Xenotransplantation could provide an unlimited supply of organs and solve the current shortage of organs for transplantation.
To become a reality in clinical practice, the immunological and physiological barriers and the risk of xenozoonosis that they possess should be resolved.
From the immunological point of view, in the last 30 years a significant progress in the production of transgenic pigs has prevented the hyperacute rejection.
About xenozoonosis, attention has been focused on the risk of transmission of porcine endogenous retroviruses; however, today, it is considered that the risk is very low and the inevitable transmission should not prevent the clinical xenotransplantation.
Regarding the physiological barriers, encouraging results have been obtained and it's expected that the barriers that still need to be corrected can be solved in the future through genetic modifications.
Los xenotrasplantes podrían proveer un suministro ilimitado de órganos y resolver la actual escasez de estos para trasplantes.
Para que los xenotrasplantes se conviertan en una realidad en la práctica clínica, se deben resolver las barreras inmunológicas, fisiológicas y el riesgo de xenozoonosis que estos poseen.
Desde el punto de vista inmunológico, en los últimos 30 años se han realizado grandes avances en la producción de cerdos transgénicos, con lo que se ha logrado evitar el rechazo hiperagudo. Acerca de la xenozoonosis, la mayor atención ha sido dirigida al riesgo de transmisión de retrovirus endógenos porcinos; sin embargo, en la actualidad, se considera que el riesgo es muy bajo y que la transmisión inevitable no debería impedir el xenotrasplante clínico. En cuanto a las barreras fisiológicas, se han obtenido resultados alentadores y se espera que las barreras que aún faltan por corregir se solucionen por medio de las modificaciones genéticas.
Xenotransplantation is the transplantation of cells, tissue or other organs between phylogenetically different species.1
Currently, the main problem in organ transplantation is the gap between the number of organs available for transplantation each year and the number of patients on waiting lists for a graft. According to the World Health Organization (WHO), more than 114000 organ transplantations are performed each year worldwide, which meets less than 10% of the current need.2
The advantage of xenotransplants is that they would provide an easily available animal source with an unlimited supply of “donor” organs. Ethically, pigs are an acceptable option for an alternative organ source.3 However, this solution is immunologically less desirable than nonhuman primates (NHP), due to the genetic distance between pigs and humans.
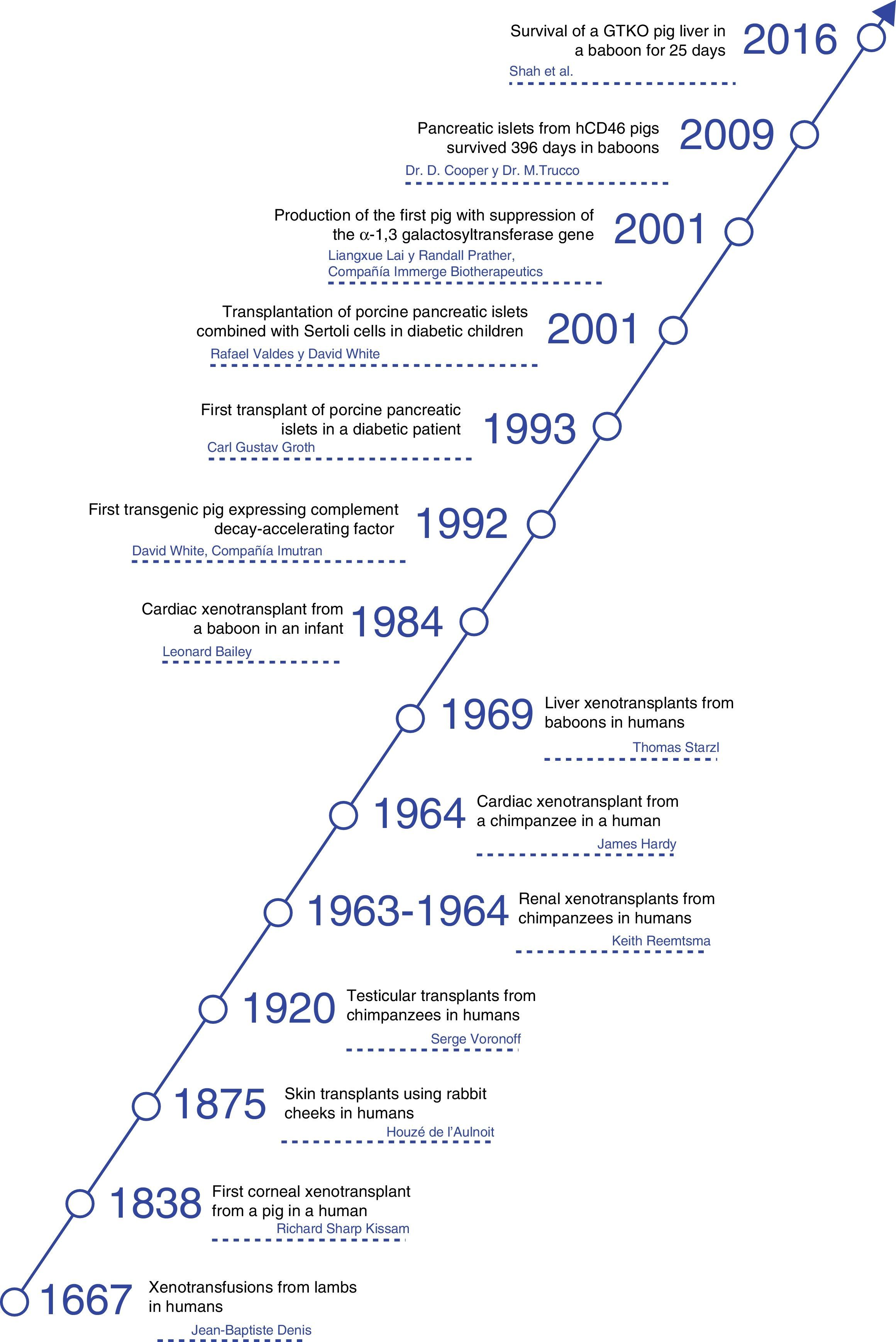
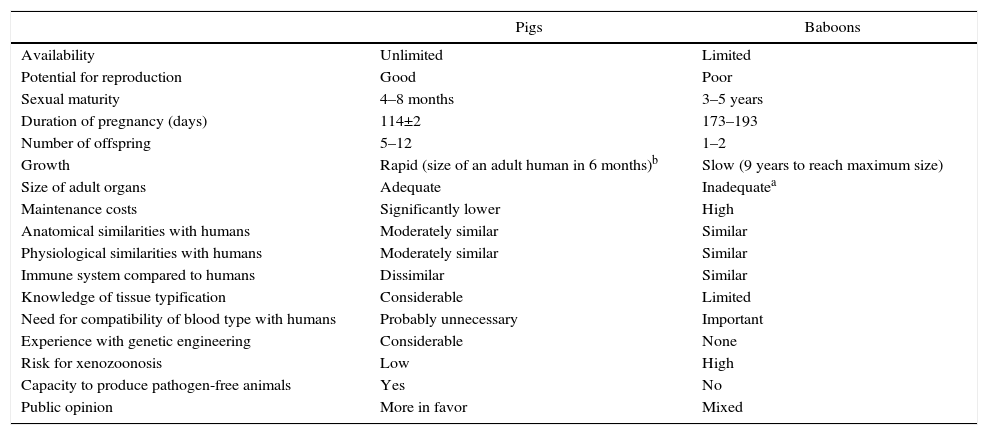
HistoryThroughout history, xenotransplants have piqued human interest and, surprisingly, there have been a number of clinical attempts made over more than the last 300 years.4
The first documented description of a transfusion in a man was a xenotransfusion conducted in 1667 by Jean-Baptiste Denis. He transfused blood from a lamb to a young man,5 curing him of a high fever. Denis carried out more xenotransfusions, obtaining mixed results, which led to xenotransfusions being banned in France for several years.6
In 1920, Serge Voronoff transplanted male chimpanzee testicles in a significant number of men.7 Reports of complications were infrequent, and, although no beneficial effects had been found, many of the men reported “remarkable rejuvenation.”
