 0.000). The median OS was not reached in stages 0, I and II, which were 26 and 12 months for stages III and IV, respectively (Fig. 3).
The variables that proved to be independent factors for a poor prognosis for OS in the Cox regression model are shown in Table 2.
Influence of the clinical and pathological variables on overall survival in gastric cancer treated with radical intent.
| Overall survival | ||||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| HR | (95%CI) | HR | (95%CI) | |
| Age >65 years | 1.791 | (1.081−2.968) | ||
| STAGES II-III (vs. I) | 2.806 | (1.382−5.698) | ||
| No perioperative CTx (in stages II and III) | 2.608 | (1.535−4.430) | 2.67 | (1.518−4.696) |
| Urgent surgery | 3.251 | (1.816−5.819) | ||
| Severe complications | 2.954 | (1.803−4.842) | 2.072 | (1.142−3.759) |
| Venous invasion | 2.696 | (1.602−4.537) | ||
| Lymphatic invasion | 2.845 | (1.723−4.696) | ||
| Perineural invasion | 2.36 | (1.449−3.843) | ||
| R1 resection margins | 2.44 | (1.238−4.810) | ||
| Tumour size ≥4 cm | 2.824 | (1.616−4.935) | ||
| pT ≥ T3 | 5.889 | (2.074−11.659) | ||
| pN+ | 4.169 | (2.284−7.609) | ||
| Lymph node ratio 0% | 1 (reference) | pgroup = 0.003 | ||
| Lymph node ratio <20% | 2.944 | (1.414−6.129) | 2.118 | (1.290−8.257) |
| Lymph node ratio ≥20% | 7.2 | (3.514−14.752) | 4.221 | (1.930−9.229) |
| Initial pathologic stage (0-I) | 1 (reference) | pgroup = 0.017 | ||
| Advanced pathologic stage (II-III) | 5.369 | (2.416−11.930) | 2.981 | (1.076−8.257) |
| Metastatic pathologic stage (IV) | 12.108 | (4.245−34.533) | 6.135 | (1.775−21.203) |
HR: hazard ratio; 95%CI: 95% confidence interval; CTx: chemotherapy; pN pathologic lymph node involvement; pT: pathologic tumour invasion.
Table 3 shows the types of LND/, with the number of isolated lymph nodes (LN) that were positive according to the LND performed. The difference in 5-year OS in pathological stages II and III according to the type of LND performed was clinically relevant (17% in LND < D1+ vs 43.3% in LND ≥ D1+), even without having reached statistical significance (P = 0.4).
DiscussionThere is a strikingly limited amount of information about healthcare quality rates in GC surgery in the Western world, especially nationally, including data as basic and important as morbidity and mortality or SV. Although the incidence of GC in Spain is relatively low (6.8 cases/100 000 inhabitants/year),9 approximately 40% of patients present with distant metastases,10–12 SV is poor (5-year OS is 25.1% in Europe for all diagnosed GC, according to the EUROCARE-5 study)2 and treatment is complex and multidisciplinary. Even with these factors that require special attention, there is a great lack of information on the results of surgical treatment of these patients in our country. Furthermore, if our results are assumed to be the same as the best published results (which are generally from eastern Asian series), this could lead to erroneous ideas about our patients’ prognoses.
Despite advances made in diagnostic accuracy, postoperative care, and complementary perioperative treatment in recent decades, the prognosis of GC remains grim, ranking third amongst the leading causes of cancer mortality worldwide.9 According to a multi-institutional study published in 2015 that included 807 patients operated on between 2000 and 2012,13 the 5-year OS rate after gastric resection in the United States is 30%, with 4.7% metastatic GC. In Japan, however, the 1991 registry reported a 5-year OS rate for all operated patients (7935 patients, 113 hospitals) of 68.2%, which also included 15.1% metastatic patients.14 This was fundamentally due to the clearly higher rate of early GC diagnoses (48.8%) made by screening programs, although the rate was also higher when compared by tumour stage. This 5-year OS rate remained unchanged in a more recent study that included 118 367 patients treated surgically between 2001 and 2007.15 In Europe, a study based on the Belgian national registry published in 2015 to identify quality indicators in esophagogastric surgery (n = 4847 patients) operated on between 2004 and 2008,16 the 5-year OS rate observed was 19.4% at the beginning of the study and 27.4% at its conclusion.
