Permanent synthetic materials are currently of choice for abdominal wall hernia repair. However, they are not ideal as short- and long-term complications with these have been reported. Extracellular matrix-derived biological implants (EMDBI) have emerged as a result of research and development into new materials. Several types of EMDBI have appeared in the last few years, each with its own manufacture characteristics and different from the rest. The current panorama of the xenogeneic EMDBI available in Spain is analyzed, their complications, the unknown factors arising in the long-term, and the clinical experience available on incisional and inguinal hernias.
Los materiales sintéticos permanentes son de elección en la actualidad para la reparación de las hernias de la pared abdominal. Sin embargo, no son ideales y se describen complicaciones relacionadas con ellos a corto y largo plazo. Fruto de la investigación y desarrollo de nuevos materiales han surgido los implantes biológicos derivados de la matriz extracelular (IBMEC). En los últimos años han aparecido varios tipos de IBMEC, cada uno con características propias de manufacturación y diferentes a los demás. En este trabajo se analiza el panorama actual de los IBMEC xenogénicos disponibles en nuestro medio, el laberinto que suponen, las incógnitas que plantean a largo plazo y la experiencia clínica disponible en las hernias incisionales e inguinales.
Nowadays, the placement of permanent synthetic mesh is considered the best method for hernia repair.1–4 Most synthetic mesh strengthens the abdominal wall, but the biological response to the material can cause complications (intestinal fistula, mesh contraction, intraperitoneal adhesions, sensation of the presence of the mesh and/or increased rigidity of the abdominal wall).5–10
Infections of the synthetic prosthetic material are feared by both patients and surgeons. Microorganisms adhere to the polymer and generate a biofilm that guarantees chronic infection.11 The morbidity and costs associated with infected synthetic mesh make surgeons avoid using them in contaminated settings or in high risk cases.
There has been much research on ways to minimize the complications related with synthetic material.12 The search for alternative materials that provide a durable, stable repair of the defect in one single operation (especially in the context of bacterial contamination), a functional abdominal wall and better quality of life for patients has led to the current era of so-called extracellular matrix (ECM) derived biological implants. ECM implants can be human in origin (allogeneic, not authorized in Spain) or animal in origin (xenogeneic: bovine, porcine, authorized in Spain). The reasons for its use in hernia repair are: to avoid acute and/or chronic infection, chronic inflammation and the formation of dense fibrous tissue with a material that is integrated/biodegradable.13 Its potential benefits are very attractive and include minimal inflammatory response, less adhesion formation and a lower risk of infection in contaminated or potentially contaminated situations.
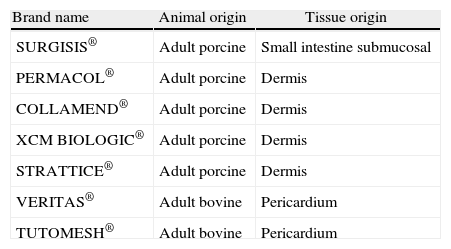
In recent years, several types of xenogeneic ECM-derived implants have appeared in our country, each with their own manufacturing characteristics and different from the others (Table 1). Unfortunately, ECM implants entail very high costs. The aim of this study is to analyze the xenogeneic ECM materials available in our setting, their potential indications, their unknown long-term outcomes and the clinical experience available in incisional and inguinal hernias.
Types of Biological Implants Derived from the Extracellular Xenogeneic Matrices Available for Hernia Repair in Spain.
| Brand name | Animal origin | Tissue origin |
| SURGISIS® | Adult porcine | Small intestine submucosal |
| PERMACOL® | Adult porcine | Dermis |
| COLLAMEND® | Adult porcine | Dermis |
| XCM BIOLOGIC® | Adult porcine | Dermis |
| STRATTICE® | Adult porcine | Dermis |
| VERITAS® | Adult bovine | Pericardium |
| TUTOMESH® | Adult bovine | Pericardium |
The different processes for obtaining and manufacturing ECM implants determine intrinsic properties for each implant that can cause different biological responses after their implantation in vivo. To speak of ECM implants exclusively as “acellular matrices” or “collagen scaffold” may be inadequate if the specific characteristics of each implant and their biological behaviors are not well known. But, what it makes the understanding of these materials and their correct selection seems like an absolute maze?