Between 1963 and 1964, Dr. Keith Reemtsma carried out 13 NHP kidney transplantations in humans.8 Most patients died in the following weeks due to organ rejection or infectious complications. One patient lived 9 months, until he suffered a sudden death; no signs of rejection were evident during autopsy, and he was thought to have probably died of a water-electrolyte imbalance.
In 1969, Thomas Starzl performed liver xenotransplantations from baboons in young patients, although long-term survival was not achieved. When tacrolimus became part of the therapeutic armamentarium, Starzl and his team performed 2 baboon liver transplantations in the 1990s: one of these patients managed to survive 70 days.9
James Hardy transplanted the first cardiac chimpanzee xenograft in 1964 in a dying patient for whom a human donor was not able to be found. The heart was not sufficient to support circulation, and the patient died within a few hours.10
In 1977, Barnard et al. carried out 2 heterotopic cardiac xenotransplantations from a chimpanzee and baboon.11,12 The most famous cardiac xenotransplantations occurred in 1984, when Leonard Bailey transplanted the heart of a baboon in an infant. The procedure was technically successful, although graft rejection occurred and the patient died 20 days later.13
In 1993, Carl Gustav Groth transplanted islets from a pig into a diabetic patient. However, although C-peptide was documented in the patient's blood, indicating that some islets survived, no clinical benefit was observed.14
In 2001, Rafael Valdes and David White transplanted islets from pigs combined with testicular Sertoli cells in children with type 1 diabetes mellitus.15 During the follow-up of more than one year, one 17-year-old girl did not require insulin or other medications (Fig. 1).
Animals UsedFrom an immunological standpoint, although NHP would be a preferable source of organs for humans, in practice NHP are too small to provide organs that would be adequate for use in adult humans.3 Furthermore, concerns have been raised about the transmission of infectious agents from NHP to humans, especially since most NHP are captured in the wild or have been living in colonies with short generational histories and, additionally, there is a lack of experience in their genetic modification (Table 1).
Advantages and Disadvantages of Pigs as a Potential Source of Organs and Cells for Humans, Compared to Baboons.
| Pigs | Baboons | |
|---|---|---|
| Availability | Unlimited | Limited |
| Potential for reproduction | Good | Poor |
| Sexual maturity | 4–8 months | 3–5 years |
| Duration of pregnancy (days) | 114±2 | 173–193 |
| Number of offspring | 5–12 | 1–2 |
| Growth | Rapid (size of an adult human in 6 months)b | Slow (9 years to reach maximum size) |
| Size of adult organs | Adequate | Inadequatea |
| Maintenance costs | Significantly lower | High |
| Anatomical similarities with humans | Moderately similar | Similar |
| Physiological similarities with humans | Moderately similar | Similar |
| Immune system compared to humans | Dissimilar | Similar |
| Knowledge of tissue typification | Considerable | Limited |
| Need for compatibility of blood type with humans | Probably unnecessary | Important |
| Experience with genetic engineering | Considerable | None |
| Risk for xenozoonosis | Low | High |
| Capacity to produce pathogen-free animals | Yes | No |
| Public opinion | More in favor | Mixed |
Now the attention is aimed at pigs as a potential source of organs and cells. In this field, great progress has been made in the past 30 years. In 1992, the first transgenic sow expressing human decay-accelerating factor (hDAF) was produced.16 Subsequently, in 2001, the α1,3-galactosyltransferase (GTKO) gene was inhibited, and this has reduced hyperacute rejection of xenotransplantation (Fig. 2).
