Soft tissue sarcomas (STS) are a group of rare and heterogeneous neoplasms (representing less than 1% of cancer in adults and 15% in pediatric patients), for which there is no updated records in the Latin American population. This study aims to describe the current situation of patients treated at a cancer institute in Latin America.
MethodsWe obtained records from 250 patients with a diagnosis of STS, treated at the National Institute of Neoplastic Diseases of Peru (INEN) during the period 2009–2013, with a mean follow-up of 62 months. The following data were recorded: epidemiological, clinical, treatment and follow-up. The analysis of global survival was done with the Cox proportional hazards model.
ResultsSTS showed a greater frequency in males (60.8%), with a peak incidence after 50 years of age (69.6%). Tumor location was predominantly in the lower extremities (64.4%), and the most frequent histologic subtypes were: undifferentiated pleomorphic sarcoma (34%) and liposarcomas (25.6%); clinical stage III was the most frequent (30.8%). The 5-year overall survival rate was 63.9%, while the statistical analysis found a significant association between global survival and the variables: age (>50 years), tumor size (>5cm), depth (subfascial), histologic grade (G3), local and distant recurrence, showing shorter survival times in these groups.
ConclusionsThis study has clarified the epidemiology, treatment and prognosis, as well as the variables that have an impact on the survival of the Latin American patients with STS studied.
Los sarcomas de partes blandas (SFTS) conforman un grupo de neoplasias poco frecuentes y heterogéneas (menos del 1% en adultos y 15% en pediátricos), de los cuales no se cuenta con registros actualizados en población latinoamericana. El estudio tiene como objetivo describir la situación actual de los pacientes tratados en un instituto oncológico de Latinoamérica.
MétodosSe obtuvo un registro de 250 pacientes con diagnóstico de SFTS, tratados en el Instituto de Enfermedades Neoplásicas del Perú (INEN), durante el periodo 2009-2013, con una media de seguimiento de 62 meses, registrándose datos: epidemiológicos, clínicos, tratamiento y seguimiento; realizándose el análisis de sobrevida global mediante el modelo proporcional de Cox.
ResultadosLos SFTS mostraron una mayor frecuencia en género masculino (60,8%), con un pico incidencia a partir de los 50 años (69,6%), la localización tumoral predominante fue en extremidades inferiores (64,4%), siendo los subtipos histológicos más frecuentes: sarcoma pleomórfico indiferenciado (34%) y liposarcomas (25,6%); el estadio clínico iii es el de mayor registro (30,8%). Se obtuvo una tasa de sobrevida global a 5 años del 63,9%, encontrándose en el análisis estadístico asociación significativa entre la sobrevida global y las variables: edad (>50 años), tamaño tumoral (>5cm), profundidad (subfascial), grado histológico (G3), recurrencia local y a distancia, mostrando en este grupo rangos menores de sobrevida.
ConclusionesSe ha logrado precisar la epidemiología, el tratamiento y el pronóstico, así como las variables que repercuten en la sobrevida de los pacientes latinoamericanos con SFTS estudiados.
Sarcomas are rare heterogeneous mesenchymal neoplasms (representing less than 1% of neoplasms in adults and 15% in pediatric patients) that include soft tissue sarcomas (STS), with more than 50 histological subtypes. In the United States, some 10390 new cases are identified annually, with a global incidence of 6 per 100000 inhabitants.1 In our country, the reporting of cases is deficient and the rate is 2.4 cases per 100000 inhabitants,2 most of whom are treated at the National Institute of Neoplastic Diseases of Peru (INEN).
The objective of this study is to determine the current situation of the disease, focusing on the following variables: epidemiological, clinical, treatment-related and prognostic. Likewise, it is our intention to identify variables that have an impact on the survival of patients with STS treated at the INEN.
MethodsThis descriptive and retrospective study is based on a systematic review of the INEN patient registry; 250 patients were identified with a histological diagnosis of STS from 2009 to 2013, and the mean follow-up was 62 months. We excluded patients treated at other institutions, patients who had neoadjuvant or adjuvant therapy elsewhere, patients who did not have surgical treatment at the institution, those outside the range of follow-up appointments (>6 months), and patient with desmoid tumors and dermatofibrosarcoma protuberans.
Epidemiological data (gender and age of presentation) were collected, as well as clinical characteristics: tumor size, depth of invasion, location and TNM stage (AJCC Cancer Staging Manual, 7th Edition).3
The patients were evaluated by a multidisciplinary committee that included surgical oncologists specialized in soft tissue tumor surgery, orthopedic oncologists, plastic and reconstructive surgeons, radiologists, oncologists and radiotherapists. Following national and international reference guidelines,4,5 the committee defined patient treatment, which included: surgical (type of resection, surgical reconstruction), neoadjuvant and adjuvant treatment (type and dose), based on the biological characteristics of the tumor, staging, previous treatments and comorbidities.
The operative equipment was available in the operating room to evaluate surgical margins both macroscopically and microscopically. These were reported as: wide, when surgical margins free of malignancy were macroscopically distant (equal to or greater than 1cm); marginal, when surgical margins free of malignancy were microscopically close; and compromised, when the surgical margins were in contact with malignant neoplasm. Surgical specimens were evaluated with hematoxylin–eosin staining and immunohistochemical panels.
The postoperative follow-up consisted of periodic clinical evaluations every 3 months (the first 2 years), every 6 months (until the 5th year) and subsequent annual evaluations. Complementary examinations during the follow-up were aimed at evaluating locoregional recurrence (soft tissue ultrasound and magnetic resonance imaging [MRI] with contrast agents) and distant recurrence (tomographic studies of thorax, abdomen and pelvis with contrast medium).
Statistical AnalysisA descriptive analysis of the data was conducted using frequencies, percentages and summary measures (mean, median and range). Overall survival (OS) curves according to the characteristics being studied were estimated with the Kaplan–Meier method, and the differences between them were evaluated with the log rank test; the Cox proportional hazards model was used to determine prognostic characteristics for OS. A P value <.05 was considered statistically significant to determine the characteristics that were important to explain the time from diagnosis to death; it was considered that the confidence interval of the hazard ratio (HR) of the Cox model should not contain the value 1. R software was used for data analysis (R Core Team [2017], R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria).