Origin of the MaterialECM implant material is derived from extracellular matrices (ECM) of mammals. These ECM are gels made up of fibrillar proteins (some are structural, such as collagen, and others have the capacity for connection and cell recognition, such as elastin), interconnected to a network of glycosaminoglycan chains. Their main function is to confer structural scaffolding that, combined with interstitial liquid, is able to resist tensile stress (via fibers) and tissue compression (via hydrated matrix). The anisotropic fibrillar architecture of ECM affects cell behavior. The close connection between the cytoskeleton and ECM allows the cells to capture and respond to mechanical changes, converting mechanical signals into chemical signals that affect different cell functions (adhesion, migration, etc.). Depending on the origin of the ECM-derived implants (species and animal age, tissue of origin, etc.), different microstructures are obtained with diverse properties. Currently, the main challenge is to be able to control the spatial organization and dynamics in order to present the multiple signals existing in the ECM for application in tissue repair and/or substitution.14
Processing of the MaterialThe materials available are processed in different manners (occasionally only known by the manufacturer). Three aspects are of special interest:
- 1.
The process of eliminating the cells from the ECM, which can be minimal (retaining proteins – elastin, proteoglycans or fibronectin)15,16 or maximal (only the collagen fibers remain).17 In addition, ECM implants that are dried or lyophilized for storage before use may have variations in the size of their pores or in the rehydration rate, which have an impact on the infiltration and vascularization capacity of the implant by the host.17
- 2.
Artificial crosslinking: natural collagen is made up of 3 polypeptide chains in a triple helix with hydrogen bond intermolecular unions (natural crosslink). This compact chain is what gives the collagen its elevated tensile strength.18 The artificial crosslink is used in ECM implants to give stability to the collagen and thus reduce the eventual and rapid biodegradation of these materials in vivo.18 To do so, different methods are used: glutaraldehyde, hexamethylene diisocyanate (HMDI) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC). However, not all ECM implants have artificial crosslinks. There are materials that use other processing methodologies that reach clinical persistence without the need for artificial stabilization. They can all determine a different cell response in the host once implanted.
- 3.
Sterilization of the material: xenogeneic ECM implants are sterilized in their final processing in order to guarantee the absence of bacteria or virus. The process of sterilization varies for each material (ethylene oxide, gamma irradiation, e-beam irradiation). The sterilization process used can vary the final biological response of the material in the host.19
- 4.
Mechanical/physical properties: the mechanical and physical properties of ECM implants depend on their origin, processing, crosslinking and sterilization. The dimensions and thickness vary from one to another, making elasticity and resistance variable. In addition, the initial mechanical properties vary over time, meaning that they are not good indicators of the final clinical results.20
Normal healing after a surgical intervention results in acute inflammation that lasts for approximately 2 weeks, followed by a remodeling phase for weeks or years due to the slow reorganization of the repaired tissue. Exactly how this process may be affected by the addition of ECM implant material or how the process of healing affects the ECM implants will vary depending on the type of product. There have been no randomized clinical trials performed on this aspect.21 Some products have been evaluated separately at an experimental level, and at least 5 possible biological responses have been identified:
- a)
lack of integration of the ECM implants10,15,22:
- -
Encapsulation
- -
Rejection
- -
- b)
integration of the ECM implants15,16,23–28:
- -
Resorption
- -
Integration with progressive biodegradation
- -
Adoption and adaptation
- -
In our opinion, the long-term unknown aspects of current ECM implants are related to their reason for being: the search for materials that are alternatives to biopolymers and provide long-lasting, stable repair of the abdominal defect in just one operation (especially in the context of bacterial contamination), with a functional abdominal wall and better quality of life for patients after the repair.
Functional RestorationThe restoration of abdominal wall function is essential for the clinical success of a hernia repair when prosthetic material is used.4 ECM implants are not an exception to this need. Naturally, and given that today no material reproduces all the functions of the abdominal wall, when we mention functional restoration we basically refer to the ability to contain the viscera and, as a consequence, the stabilization of the torso as well as impact on improved breathing, movement and posture. These properties will ultimately depend on the mechanical characteristics of the material. We lack objective data about the long-term effect on abdominal wall function after the use of ECM implants. It is possible that current ECM implants may not be very effective for complete, long-term reestablishment of abdominal containment, and the patient may eventually feel discomfort and experience poorer quality of life.29 Recent experimental studies seem to demonstrate decreased mechanical properties of some biological implants after bacterial infection.30 Nevertheless, there are also data showing that, over time, ECM implants are able to recover mechanical function as they are “infiltrated” by cells.31,32 Despite this, the clinical utility (i.e. functional restoration) of ECM implants will in the end depend on a delicate balance between biodegradation rate and infiltration rate, which can be affected by the degree of infection. Consequently, and given that ECM implants are marketed for use especially in contexts of bacterial contamination, the mechanical properties (and therefore the long-term functional response) can be completely different depending on the septic scenario in which they are used (chronic infection, acute infection, open intestinal surgeries, fistulas or peritonitis). It is evident that more studies are needed on the long-term functional behavior of ECM implants and the characteristics that should be maintained in implants so that correct mechanical function is long-lasting.