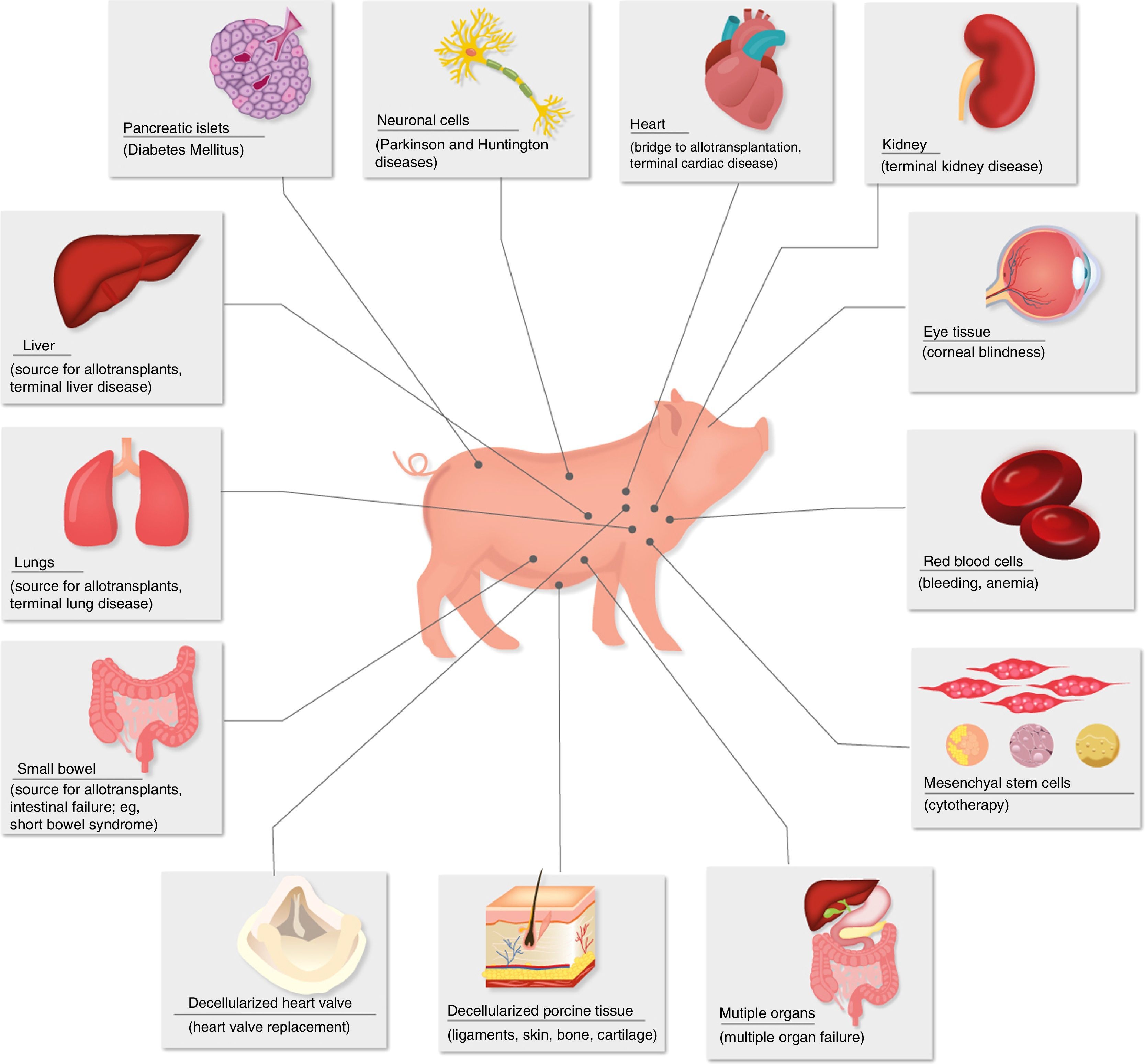
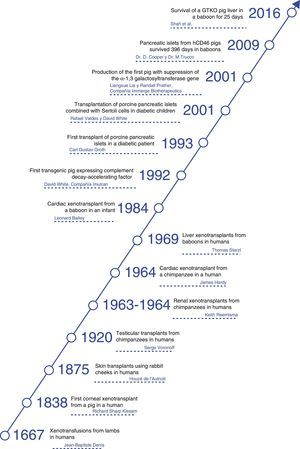
Diseases for which xenotransplants could be an alternative therapy.
New genetic techniques have recently been introduced, such as transcription activator-like effector nuclease (TALEN)17 and clustered regularly interspaced short palindromic repeats associated with protein 9 (CRISPR/Cas9),18,19 which have become the most frequently used tools in genetic editing. It is estimated that there are currently 40 different types of genetically modified pigs worldwide, some expressing up to 5 or 6 modifications20 (Table 2).