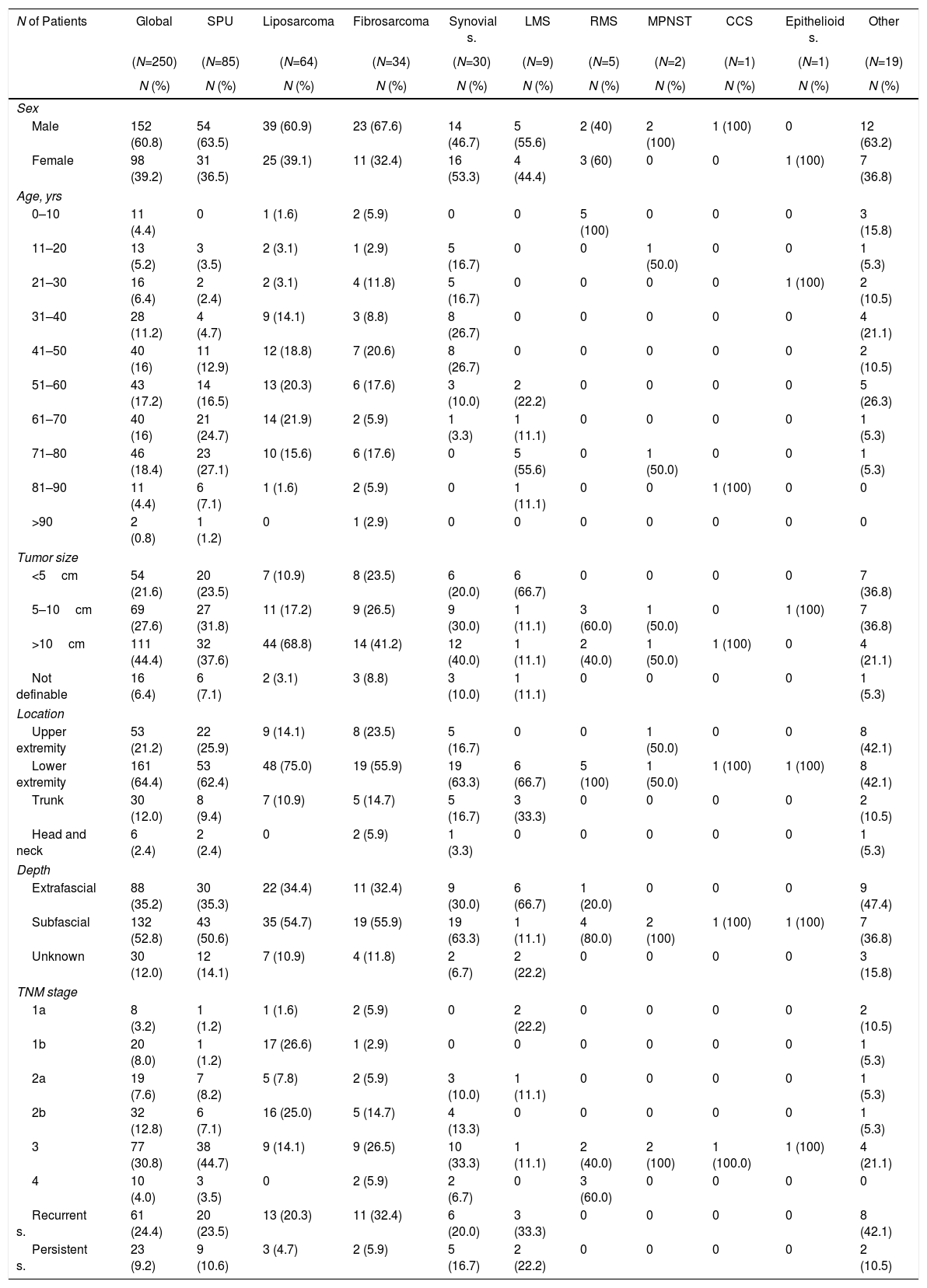
ResultsWe identified 625 INEN patients who had an anatomic pathology diagnosis of STS from 2009 to 2013, 250 of whom met the inclusion criteria. Table 1 shows the clinical and epidemiological characteristics; there was a higher incidence of males (60.8%) and a higher proportion of patients older than 50 years (69.6%), with a peak incidence at 70 years and a mean age of 52.6 years. The histological subtypes with the most registries were: undifferentiated pleomorphic sarcoma (UPS) (34%) and liposarcoma (25.6%), with a greater proportion of patients older than 50, while rhabdomyosarcoma (RMS) and synovial sarcomas had the highest incidence in pediatric ages and young adults (<50 years). 72% of cases presented tumors larger than 5cm, and the lower extremities were most frequently affected (64.4%), predominantly by the subtypes UPS, liposarcoma, fibrosarcoma and synovial sarcoma in this location. MRI revealed a subfascial compromise rate of 52.8%, and clinical stage 3 was the most frequent (30.8%).
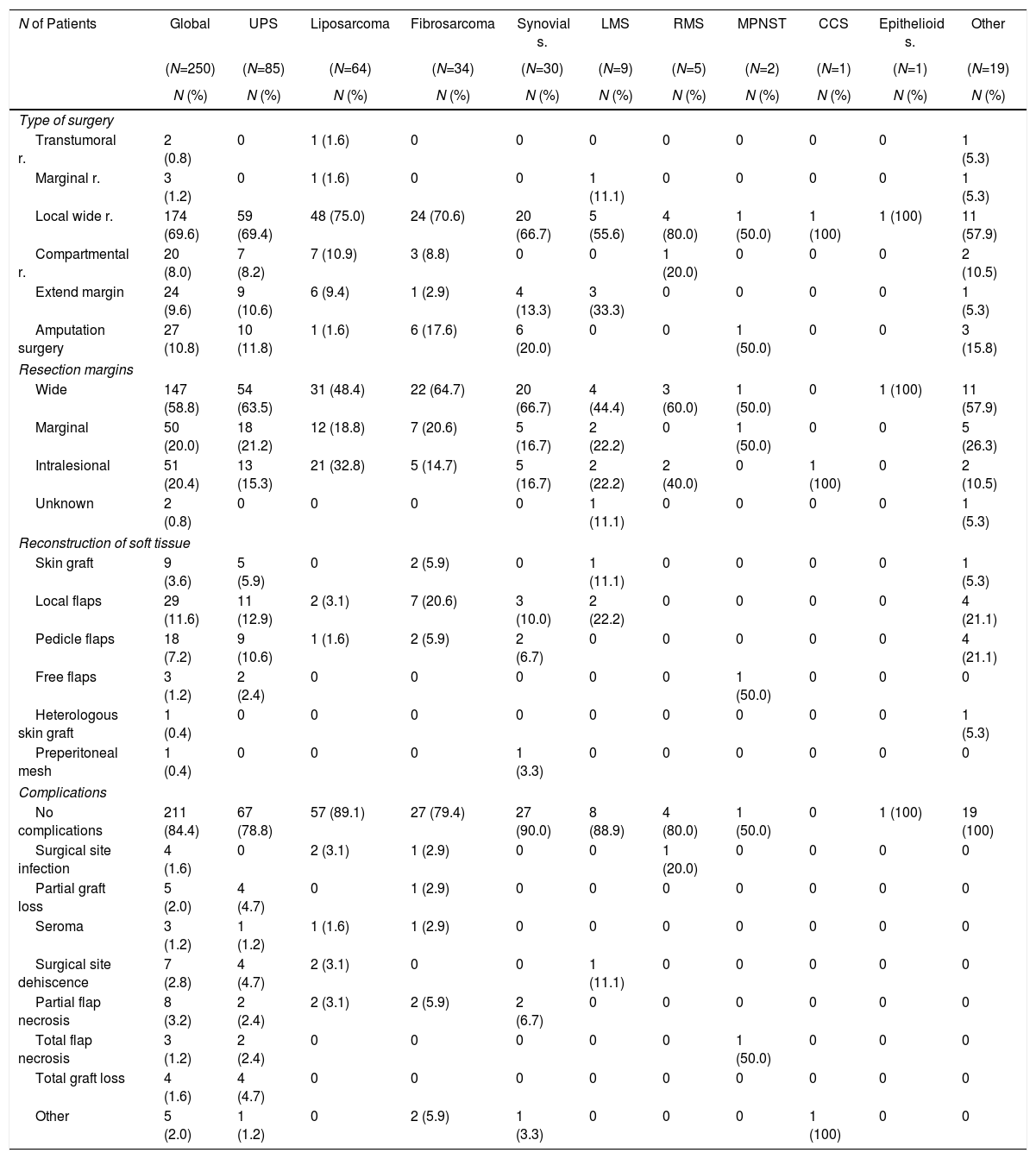
Clinical and Epidemiological Characteristics According to Histologic Subtype.