The 5-year OS of our series (53.8%) was much higher than that published in the United States (30%), despite including more patients in stage IV (8.2% vs 4.7%), and higher than the Belgian registry (27.4% at the end of the study). However, it was lower than the OS recorded by the Japanese registry (68.2%), which included 15.1% of patients operated on with stage IV. In a European multicentre registry published in 201317 that included patients operated on from the national registries of the Netherlands, Sweden, Denmark, and England (stage IV 7.6%–17.1%), the 2-year OS rates were 51.9%, 51.7%, 53.7% and 56.3%, respectively, while the 2-year OS of our series was close to 70% (stage IV 8.2%). These global comparisons are merely illustrative due to the biases that arise in the cross comparisons between studies, especially when the distribution by stages is not exactly the same in all the series, and the rates of curative/palliative surgery are not specified in most.
Nevertheless, the comparison of SV by stages may provide better clarification. In the 2015 multi-institutional study from the US, 13 5-year OS was 62% for stage I (27.9% of patients), 43% for stage II (24.9%), 21% for stage III (42.5%) and 4% for stage IV (4.7%). In the stage analysis of the Belgian registry, 16 the 5-year OS rates for men/women were 57.9%/58.3% for stage I, 40.7%/36.5% for stage II, 17.6%/17.1% for stage III and 3.7%/2.8% for stage IV. In the results published by the JGCA,15 5-year OS was 91.5% for stage IA (43.7% patients), 83.3% for stage IB (15.5%), 68.9% for stage II (13.1%), 49.6% for stage IIIA (8.6%), 32.3% for stage IIIB (3.4%) and 17% for stage IV (14.2%).
In our series, the 5-year OS was 100% for stage 0 (8.2% of patients), 88.1% for stage I (20.2%), 62.2% for stage II (26.1%), 21.1% for stage III (37.3%) and 17% for stage IV (8.2%). These rates were higher than those published by the national registries of the United States13 and Belgium.16 They are also similar to the figures registered by Japan15 for stages I, II and IV, although the stage III rate was much lower.
The Spanish EURECCA group for esophagogastric cancer7 published a mean OS of 39.5 months in 2019, without including patients in stage IV and without specifying 5-year OS rates or by stage. The study based on the Valencian Community registry for GC6 also did not include OS results with which we could compare. Although the need to know and audit one’s own results in complex oncological surgery might seem indisputable, the reality in our setting is very different due to the patient overload, the lack of a tradition of measuring results and, above all, the absence of the essential administrative infrastructure for data collection (which therefore depends on personal initiative and overworking). The information that is transmitted to patients about these surgical procedures is usually based on data from the literature and not one’s own, so it can be completely biased and, in addition, induce a false feeling of security (in both doctors and patients) by assuming that our results are equal to the best published results. The registration and analysis of one’s own activity is essential to know the local results and promote improvement actions in order to optimise onco-surgical treatment.
The independent poor prognostic factors for OS that we have obtained (absence of perioperative CT in stages II and III, severe complications, and lymph node ratio) are similar to those of other series. Table 2 only shows the factors that were statistically significant, at least in the univariate analysis. Other variables traditionally related to 5-year OS have not been significant in our series and are therefore not shown in said table. These include the proximal location, Lauren diffuse type, degree of differentiation, type of gastrectomy, extension of the LND or the isolation of less than 15 L N, although the number of isolated LN and positive LN increased depending on the type of LND performed (Table 3), and the lymph node ratio was an independent prognostic factor for OS (Table 2).
The favourable SV results in our series are probably due to a combination of measures, which include taking all treatment decisions in a joint manner in a Multidisciplinary Tumour Committee, optimal staging based on laparoscopy in a high percentage of cases, oncology-surgery coordination (making it possible for most patients to receive perioperative CTx when indicated), and the high number of extensive LND.
We therefore conclude that our OS rates and those obtained by stage are among the best published in the Western literature, although very wide ranges and great variability between countries have been described. The results are close to those obtained in the Japanese registry in stages I, II, and IV, but are markedly lower in stage III, which is probably why there is a difference in gross OS. They have not been able to be compared with national results due to the lack of information for oncological results in GC surgery in our setting. The analysis of the results obtained in complex oncological surgery allows us to analyse the opportunities for improvement in order to optimise diagnostic and therapeutic decisions in these patients.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Rihuete-Caro C, Pereira-Pérez F, Manzanedo-Romero I, Carrión-Álvarez L. Auditoría interna de resultados oncológicos en la cirugía del cáncer gástrico. Cir Esp. 2022;100:133–139.