Currently Available Pigs That Have Been Genetically Modified for Xenotransplant Research.
| Regulation of the complement by means of the expression of a human complement regulatory gene |
| CD46 (membrane cofactor protein) |
| CD55 (decay-accelerating factor) |
| CD59 (protectin) |
| Deletion or suppression of Gal or nonGal antigens |
| Expression of the human H-transferase gene (expression of the O blood group antigen) |
| Endo-beta-galactosidase C (reduction of Gal antigen expression) |
| Inactivation of the α-galactosyltransferase gene (GTKO) |
| Inactivation of the cytidine monophosphate-N-acetylneuraminic acid gene (NeuGcKo) |
| Inactivation of β1,4 N-acetylglucosaminyltransferase |
| Expression of the N-acetylglucosaminyltransferase III gene (GnT-III) |
| Expression of tumor necrosis factor α receptor 1 |
| Suppression of the cellular immune response by the expression or regulation of genes |
| CIITA-DN (suppression of class II CMH, which results in suppression of class IIleukocyte antigen) |
| Suppression of class I CMH |
| HLA-E/human microglobulin-β2 (inhibition of human natural killer cells and macrophage-mediated cytotoxicity) |
| Human Fas ligands (CD95L) |
| Porcine CTLA4-Ig (cytotoxic T lymphocyte antigen-4 or CD152) |
| Human TRAIL (apoptosis-inducing ligand related with tumor necrosis factor) |
| Expression or deletion of anticoagulant or anti-inflammatory genes |
| von Willebrand factor deficiency (natural mutation) |
| Human tissue factor pathway inhibitor (TFPI) |
| Human thrombomodulin |
| Human endothelial protein C receptor (EPCR) |
| Human CD39 (ectonucleoside triphosphate diphosphohydrolase-1) |
| Expression of anticoagulants, anti-inflammatories and anti-apoptosis genes |
| Human A20 (tumor necrosis factor α inducer of protein 3) |
| Heme oxygenase 1 (HO-1) |
| Human CD47 (species-specific interaction with SIRP α inhibiting phagocytosis) |
| Suppression of the asialoglycoprotein 1 porcine receptor gene (ASGR1-KOO) (decreased platelet phagocytosis) |
| Signal regulatory protein α (SIRPα) (decreased platelet phagocytosis by self-recognition) |
| Prevention of porcine endogenous retrovirus (PERV) activation PERV siRNA |
MHC: major histocompatibility complex (MHC).
Anatomically, pig hearts are similar but not identical to human hearts. Physiologically, the cardiac output and systolic volume are comparable between the 2 species.21 Mean blood pressure, heart rate, and blood flow are almost identical. Unlike pigs, the erect posture of humans has had an evolutionary impact on the structure of human heart valves; however, this difference has been proven to be an insignificant problem in studies between pigs and NHP.22
There is a different potential for action between cardiomyocytes that is related to the morphological differences in the atrioventricular node between species, which could result in increased arrhythmogenicity in a transplanted swine heart in a human. However, the observations in NHP have not demonstrated that this is a problem.23
The anatomy of porcine kidneys is very similar to human kidneys. The maximum concentration capacity (1080mOsm/L) and glomerular filtration rate (126–175μm/h) of porcine kidneys are similar to those of humans.21 Although potassium in pigs is slightly higher, other serum electrolytes, creatinine and urea nitrogen are similar. Porcine kidneys have been shown to maintain homeostasis, electrolyte balance and adequate osmolarity in NHP.
Porcine liver anatomy is slightly different from human liver anatomy. Pig livers have 3 lobes (right, middle and left). The middle lobe is divided by a deep umbilical fissure that extends almost to the hilum. The inferior vena cava is intraparenchymal and runs to the caudate lobe. There are similarities in porcine and human liver function,24 although pigs have high levels of alkaline phosphatase, lactate dehydrogenase, and gamma-glutamyltransferase.
Studies by Ekser et al.25 and Kim et al.26 indicate that porcine livers can function adequately in NHP.
Although the experience with porcine lung xenotransplantation is limited, largely due to the severe and early vasoconstriction that occurs in transplanted lungs, porcine lungs have been shown to provide adequate oxygenation and gas exchange in NHP.27
Porcine insulin differs from human insulin in only one amino acid, and it has been used for many years in patients with type 1 diabetes mellitus.
Currently, special attention has been paid to the transplantation of islets of Langerhans as a source of insulin for patients with diabetes mellitus.28–30 Both islets of unmodified pigs and those of transgenic pigs have been shown to maintain normoglycemia in diabetic monkeys for periods of more than one year.31
Porcine corneas have a refractive power, size and tensile strength similar to human corneas.32 The cornea is an unusual tissue in terms of its immunological characteristics: it is avascular, has weak expression of major histocompatibility complex antigens and has immunomodulatory molecules in the aqueous humor.33 However, in spite of these advantageous characteristics, the antigenicity of pig corneas has been an obstacle for xenotransplantation.34 Recently, a Chinese group has achieved encouraging results using decellularized porcine corneas as grafts in anterior lamellar keratoplasty to treat corneal ulcers in humans.35 With these advances, as well as with the progress of immunosuppressants and the availability of transgenic pigs, clinical corneal xenotransplantation may be a solution in the near future to resolve the shortage of these grafts.34
Immunological BarriersHyperacute RejectionWhen a porcine organ is transplanted into a human or NHP, an immediate immune response occurs with hyperacute rejection. This has been defined as destruction of the graft in less than 24h, however, it usually occurs within the first hour.