| N of Patients | Global | SPU | Liposarcoma | Fibrosarcoma | Synovial s. | LMS | RMS | MPNST | CCS | Epithelioid s. | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N=250) | (N=85) | (N=64) | (N=34) | (N=30) | (N=9) | (N=5) | (N=2) | (N=1) | (N=1) | (N=19) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Sex | |||||||||||
| Male | 152 (60.8) | 54 (63.5) | 39 (60.9) | 23 (67.6) | 14 (46.7) | 5 (55.6) | 2 (40) | 2 (100) | 1 (100) | 0 | 12 (63.2) |
| Female | 98 (39.2) | 31 (36.5) | 25 (39.1) | 11 (32.4) | 16 (53.3) | 4 (44.4) | 3 (60) | 0 | 0 | 1 (100) | 7 (36.8) |
| Age, yrs | |||||||||||
| 0–10 | 11 (4.4) | 0 | 1 (1.6) | 2 (5.9) | 0 | 0 | 5 (100) | 0 | 0 | 0 | 3 (15.8) |
| 11–20 | 13 (5.2) | 3 (3.5) | 2 (3.1) | 1 (2.9) | 5 (16.7) | 0 | 0 | 1 (50.0) | 0 | 0 | 1 (5.3) |
| 21–30 | 16 (6.4) | 2 (2.4) | 2 (3.1) | 4 (11.8) | 5 (16.7) | 0 | 0 | 0 | 0 | 1 (100) | 2 (10.5) |
| 31–40 | 28 (11.2) | 4 (4.7) | 9 (14.1) | 3 (8.8) | 8 (26.7) | 0 | 0 | 0 | 0 | 0 | 4 (21.1) |
| 41–50 | 40 (16) | 11 (12.9) | 12 (18.8) | 7 (20.6) | 8 (26.7) | 0 | 0 | 0 | 0 | 0 | 2 (10.5) |
| 51–60 | 43 (17.2) | 14 (16.5) | 13 (20.3) | 6 (17.6) | 3 (10.0) | 2 (22.2) | 0 | 0 | 0 | 0 | 5 (26.3) |
| 61–70 | 40 (16) | 21 (24.7) | 14 (21.9) | 2 (5.9) | 1 (3.3) | 1 (11.1) | 0 | 0 | 0 | 0 | 1 (5.3) |
| 71–80 | 46 (18.4) | 23 (27.1) | 10 (15.6) | 6 (17.6) | 0 | 5 (55.6) | 0 | 1 (50.0) | 0 | 0 | 1 (5.3) |
| 81–90 | 11 (4.4) | 6 (7.1) | 1 (1.6) | 2 (5.9) | 0 | 1 (11.1) | 0 | 0 | 1 (100) | 0 | 0 |
| >90 | 2 (0.8) | 1 (1.2) | 0 | 1 (2.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tumor size | |||||||||||
| <5cm | 54 (21.6) | 20 (23.5) | 7 (10.9) | 8 (23.5) | 6 (20.0) | 6 (66.7) | 0 | 0 | 0 | 0 | 7 (36.8) |
| 5–10cm | 69 (27.6) | 27 (31.8) | 11 (17.2) | 9 (26.5) | 9 (30.0) | 1 (11.1) | 3 (60.0) | 1 (50.0) | 0 | 1 (100) | 7 (36.8) |
| >10cm | 111 (44.4) | 32 (37.6) | 44 (68.8) | 14 (41.2) | 12 (40.0) | 1 (11.1) | 2 (40.0) | 1 (50.0) | 1 (100) | 0 | 4 (21.1) |
| Not definable | 16 (6.4) | 6 (7.1) | 2 (3.1) | 3 (8.8) | 3 (10.0) | 1 (11.1) | 0 | 0 | 0 | 0 | 1 (5.3) |
| Location | |||||||||||
| Upper extremity | 53 (21.2) | 22 (25.9) | 9 (14.1) | 8 (23.5) | 5 (16.7) | 0 | 0 | 1 (50.0) | 0 | 0 | 8 (42.1) |
| Lower extremity | 161 (64.4) | 53 (62.4) | 48 (75.0) | 19 (55.9) | 19 (63.3) | 6 (66.7) | 5 (100) | 1 (50.0) | 1 (100) | 1 (100) | 8 (42.1) |
| Trunk | 30 (12.0) | 8 (9.4) | 7 (10.9) | 5 (14.7) | 5 (16.7) | 3 (33.3) | 0 | 0 | 0 | 0 | 2 (10.5) |
| Head and neck | 6 (2.4) | 2 (2.4) | 0 | 2 (5.9) | 1 (3.3) | 0 | 0 | 0 | 0 | 0 | 1 (5.3) |
| Depth | |||||||||||
| Extrafascial | 88 (35.2) | 30 (35.3) | 22 (34.4) | 11 (32.4) | 9 (30.0) | 6 (66.7) | 1 (20.0) | 0 | 0 | 0 | 9 (47.4) |
| Subfascial | 132 (52.8) | 43 (50.6) | 35 (54.7) | 19 (55.9) | 19 (63.3) | 1 (11.1) | 4 (80.0) | 2 (100) | 1 (100) | 1 (100) | 7 (36.8) |
| Unknown | 30 (12.0) | 12 (14.1) | 7 (10.9) | 4 (11.8) | 2 (6.7) | 2 (22.2) | 0 | 0 | 0 | 0 | 3 (15.8) |
| TNM stage | |||||||||||
| 1a | 8 (3.2) | 1 (1.2) | 1 (1.6) | 2 (5.9) | 0 | 2 (22.2) | 0 | 0 | 0 | 0 | 2 (10.5) |
| 1b | 20 (8.0) | 1 (1.2) | 17 (26.6) | 1 (2.9) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.3) |
| 2a | 19 (7.6) | 7 (8.2) | 5 (7.8) | 2 (5.9) | 3 (10.0) | 1 (11.1) | 0 | 0 | 0 | 0 | 1 (5.3) |
| 2b | 32 (12.8) | 6 (7.1) | 16 (25.0) | 5 (14.7) | 4 (13.3) | 0 | 0 | 0 | 0 | 0 | 1 (5.3) |
| 3 | 77 (30.8) | 38 (44.7) | 9 (14.1) | 9 (26.5) | 10 (33.3) | 1 (11.1) | 2 (40.0) | 2 (100) | 1 (100.0) | 1 (100) | 4 (21.1) |
| 4 | 10 (4.0) | 3 (3.5) | 0 | 2 (5.9) | 2 (6.7) | 0 | 3 (60.0) | 0 | 0 | 0 | 0 |
| Recurrent s. | 61 (24.4) | 20 (23.5) | 13 (20.3) | 11 (32.4) | 6 (20.0) | 3 (33.3) | 0 | 0 | 0 | 0 | 8 (42.1) |
| Persistent s. | 23 (9.2) | 9 (10.6) | 3 (4.7) | 2 (5.9) | 5 (16.7) | 2 (22.2) | 0 | 0 | 0 | 0 | 2 (10.5) |
LMS: leiomyosarcoma; MPNST: malignant peripheral nerve sheath tumor; RMS: rhabdomyosarcoma; s.: sarcoma; CCS: clear-cell sarcoma; SPU: undifferentiated pleomorphic sarcoma; T: tumor.
Table 2 summarizes the surgical statistics according to the histological subtype. Limb preservation surgery was the most frequently used treatment option (89.2%), and amputation surgery reached 10.8%. The status of the surgical margins was wide in 58.8%, marginal in 20% and compromised in 20.4%. Surgical soft tissue reconstruction was required in 61 patients (24.4%) with local flaps (11.6%) and pedicle flaps (7.2%), which were mostly used in cases of UPS (31.8%) and fibrosarcoma (32.4%). The percentage of surgical complications was 15.6%, occurring more frequently in patients with surgical reconstructions (partial flap necrosis 3.2% and dehiscence of the surgical site 2.8%).