BiodegradationAn ideal material for abdominal wall repair should, once implanted, carry out the function of repairing the damaged abdominal wall (both tissue and function) and afterwards disappear. Finally, the clinical utility (results) of the biodegradable material would depend on a delicate balance between the biodegradation rate and the infiltration rate. If the material biodegrades prior to adequate cell infiltration, neovascularization and deposit and differentiation of collagen, the overall quality and resistance of the newly formed tissue can be insufficient to ensure a long-lasting and stable repair of the abdominal wall. The biodegradation can be affected by crosslinking.
InfectionInfection can accelerate the biodegradation rate, and therefore preventing infection of the ECM material is considered an important necessity. Preventing the infection of ECM implants can be particularly challenging, especially because these implants are used in contaminated surgeries or in patients with a high risk for infection (immunosuppressed). These materials do not have intrinsic properties that make them resistant to infection. Nevertheless, studies in the literature mention a certain resistance or defense capacity of ECM implants against infection.33–38 The resistance of ECM implants to infection may depend on their capability to release antimicrobial peptides when they biodegrade in vivo and on the quick and early stimulation of neovascularization. However, when ECM implants are colonized by large bacterial inocula, they will rapidly biodegrade.38 In other words, the ability of ECM implants to successfully become incorporated in the repair of an abdominal wall that is contaminated or infected will depend on the characteristics of the contamination or infection (major or minor bacterial inocula) and the speed of angiogenesis. Clinically, it seems that, depending on the degree of infection37,38 and the consequent inflammation that it produces (recent evidence suggests that the inflammatory response to infection can be as pernicious for the tissue as the bacteria that cause it),39 ECM implants can alter their normal architecture, leading to a biodegradation of the implant and failure of the repair. When this occurs, not only will the patient have a relapse of the original hernia, but also the added morbidity of additional surgery.
This information suggests that ECM implants may not be a good option in the context of infection with the presence of large bacterial inocula that cause intense secondary inflammatory processes. Future research should incorporate the development of implants that resist bacterial colonization and can minimize vigorous inflammatory responses in order to avoid rapidly biodegradation in situations with large bacterial inocula, thus maintaining the capability to reinforce the abdominal wall and promote tissue remodeling.
CrosslinkingAs mentioned previously, crosslinking was added to the ECM implants with the aim to increase the durability of these materials. When studied in experimental models,15,40–46 cross-linked materials may not provide the same amount of cell infiltration and neovascularization as the non-cross-linked materials, which will determine high rates of implant encapsulation with possible persistence in vivo. This response can be desirable if the desired clinical results are consistent with those derived from the use of non-biodegradable polymers. However, encapsulated ECM implants can theoretically produce the same clinical complications as other permanent materials that react with the classic response to a foreign object: migration, extrusion10,22,47 or destructuring of the material secondary to the chronic inflammation.48
Clinical Experience With Extracellular Matrix-Derived Implants in Incisional/Ventral HerniasThe first publication about the clinical use of ECM implants was published in 1995.49 In 2004, the use of these materials was reported as an alternative in abdominal wall reconstruction.50 Since then, the only systematic review about the benefit of ECM implants in different types of abdominal wall hernia was published in 2009.21 This review shows that there are only data in humans with 4 types of ECM implants: human dermal (allogeneic), cross-linked porcine dermal (xenogeneic), small-intestinal submucosal (xenogeneic) and bovine pericardial (xenogeneic). All the studies that are reviewed in the article add up to a total of 80 publications. Unfortunately, the data derived from studies about allogeneic materials are not very useful in our setting for the decision-making process since, as previously mentioned, the allogeneic materials are not commercialized in our country or in our region (i.e. Europe). Therefore, the review mentioned provides us with useful data for the use in our country of only 3 ECM implants, constituting a total of 48 publications, 16 of which are about incisional/ventral hernias. Several considerations arise from this study:
- 1)
The recurrence rates for the xenogeneic materials studied are difficult to interpret due to the disparity and heterogeneity of the data (i.e. contexts of treatment, materials, patients, techniques).
- 2)
Most of the studies use these materials in clean surgical contexts. It is possible, as most of the studies are from the United States, that this circumstance may be due to the legislation and regulation of the use of these materials for hernia repair in the US which limit the approval of the clinical use of materials in specific situations (i.e. infected wounds).51
- 3)
Most of the clinical studies published use a type of ECM implant that is not available in our setting (human dermal).