This is due to the binding of the preformed antiporcine antibodies (AB) to the endothelial cells of the graft. AB deposits initiate a complement-mediated response with endothelial injury, resulting in thrombosis, interstitial hemorrhage and edema, with subsequent graft dysfunction.36
Later, it was determined that AB bind to the carbohydrate epitope, galactose–α1,3-galactose (Gal), expressed in the porcine vascular endothelium. This oligosaccharide is present in other mammals, except humans and primates. These AB are produced in response to viruses and microorganisms that express Gal and colonize the gastrointestinal tract of primates.
Humoral Acute Rejection and Adaptive Immune ResponseAlthough hyperacute rejection is prevented, a similar reaction is generated days or weeks later. This is also due to the AB and complement deposits that activate the endothelium and, in addition, there is infiltration of innate immunity cells (polymorphonuclear cells, macrophages, natural killer cells) which together destroy tissue.37
If immunosuppressive therapy is inadequate, a response develops of preformed AB dependent on T cells, resulting in high levels of anti-porcine IgG.38 The union of these AB with the vascular endothelium generates histopathological changes that are indistinguishable from acute rejection.37
Surprisingly, acute cell rejection, as seen in most transplants, has almost never occurred in xenotransplants from pigs to NHP. This is probably because the humoral response is faster and overlaps the cell response.39
Coagulation DysfunctionThe altered coagulation in the graft vessels plays an important role in its failure. Altered coagulation leads to thrombotic microangiopathy, in which the organ vasculature is constantly occluded by thrombi, resulting in ischemic tissue necrosis.40
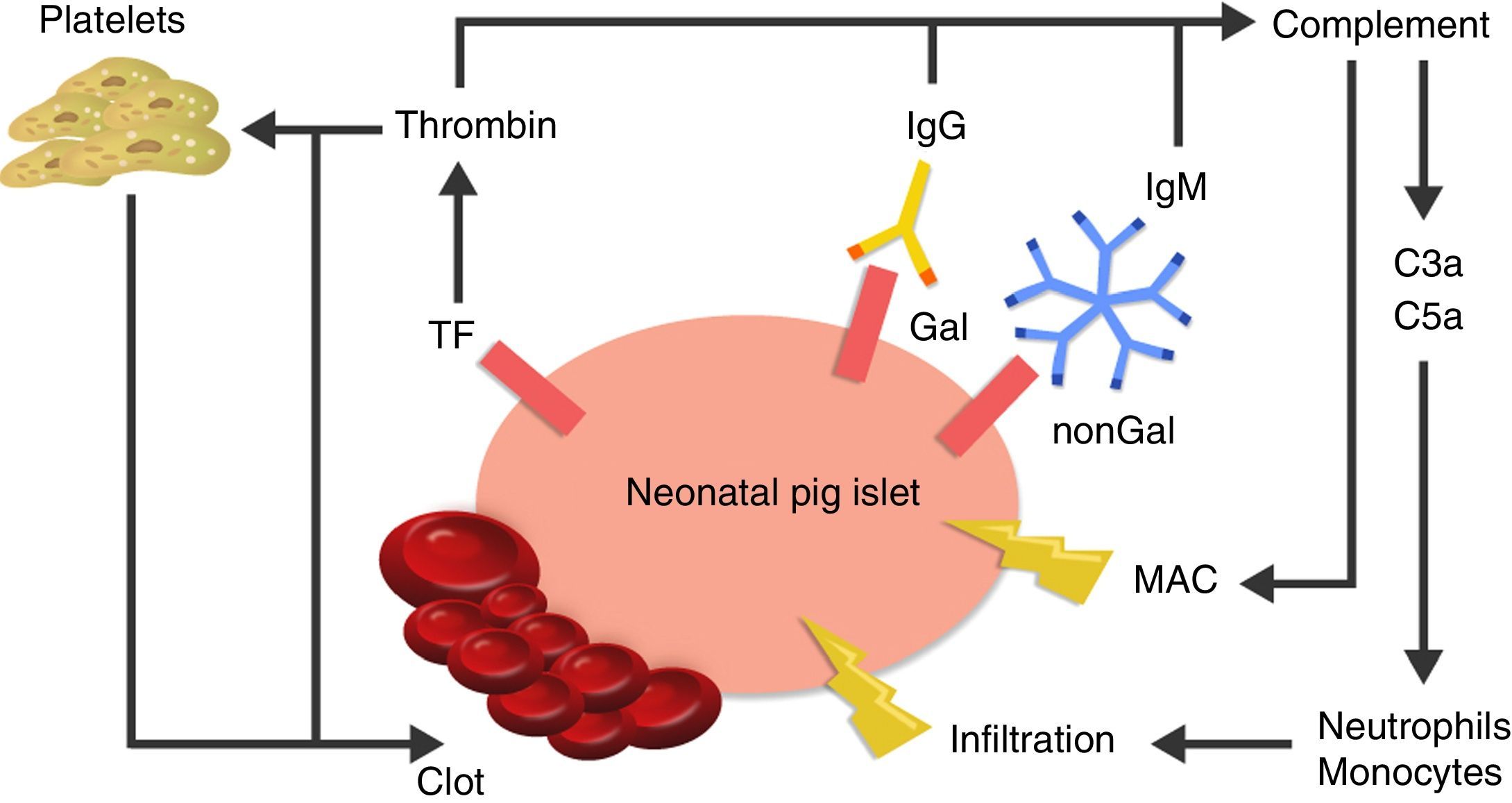
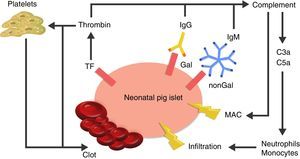
The cause of thrombotic microangiopathy is the activation of vascular endothelial cells by AB, complement, or cells of the innate immune response, which change the phenotype of cells from an anticoagulant state to a procoagulant state41 (Fig. 3).