Surgical Statistics According to Histologic Subtype of STS.
| N of Patients | Global | UPS | Liposarcoma | Fibrosarcoma | Synovial s. | LMS | RMS | MPNST | CCS | Epithelioid s. | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N=250) | (N=85) | (N=64) | (N=34) | (N=30) | (N=9) | (N=5) | (N=2) | (N=1) | (N=1) | (N=19) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Type of surgery | |||||||||||
| Transtumoral r. | 2 (0.8) | 0 | 1 (1.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.3) |
| Marginal r. | 3 (1.2) | 0 | 1 (1.6) | 0 | 0 | 1 (11.1) | 0 | 0 | 0 | 0 | 1 (5.3) |
| Local wide r. | 174 (69.6) | 59 (69.4) | 48 (75.0) | 24 (70.6) | 20 (66.7) | 5 (55.6) | 4 (80.0) | 1 (50.0) | 1 (100) | 1 (100) | 11 (57.9) |
| Compartmental r. | 20 (8.0) | 7 (8.2) | 7 (10.9) | 3 (8.8) | 0 | 0 | 1 (20.0) | 0 | 0 | 0 | 2 (10.5) |
| Extend margin | 24 (9.6) | 9 (10.6) | 6 (9.4) | 1 (2.9) | 4 (13.3) | 3 (33.3) | 0 | 0 | 0 | 0 | 1 (5.3) |
| Amputation surgery | 27 (10.8) | 10 (11.8) | 1 (1.6) | 6 (17.6) | 6 (20.0) | 0 | 0 | 1 (50.0) | 0 | 0 | 3 (15.8) |
| Resection margins | |||||||||||
| Wide | 147 (58.8) | 54 (63.5) | 31 (48.4) | 22 (64.7) | 20 (66.7) | 4 (44.4) | 3 (60.0) | 1 (50.0) | 0 | 1 (100) | 11 (57.9) |
| Marginal | 50 (20.0) | 18 (21.2) | 12 (18.8) | 7 (20.6) | 5 (16.7) | 2 (22.2) | 0 | 1 (50.0) | 0 | 0 | 5 (26.3) |
| Intralesional | 51 (20.4) | 13 (15.3) | 21 (32.8) | 5 (14.7) | 5 (16.7) | 2 (22.2) | 2 (40.0) | 0 | 1 (100) | 0 | 2 (10.5) |
| Unknown | 2 (0.8) | 0 | 0 | 0 | 0 | 1 (11.1) | 0 | 0 | 0 | 0 | 1 (5.3) |
| Reconstruction of soft tissue | |||||||||||
| Skin graft | 9 (3.6) | 5 (5.9) | 0 | 2 (5.9) | 0 | 1 (11.1) | 0 | 0 | 0 | 0 | 1 (5.3) |
| Local flaps | 29 (11.6) | 11 (12.9) | 2 (3.1) | 7 (20.6) | 3 (10.0) | 2 (22.2) | 0 | 0 | 0 | 0 | 4 (21.1) |
| Pedicle flaps | 18 (7.2) | 9 (10.6) | 1 (1.6) | 2 (5.9) | 2 (6.7) | 0 | 0 | 0 | 0 | 0 | 4 (21.1) |
| Free flaps | 3 (1.2) | 2 (2.4) | 0 | 0 | 0 | 0 | 0 | 1 (50.0) | 0 | 0 | 0 |
| Heterologous skin graft | 1 (0.4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.3) |
| Preperitoneal mesh | 1 (0.4) | 0 | 0 | 0 | 1 (3.3) | 0 | 0 | 0 | 0 | 0 | 0 |
| Complications | |||||||||||
| No complications | 211 (84.4) | 67 (78.8) | 57 (89.1) | 27 (79.4) | 27 (90.0) | 8 (88.9) | 4 (80.0) | 1 (50.0) | 0 | 1 (100) | 19 (100) |
| Surgical site infection | 4 (1.6) | 0 | 2 (3.1) | 1 (2.9) | 0 | 0 | 1 (20.0) | 0 | 0 | 0 | 0 |
| Partial graft loss | 5 (2.0) | 4 (4.7) | 0 | 1 (2.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Seroma | 3 (1.2) | 1 (1.2) | 1 (1.6) | 1 (2.9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surgical site dehiscence | 7 (2.8) | 4 (4.7) | 2 (3.1) | 0 | 0 | 1 (11.1) | 0 | 0 | 0 | 0 | 0 |
| Partial flap necrosis | 8 (3.2) | 2 (2.4) | 2 (3.1) | 2 (5.9) | 2 (6.7) | 0 | 0 | 0 | 0 | 0 | 0 |
| Total flap necrosis | 3 (1.2) | 2 (2.4) | 0 | 0 | 0 | 0 | 0 | 1 (50.0) | 0 | 0 | 0 |
| Total graft loss | 4 (1.6) | 4 (4.7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 5 (2.0) | 1 (1.2) | 0 | 2 (5.9) | 1 (3.3) | 0 | 0 | 0 | 1 (100) | 0 | 0 |
LMS: leiomyosarcoma; MPNST: malignant peripheral nerve sheath tumor; RMS: rhabdomyosarcoma; s.: sarcoma; CCS: clear-cell sarcomas; STS: soft-tissue sarcoma; UPS: undifferentiated pleomorphic sarcoma.
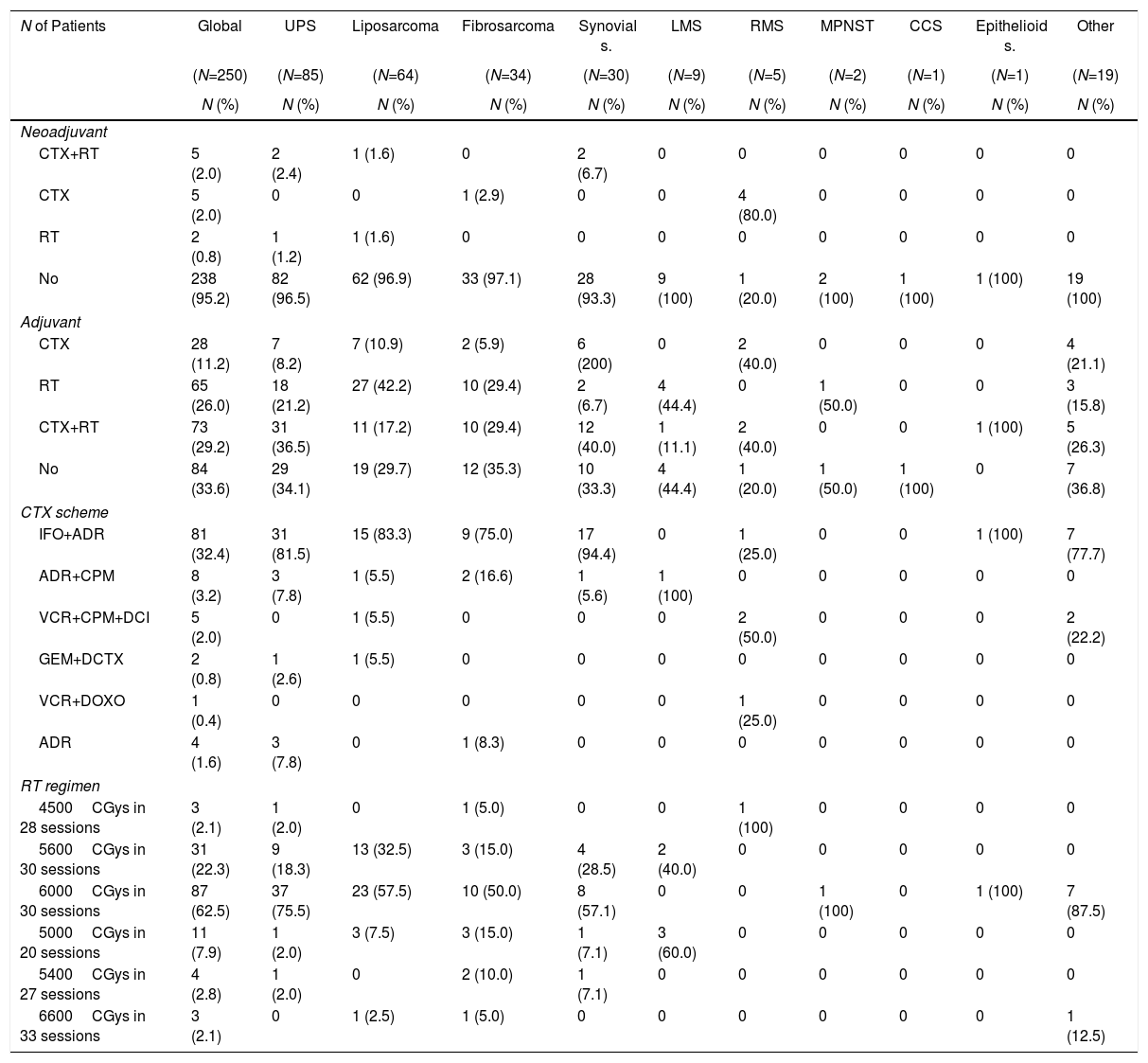
Table 3 summarizes the neoadjuvant and adjuvant treatment given.
Statistical Data About the Neoadjuvant and Adjuvant Treatment, According to Histologic Subtype of STS.
| N of Patients | Global | UPS | Liposarcoma | Fibrosarcoma | Synovial s. | LMS | RMS | MPNST | CCS | Epithelioid s. | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N=250) | (N=85) | (N=64) | (N=34) | (N=30) | (N=9) | (N=5) | (N=2) | (N=1) | (N=1) | (N=19) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Neoadjuvant | |||||||||||
| CTX+RT | 5 (2.0) | 2 (2.4) | 1 (1.6) | 0 | 2 (6.7) | 0 | 0 | 0 | 0 | 0 | 0 |
| CTX | 5 (2.0) | 0 | 0 | 1 (2.9) | 0 | 0 | 4 (80.0) | 0 | 0 | 0 | 0 |
| RT | 2 (0.8) | 1 (1.2) | 1 (1.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No | 238 (95.2) | 82 (96.5) | 62 (96.9) | 33 (97.1) | 28 (93.3) | 9 (100) | 1 (20.0) | 2 (100) | 1 (100) | 1 (100) | 19 (100) |
| Adjuvant | |||||||||||
| CTX | 28 (11.2) | 7 (8.2) | 7 (10.9) | 2 (5.9) | 6 (200) | 0 | 2 (40.0) | 0 | 0 | 0 | 4 (21.1) |
| RT | 65 (26.0) | 18 (21.2) | 27 (42.2) | 10 (29.4) | 2 (6.7) | 4 (44.4) | 0 | 1 (50.0) | 0 | 0 | 3 (15.8) |
| CTX+RT | 73 (29.2) | 31 (36.5) | 11 (17.2) | 10 (29.4) | 12 (40.0) | 1 (11.1) | 2 (40.0) | 0 | 0 | 1 (100) | 5 (26.3) |
| No | 84 (33.6) | 29 (34.1) | 19 (29.7) | 12 (35.3) | 10 (33.3) | 4 (44.4) | 1 (20.0) | 1 (50.0) | 1 (100) | 0 | 7 (36.8) |
| CTX scheme | |||||||||||
| IFO+ADR | 81 (32.4) | 31 (81.5) | 15 (83.3) | 9 (75.0) | 17 (94.4) | 0 | 1 (25.0) | 0 | 0 | 1 (100) | 7 (77.7) |
| ADR+CPM | 8 (3.2) | 3 (7.8) | 1 (5.5) | 2 (16.6) | 1 (5.6) | 1 (100) | 0 | 0 | 0 | 0 | 0 |
| VCR+CPM+DCI | 5 (2.0) | 0 | 1 (5.5) | 0 | 0 | 0 | 2 (50.0) | 0 | 0 | 0 | 2 (22.2) |
| GEM+DCTX | 2 (0.8) | 1 (2.6) | 1 (5.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VCR+DOXO | 1 (0.4) | 0 | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 0 | 0 | 0 |
| ADR | 4 (1.6) | 3 (7.8) | 0 | 1 (8.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| RT regimen | |||||||||||
| 4500CGys in 28 sessions | 3 (2.1) | 1 (2.0) | 0 | 1 (5.0) | 0 | 0 | 1 (100) | 0 | 0 | 0 | 0 |
| 5600CGys in 30 sessions | 31 (22.3) | 9 (18.3) | 13 (32.5) | 3 (15.0) | 4 (28.5) | 2 (40.0) | 0 | 0 | 0 | 0 | 0 |
| 6000CGys in 30 sessions | 87 (62.5) | 37 (75.5) | 23 (57.5) | 10 (50.0) | 8 (57.1) | 0 | 0 | 1 (100) | 0 | 1 (100) | 7 (87.5) |
| 5000CGys in 20 sessions | 11 (7.9) | 1 (2.0) | 3 (7.5) | 3 (15.0) | 1 (7.1) | 3 (60.0) | 0 | 0 | 0 | 0 | 0 |
| 5400CGys in 27 sessions | 4 (2.8) | 1 (2.0) | 0 | 2 (10.0) | 1 (7.1) | 0 | 0 | 0 | 0 | 0 | 0 |
| 6600CGys in 33 sessions | 3 (2.1) | 0 | 1 (2.5) | 1 (5.0) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (12.5) |
ADR: adriamycin; CPM: cyclophosphamide; DCI: dactinomycin; DCTX: docetaxel; DOXO: doxorubicin; GEM: gemcitabine; IFO: ifosfamide; LMS: leiomyosarcoma; MPNST: malignant peripheral nerve sheath tumor; CTx: chemotherapy; RMS: rhabdomyosarcoma; RT: radiotherapy; s.: sarcoma; CCS: clear-cell sarcomas; STS: soft-tissue sarcoma; UPS: undifferentiated pleomorphic sarcoma; VCR: vincristine.
The neoadjuvant treatment was indicated in the multidisciplinary evaluation at admission for 4.8% (RMS 80% and synovial sarcoma 6.7%). Adjuvant therapy was administered in 66.4%, and the combined use of chemotherapy and radiotherapy was most frequent (29.2%). The most frequently used chemotherapy scheme was ifosfamide associated with adriamycin. The most commonly used external radiotherapy dose was 6000CGys in 30 sessions.
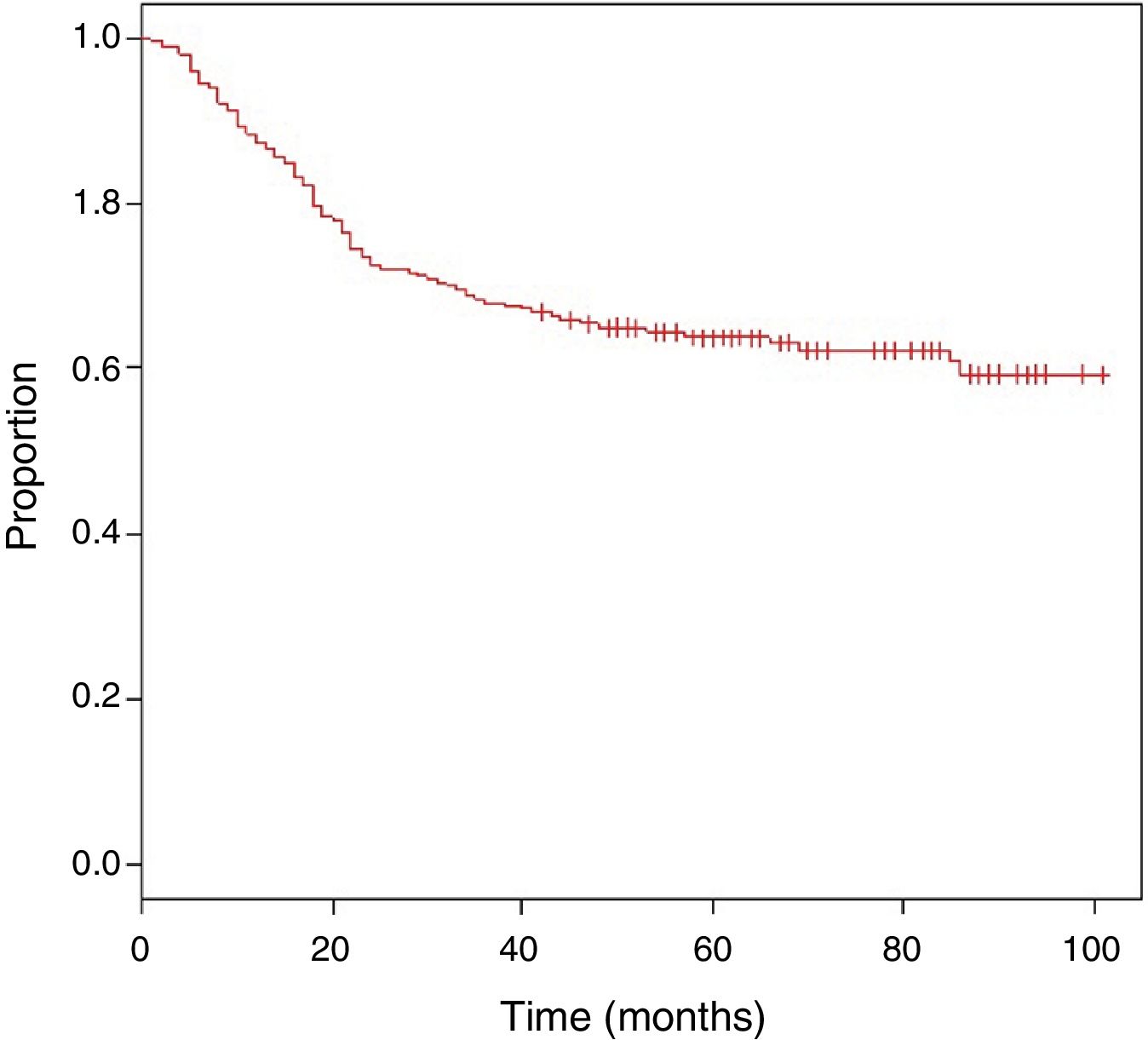
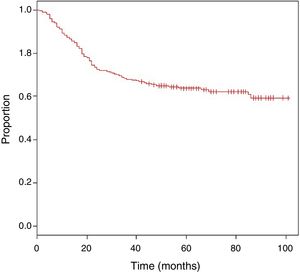
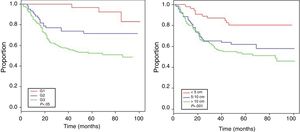
The mean follow-up was 62 months, with an estimated OS of 63.9% (Fig. 1).