Diagram of the immediate response after xenotransplantation. The expression of tissue factor and the binding of antibodies to Gal and nonGal antigens in the neonatal porcine islets leads to activation of the complement and the coagulation cascade. Clot formation and direct damage of the cell membrane is generated through the membrane attack complex (MAC), as well as recruitment of macrophages and monocytes through chemoattractants C3a and C5a. TF: tissue factor.
Chronic rejection has been documented in cardiac xenografts from pigs that have functioned for more than 3 months in baboons37 and have shown evidence of histopathological changes similar to those that appear after allotransplantation.
Induction of ToleranceThe goal in both xenotransplantation and allotransplantation is to achieve “immune tolerance”, in which the recipient's immune system is manipulated for the body to accept the transplanted cells or organ without effort or attempts at rejection. Efforts in this regard have been made through the induction of chimerism in hematopoietic cells42 or through transplantation of porcine thymus,43 but to date they have not been successful.44
Risk of InfectionThe term “xenozoonosis” is used to refer to the possibility of the appearance of new pathogenic agents in xenotransplants.
The infectious risk of clinical xenotransplantation is unknown due to the lack of clinical trials. Based on experience with human allotransplants, there is an assumed potential risk of infection transmission through xenografts.45 The risk is amplified by the danger of transmitting zoonotic infections from animals (pigs) to human receptors, for which we have no diagnostic tools and whose behavior in the immunosuppressed recipient is unpredictable.
The results of clinical trials such as that by Wynyard et al.46 indicate that transmission episodes are uncommon and may be unrecognizable among the infections expected to occur in immunosuppressed recipients.47 In addition, significant progress has been made in the study of the microbiology of xenotransplants, especially in the screening of animals from clinical trials, and much microbiological evidence is currently available.
Both pigs and humans possess retroviruses in their genome, called porcine endogenous retroviruses (PERV) in pigs. These are the result of retroviral infection of pig germ cells, so that they become part of the genetic code and are transmitted from one generation to another.
Concern over the transmission of retroviruses in xenotransplants is due to the possibility of “silent” transmission that can cause altered gene regulation, oncogenesis or genetic recombination.
Three subgroups of gammaretroviruses (PERV A–B–C) have been identified with infectious potential.48 Of these, PERV A and B have the ability to infect porcine cells and human cells in in vitro cultures, whereas PERV C only infects porcine cells.
There is no evidence of retroviral infection in human cells in vivo, but it appears that they could be susceptible to certain antiviral agents.49
The approach to ensuring the safety of clinical xenotransplants has been derived from the approach used in human allotransplants. However, in both cases absolute prevention of transmission of infection with transplantation is not possible. Screening for all potential pathogens is impossible. In clinical xenotransplantation, the level of safety has developed beyond that available for allotransplants. Modern-day porcine production ensures the birth of piglets that are completely free of specific pathogens and can be routinely tested for an extensive battery of human pathogens.
Solutions for BarriersInnate Immune ResponseSince the factors associated with hyperacute xenograft rejection are similar to those of ABO-incompatible allograft rejection, plasmapheresis was used to deplete the anti-pig antibody receptor and, afterwards, an attempt was made to specifically deplete the anti-Gal antibodies using immunoaffinity columns.50 However, these treatments were only partially successful, since they delayed rejection, but the grafts were lost when antibody levels recovered.
Another approach was to administer an agent that inhibited the complement, such as the cobra venom factor; this increased graft survival, however, but only had a temporary effect.51
Subsequently, when porcine genetic modification was possible, a different approach was proposed to avoid rejection by means of complement regulatory proteins. Examples of these are the complement decay-accelerating factor (CD55 or DAF) or the membrane cofactor protein (MCP or CD46).52
When the importance of Gal in rejection was established, it was decided to attack the gene that produces the enzyme binding Gal to the terminals of the oligosaccharide chain, called α1,3-galactosyltransferase: in 2001, the first pig was produced with suppression of this gene (GTKO), and until now studies have shown protection from hyperacute rejection.53
Adaptive Immune ResponseThe T-cell response to an allograft or xenograft consists of a response to the graft antigens, but also requires a second response, known as co-stimulation. Prevention of co-stimulation results in inhibition of T-cells.54
Although high doses of conventional immunosuppression have delayed graft rejection, they have been associated with a high incidence of infectious complications. A more successful approach has been generated with the genetic engineering of transgenic pigs expressing T-cell stimulating agent (CTLA4-Ig),55 but because of the high levels of immunosuppression produced, this impedes long-term survival. Subsequent efforts have been directed at expressing CTLA4-Ig in selected tissues.
NonGal Targets*Even when the innate and adaptive responses are controlled thanks to a combination of genetic engineering and a suitable regimen of immunosuppressants, there is still a low degree of activation of the vascular endothelial cells of the graft due to the binding of AB to other porcine antigens.
Humans have at least two other natural AB, called N-glycolylneuraminic acid (NeuGc) and β 1,4N-acetylgalactosaminyltransferase.56,57 The recent production of pigs in which the gene coding for NeuGc has been eliminated has given rise to the initiation of in vitro studies that are underway.58,59
Coagulation DysfunctionEfforts have been made to control this problem by administering the recipient anticoagulants or antithrombotic agents, with partially successful results. Evidence indicates that increased immunosuppression is more effective than anticoagulant agents.