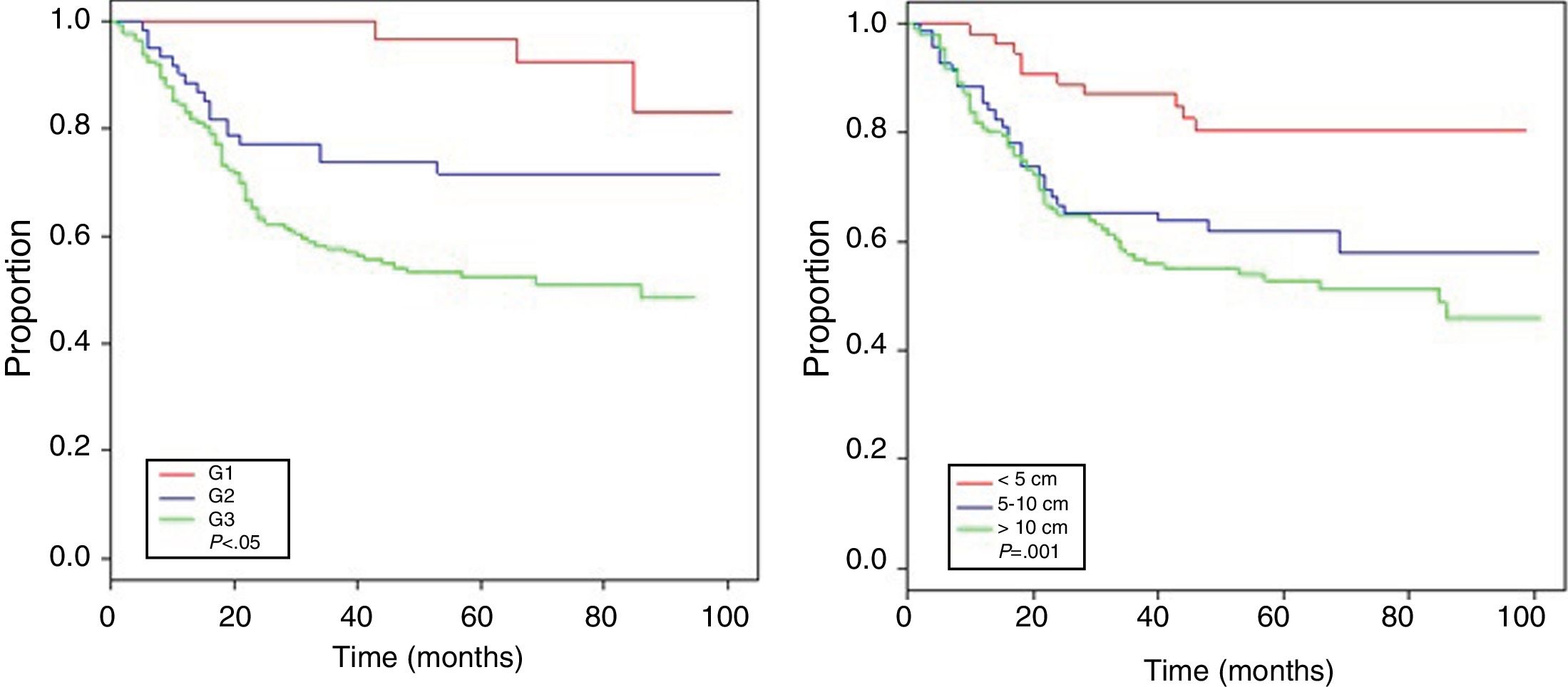
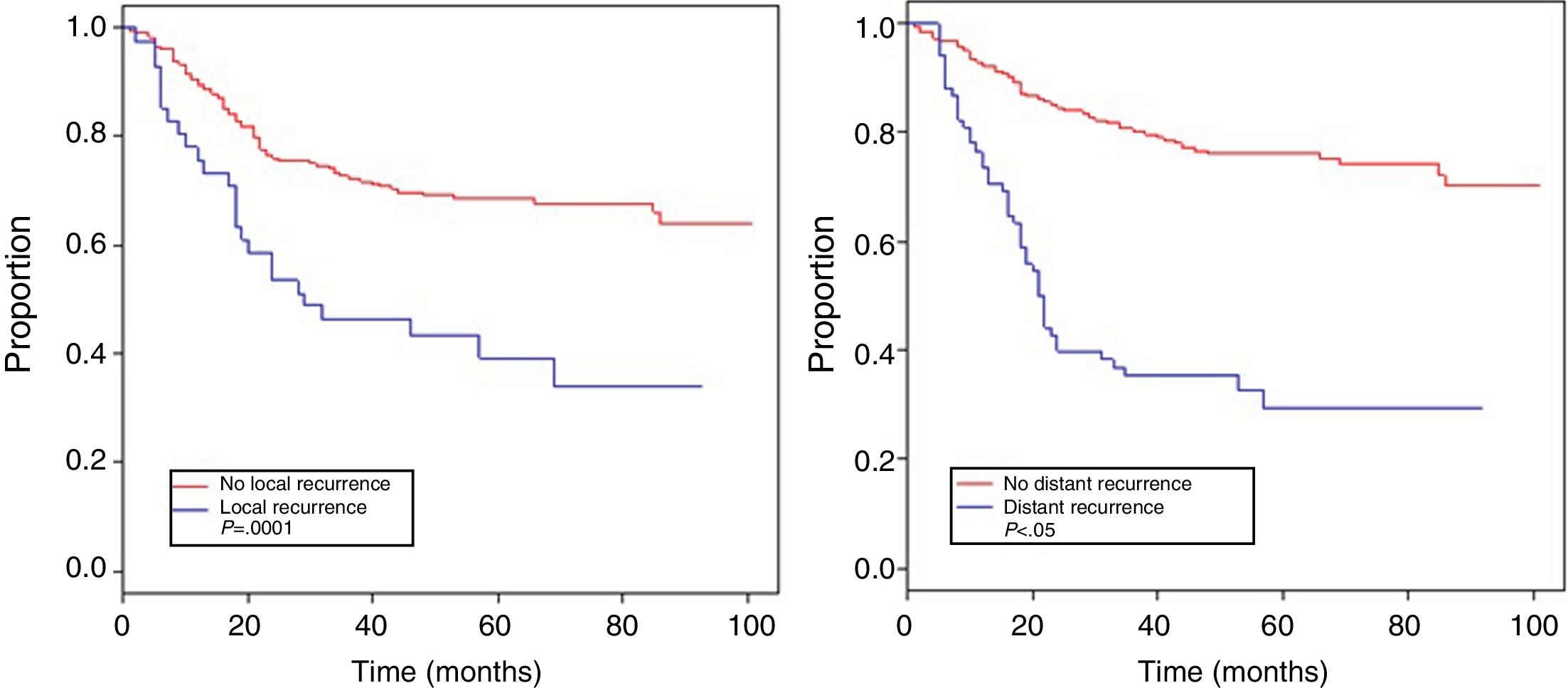
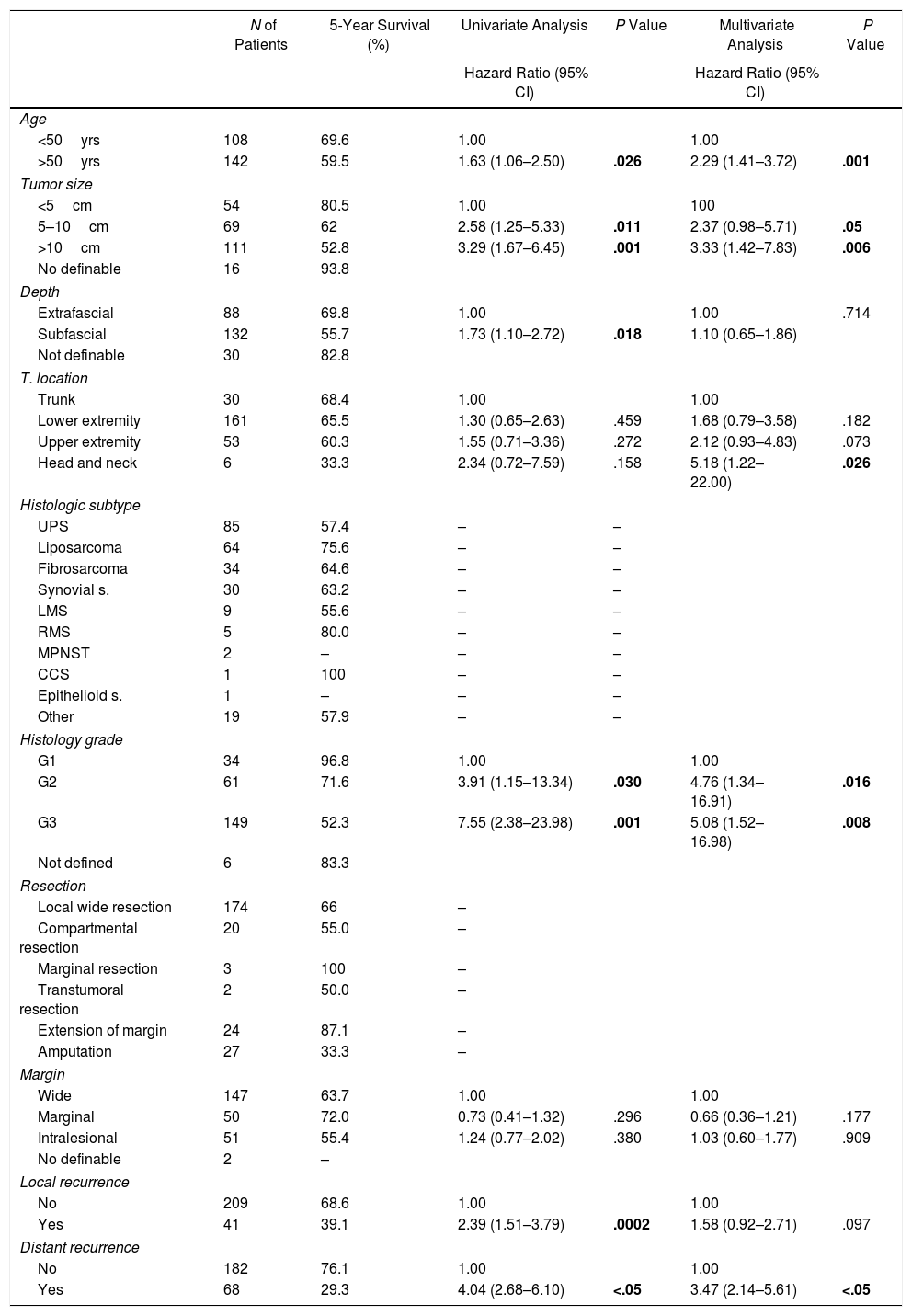
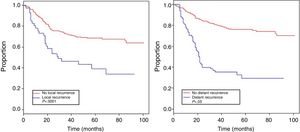
Table 4 shows the Cox proportional hazards analysis and the adjusted HR ranges for OS, based on 250 cases and correlated with the variables: age, tumor size, depth of tumor invasion, location, histological subtype, histological grade, type of surgical resection, surgical margins, local and distant recurrence. In the analysis, patients aged >50 years had shorter survival (59.5%; HR: 1.63; 95% confidence interval [CI] 1.06–2.50; P=.026), tumors in ranges >5–10cm and >10cm presented worse prognosis for OS compared to those with a smaller diameter (62%; HR: 2.58; 95% CI; 1.25–5.33; P=.011 and 52.8%; HR: 3.29; 95% CI 1.67–6.45; P=.001) (Fig. 2); for compromise of tumor invasion, subfascial tumors presented lower survival (55.7%; HR: 1.73; 95% CI 1.10–2.72; P=.018). In the evaluation of the anatomic presentation site, no statistical difference was observed in terms of OS; however, the multivariate analysis showed a variable to be considered, as the presentation of tumors in the head and neck had 5 times greater risk of death than in other locations (HR: 5.18; 95% CI 1.22–22.00; P=.026). The histological subtypes that showed the lowest OS rate were: malignant peripheral nerve sheath tumor (MPNST) and epithelioid sarcoma; however, due to the limited number of patients in certain histological subtypes, a statistical analysis could not be conducted. Despite this, it was observed that high nuclear grade tumors (G2 and G3) presented a poorer prognosis (71.6%; HR: 3.91; 95% CI 1.15–13.34; P=.030 and 52.3%; HR: 7.55; 95% CI 2.38–23.9; P=.001) (Fig. 2). Amputation surgery presented lower OS compared with limb preservation surgery (33% vs 66% in patients with wide local resection) and the surgical margins analyzed showed an effect in the OS of patients with compromised margins (55.4%; HR: 1.24; 95% CI 0.77–2.02; P=.38). The rates of local and distant recurrence (39.1% and 24%, respectively) reduced OS ranges significantly in these patients (39.1%; HR: 2.39; 95% CI 1.51–3.79; P=.0002 and 29.3%; HR: 4.04; 95% CI 2.68–6.10; P<.05) (Fig. 3).
Overall Survival and Proportional Risk Model (N=250).
| N of Patients | 5-Year Survival (%) | Univariate Analysis | P Value | Multivariate Analysis | P Value | |
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |||||
| Age | ||||||
| <50yrs | 108 | 69.6 | 1.00 | 1.00 | ||
| >50yrs | 142 | 59.5 | 1.63 (1.06–2.50) | .026 | 2.29 (1.41–3.72) | .001 |
| Tumor size | ||||||
| <5cm | 54 | 80.5 | 1.00 | 100 | ||
| 5–10cm | 69 | 62 | 2.58 (1.25–5.33) | .011 | 2.37 (0.98–5.71) | .05 |
| >10cm | 111 | 52.8 | 3.29 (1.67–6.45) | .001 | 3.33 (1.42–7.83) | .006 |
| No definable | 16 | 93.8 | ||||
| Depth | ||||||
| Extrafascial | 88 | 69.8 | 1.00 | 1.00 | .714 | |
| Subfascial | 132 | 55.7 | 1.73 (1.10–2.72) | .018 | 1.10 (0.65–1.86) | |
| Not definable | 30 | 82.8 | ||||
| T. location | ||||||
| Trunk | 30 | 68.4 | 1.00 | 1.00 | ||
| Lower extremity | 161 | 65.5 | 1.30 (0.65–2.63) | .459 | 1.68 (0.79–3.58) | .182 |
| Upper extremity | 53 | 60.3 | 1.55 (0.71–3.36) | .272 | 2.12 (0.93–4.83) | .073 |
| Head and neck | 6 | 33.3 | 2.34 (0.72–7.59) | .158 | 5.18 (1.22–22.00) | .026 |
| Histologic subtype | ||||||
| UPS | 85 | 57.4 | – | – | ||
| Liposarcoma | 64 | 75.6 | – | – | ||
| Fibrosarcoma | 34 | 64.6 | – | – | ||
| Synovial s. | 30 | 63.2 | – | – | ||
| LMS | 9 | 55.6 | – | – | ||
| RMS | 5 | 80.0 | – | – | ||
| MPNST | 2 | – | – | – | ||
| CCS | 1 | 100 | – | – | ||
| Epithelioid s. | 1 | – | – | – | ||
| Other | 19 | 57.9 | – | – | ||
| Histology grade | ||||||
| G1 | 34 | 96.8 | 1.00 | 1.00 | ||
| G2 | 61 | 71.6 | 3.91 (1.15–13.34) | .030 | 4.76 (1.34–16.91) | .016 |
| G3 | 149 | 52.3 | 7.55 (2.38–23.98) | .001 | 5.08 (1.52–16.98) | .008 |
| Not defined | 6 | 83.3 | ||||
| Resection | ||||||
| Local wide resection | 174 | 66 | – | |||
| Compartmental resection | 20 | 55.0 | – | |||
| Marginal resection | 3 | 100 | – | |||
| Transtumoral resection | 2 | 50.0 | – | |||
| Extension of margin | 24 | 87.1 | – | |||
| Amputation | 27 | 33.3 | – | |||
| Margin | ||||||
| Wide | 147 | 63.7 | 1.00 | 1.00 | ||
| Marginal | 50 | 72.0 | 0.73 (0.41–1.32) | .296 | 0.66 (0.36–1.21) | .177 |
| Intralesional | 51 | 55.4 | 1.24 (0.77–2.02) | .380 | 1.03 (0.60–1.77) | .909 |
| No definable | 2 | – | ||||
| Local recurrence | ||||||
| No | 209 | 68.6 | 1.00 | 1.00 | ||
| Yes | 41 | 39.1 | 2.39 (1.51–3.79) | .0002 | 1.58 (0.92–2.71) | .097 |
| Distant recurrence | ||||||
| No | 182 | 76.1 | 1.00 | 1.00 | ||
| Yes | 68 | 29.3 | 4.04 (2.68–6.10) | <.05 | 3.47 (2.14–5.61) | <.05 |
LMS: leiomyosarcoma; MPNST: malignant peripheral nerve sheath tumor; CTX: chemotherapy; RMS: rhabdomyosarcoma; s.: sarcoma; CCS: clear-cell sarcomas; UPS: undifferentiated pleomorphic sarcoma.
Data with statistical significance are shown in bold in the statistical assessment table.
The study shows the clinical characteristics, treatment and factors that significantly influenced the survival of 250 patients treated at a Latin American oncology institute (INEN) from 2009 to 2013. It also reflects the multidisciplinary treatment of this pathology in accordance with international treatment standards.5
The clinical characteristics analyzed showed a male-to-female ratio at the time of diagnosis of 1.5:1, with a predominance of males. Nationally, patients >60 years represent 9.2% of the population.6 In spite of this, STS show an increase in the incidence rate after the age of 50, with a peak at 70 years, a variable that has an impact on the OS of patients (HR: 1.63; 95% CI, 1.06–2.50), similar to that described by Brennan et al.,7 which could be related to comorbidities and the functional status of this age group. The tumor diameter (T), depth of tumor invasion and the nuclear grade of STS are important oncological factors for AJCC staging.3 In the registry, advanced clinical stages were observed, with a predominance of EC III (30.8%), which correlated with tumors showing diameters greater than or equal to 5cm (72%) that were subfascial (52.8%) and had nuclear grades 2 and 3 (71.6% and 52.3%, respectively); these variables had a significant impact on the survival of these patients in the univariate and multivariate analyses. The most frequent anatomical location was the lower limbs (64.4%), which is similar to descriptions from a series of cases reported nationally.8 This variable was important in the multivariate analysis, showing an increase in the risk of death and in head and neck locations. This is related to the subtypes registered in this anatomical location (UPS, fibrosarcoma), which present characteristics of accelerated growth, infiltration capacity of vascular and nerve structures, as well as the surgical difficulty to obtain wide margins and aggressive resections due to the critical anatomy of the head and neck area.9
Surgical procedures remain the cornerstone of treatment in STS. However, they have been modified over time in order to preserve functionality. The oncological safety of limb-sparing surgery has been described in multiple publications10–12; this was used in most surgical interventions (wide local resection 69.6%), following the approaches recommended according to the anatomic site.11,13 The use of neoadjuvant treatment varies according to different cancer institute registries (2%–52%),14 showing a percentage of 4.8% in the current data. This percentage may be underestimated, as the present retrospective study did not consider those patients whose disease progressed and were not candidates for surgical treatment (exclusion criterion). The percentage of skin complications described in the neoadjuvant treatment with radiotherapy (35%)15 was assessed, and conservation surgeries with surgical reconstruction were used in extensive defects due to advanced clinical stages in 24.4% of patients. These patients presented the highest percentage of surgical complications, but there was no impact on their survival.
The adjuvant treatment included radiotherapy which, together with limb-preservation surgery, avoided amputation and achieved local control in 85.6%, which is within the ranges described by several authors (85%–90%).16–18 The most frequently used scheme was 6000CGys in 30 sessions, similar to O'Sullivan et al.19 Systemic treatment was used, indicated by the advanced stages reported, and ifosfamide associated with adriamycin was the most frequent (32.4%) scheme, as recommended internationally.20,21 Response ranges varied between 20% and 25%, compared to single-agent schemes at <10%.22
The importance of obtaining adequate local control in STS involves considering several factors, as mentioned: stage, tumor size, grade, anatomic location, adequate surgical margins and neoadjuvant/adjuvant therapy. The rate of local disease recurrence was 39.1%, despite multidisciplinary treatment. Varying rates of local recurrence have been described, ranging between 7% and 24% worldwide.23 Our rate of recurrence is likely related to the high number of advanced stages in our population. Distant recurrence occurred in 29.3%, and pulmonary metastasis was the most frequent (90.4%), which is widely described in the characteristic hematogenous dissemination of this disease.24 Both distant and local recurrence had a significant impact on patient survival (Table 4), similar to that reports by Brennan et al.25 and Pisters et al.,26 which reveals the need for multidisciplinary planning and treatment in order to improve the survival of patients with STS.27
We have reported the statistics of patients with STS, discussing their epidemiological and clinical characteristics, treatment and prognostic factors for survival, based on the registry of the Institute of Neoplastic Diseases (INEN) in Peru. Our study allows us to understand the importance of multidisciplinary therapy and obtaining updated data on the behavior of STS as well as variables that impact the survival of patients in Latin America.
Conflict of InterestsThe authors have no conflict of interests to declare with this present scientific study.
Please cite this article as: Chávez M, Ziegler G, Cotrina J, Galarreta J, de la Cruz M, Mantilla R. Situación actual de los sarcomas de partes blandas: registro de un instituto oncológico de Latinoamérica. Cir Esp. 2019;97:203–212.