In addition, this problem is being addressed through genetic manipulation by attempting to develop pigs that express coagulation regulatory proteins, such as thrombomodulin (TBM), endothelial protein C receptor (CD39) or tissue factor inhibitor. This would generate a procoagulant and anticoagulant balance with increased tissue survival.60
Current StudiesPancreatic IsletsIn 2009, van der Windt et al.61 investigated whether the transgenic expression of human complement regulatory protein (hCD46) in porcine islets would improve the outcome of islet xenotransplants in macaque monkeys with streptozotocin-induced diabetes. After the transplantation of islets from pigs without genetic modifications (n=2) or GTKO pigs (n=2), the islets survived a maximum of 46 days. Transplants from hCD46 pigs resulted in graft survival and normoglycemia at the 3-month follow-up of the experiment and for more than one year in one patient.
KidneyHigginbotham et al. studied the importance of preformed anti-porcine AB levels prior to xenotransplantation.62 They used 34 Macacos rhesus to measure antiporcine AB levels and transplanted them with kidneys from GTKO/CD55 pigs. All animals received T-cell depletion followed by immunosuppression therapy with co-stimulation blockade (anti-DC154m Ab or belatacept), mycophenolate and steroids. Animals with the highest titers presented with rejection within the first week. NHP with lower titers treated with anti-CD154 AB showed prolonged renal graft survival (>133, >126 vs 14, 21 days, respectively). Monkeys with prolonged xenograft survival had normal renal function, with no evidence of rejection at the 100-day biopsy.
Iwase et al. has reported a case of prolonged renal xenotransplant survival.63 They performed a kidney transplantation from a GTKO/CD45/CD55/TBM/EPCR/CD39 pig to a baboon, which survived 136 days with stable serum creatinine levels (0.6–1.06mg/dL) up to the end.
LiverLiver xenotransplants have received little attention due to the fatal coagulopathy that accompanies the transplantation of porcine liver in NHP,64 which has proven to be unsurmountable.25
A study by Ramírez et al.,65 in which 5 pig livers were transplanted in baboons (3 from unmodified pigs and 2 from transgenic hDAF pigs), showed that the baboons that received unmodified pig livers survived less than 12h, whereas those that received transgenic hDAF livers survived 4 and 8 days, with no evidence of hyperacute rejection. The death on day 8 was due to sepsis and coagulopathy, and the other animal was euthanized on day 4 due to an episode of vomiting and aspiration; however, adequate production of coagulation factors and protein levels had been observed during survival, and autopsy showed no signs of xenograft rejection.
In 2010, Ekser et al. reported hepatic xenotransplantation in baboons using the livers of genetically modified pigs (GTKO n=2 and DC46 n=8) in association with common immunosuppressants.25 Out of the 10 baboons, 6 survived for 4–7 days. In all cases, liver function was adequate. The main problem that prevented prolongation of survival was intense thrombocytopenia that developed after the first hour of reperfusion, leading to spontaneous bleeding.
Recently Shah et al. reported their historical experiment in which they overcame coagulopathy and achieved 25-day survival of an NHP who had received a GTKO porcine liver orthotopic xenograft.66 After xenotransplantation, exogenous coagulation factors were administered, achieving control of coagulopathy, maintaining platelet circulation and preventing thrombotic microangiopathy. The NHP was euthanized on day 25 due to cholestasis and hemolysis; post-mortem biopsy showed evidence of 30% hepatic necrosis and mild congestion; however, the portal tracts were intact, with no evidence of rejection, inflammation, or thrombotic microangiopathy. This experiment provides the first evidence that porcine liver xenotransplants could provide support for a prolonged period.67
HeartMuhammad et al.23 transplanted heterotopic hearts from genetically modified pigs into baboons. They were divided into groups of GTKO/hCD46 pigs (group A, B, C) and others that also expressed human thrombomodulin (hTBM) regulator gene (group D). The grafts from group D (n=5) have survived more than 200 days and continue in follow-up for more than one year (Fig. 4).
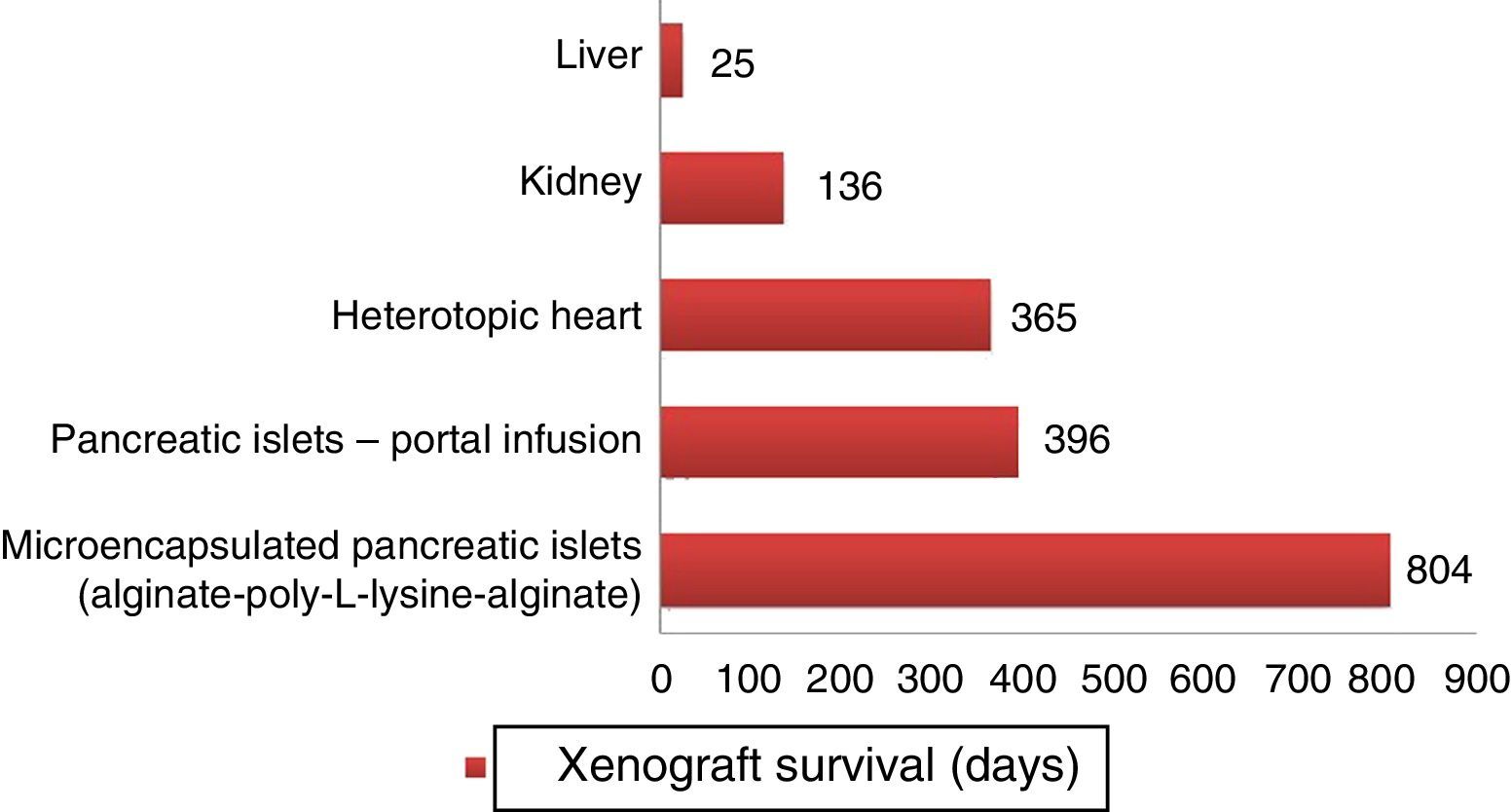
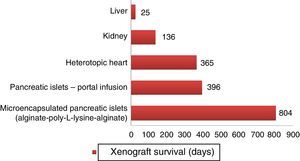
Longer survival rates of porcine xenografts in nonhuman primates. Microencapsulated pancreatic islets of unmodified pigs survived 804 days with re-transplantation and 250 days without a new transplantation.72 Pancreatic islets from hCD46 pigs survived 396 days in baboons.61 Heterotopic hearts of GTKO/hCD46/hTBM pigs obtained survivals of more than one year.23 Kidneys from GTKO/CD46/CD55/TBM/EPCR/CD39 pigs achieved survivals of 136 days with stable creatinine.63 One orthotopic xenograft from a GTKO porcine liver reached a survival of 25 days in a baboon with continuous post-transplantation administration of exogenous coagulation factors.66
The scarcity of available organs is an undeniable problem in organ transplantation. Even if we are able to significantly increase donor rates, allotransplants will never be able to provide enough islets for all diabetic patients in the world. Xenotransplantation will be a reality in the near future, which could be initiated through cell xenotransplantation or as a “bridge” to allotransplantation.68
Pig xenotransplants in NHP have progressed a great deal, and the first clinical trials of complete organ xenografts will likely involve patients with renal failure. These patients could be selected because they have a high degree of HLA sensitization,69 which prevents them from easily obtaining an allograft. Likewise, patients who no longer have optimal accesses to continue with dialysis could be selected.
As for safety against zoonosis, the greatest concern has been directed at the risk of transmission of PERV. Currently, the risk is considered to be very low, and any unavoidable transmission should not prevent clinical xenotransplantation.70
When all pathobiological barriers have been overcome, it will be much easier to study the physiological differences that may limit the efficacy of xenotransplants.71 For example, although some porcine liver products appear to function adequately in NHP,24 these livers are unlikely to meet all the metabolic needs of human recipients. In these cases, genetic engineering of source pigs could correct these differences.
In the next 5 years, experts expect to resolve the remaining barriers with the introduction of porcine genetic engineering, which is achieving genomic modifications increasingly faster and easier.
Authorship/CollaboratorsA.M. Aristizabal contributed to the concept and design of the review, composition of the manuscript and design of the diagrams and figures.
L.A. Caicedo critically reviewed the content of the manuscript and approved the final version.
J.M. Martínez and M. Moreno wrote the manuscript.
G. Echeverri contributed to the concept and design of the review, the design of the diagrams/figures and general concept, as well as the critical review of the content and the approval of the final version of the manuscript.
Conflict of InterestThe authors have no conflict of interest to declare.
Presence of natural antibodies that bind with antigens other than galactose-α-1,3-galactose (GAL).
Please cite this article as: Aristizabal AM, Caicedo LA, Martínez JM, Moreno M, Echeverri GJ. Xenotrasplantes, una realidad cercana en la práctica clínica: revisión de la literatura. Cir Esp. 2017;95:62–72.