- 4)
The quality and level of evidence of the studies is limited and is classified by the authors themselves as low.
From 2009 and up until the time of the writing of this article, we have found a few clinical studies in the literature about incisional/ventral hernia repair using xenogeneic material both with and without crosslink.52–62 Although these studies have increased our clinical understanding about the different types of ECM implants, it is possible that the benefits and limitations of these materials are not widely discussed. In our opinion, the following facts can be extracted from the analysis of the current evidence regarding these materials in incisional/ventral hernia surgery:
- 1)
There is no irrefutable clinical superiority of one ECM implant over another.
- 2)
Each ECM implant “functions” differently and may not be clinically interchangeable.
- 3)
Although clinical data with long-term results are necessary (recurrence and quality of life), it seems that the constant clinical message is that no ECM materials should be used in situations of serious infection (i.e. large bacterial inocula). It must be remembered that a serious infection can cause destruction of even the best natural connective tissues (i.e. necrotizing fasciitis).
In 2010, the Ventral Hernia Working Group (VHWG) published an incisional/ventral hernia grading system recommending interventions and type of prosthesis (including ECM implants) to use in different clinical scenarios.63 The authors themselves emphasize that their paper was based on the “best” evidence available and recognize its weaknesses. In addition, they mention that their grading system would benefit from a validated peer-reviewed study, and they state that the recommendations will be revised as evidence evolves. In our opinion, this system can be helpful for surgeons who want to use ECM materials, but right now it should not be treated as a guideline to follow when using or recommending the use of these materials.
For incisional/ventral hernia repair, there are reports of laparoscopic and open techniques with prostheses. Open techniques include: inlay, onlay or sublay. An inlay repair does not perform closure of the defect. Onlay or sublay repairs entail reinforcing a previous facial repair with the prosthetic material. Most laparoscopic techniques are inlay.
Currently, biological materials are used both for open onlay as well as sublay repair, and it is accepted that ECM IMPLANTS are not effective when used as inlays for repairing a hernial orifice with open surgery,28,64,65 the reason being that this requires previous closure of the defect in order to promote adequate cell infiltration and angiogenesis. Only studies with long-term results will be able to tell if this may limit the use of ECM implants in the laparoscopic repair of incisional/ventral hernias.
Clinical Experience With Extracellular Matrix-Derived Biological Implants in Inguinal HerniasIn the systematic review about the use of ECM implants published in 2009 and mentioned previously, 13 studies were included that referred to the use of xenogeneic biological materials in inguinal hernia repair. The data extracted from these studies are difficult to interpret due to their disparity, limitations in indications (although there are studies in contaminated situations, most are done in situations of clean surgery) and type of material (most of the studies are with small intestine submucosa), with limited use for the clinical decision-making process. Since the publication of the systematic review, other clinical papers about inguinal hernias have appeared.66–68 As mentioned for incisional/ventral hernias, we now believe that the use of ECM implants in inguinal hernia surgery should be done from the premise that they are not interchangeable materials in their clinical application, there is no superiority of one over the others and they should not be placed in cases of infections with large bacterial inocula.
Repair TechniqueThe current tendency for inguinal hernia repair is the incorporation of laparoscopic surgery, either using a transabdominal preperitoneal approach (TAPP) or a total extraperitoneal approach (TEP).69,70 In cases where a laparoscopic approach is indicated, current data do not clarify if ECM implants may be useful either now or in the future.66
SummarySynthetic mesh is clearly the material of choice in many abdominal wall surgeries.4,71 Clinically, there are data suggesting that ECM-derived material may be effective. Nonetheless, there are practically no clinical studies with maximum degree of evidence (level I), there is no consensus of how or when to use biological implants and there are no long-term data of the effects of their application (functional response/durability). From an experimental standpoint, the studies are good in general, but the parameters studied are occasionally redundant and the follow-ups are short, which make their interpretation and translation to daily practice difficult.
Given this situation, it may be reasonable to use biological implants only in selected cases where conventional synthetic mesh is contraindicated (immunocompromised patients, situations of contaminated surgeries or with chronic infections without large bacterial inocula). The use of ECM implants is not recommended in situations of infection with large bacterial inocula. The indications for their use may possibly expand, but prospective, randomized studies with long follow-up periods are necessary to determine the efficacy, applicability, risk/benefit relationship and quality of life derived from the use of these materials.72 Registries of their use will also provide data to be used as guidelines.73
With the current lack of strong scientific evidence, the selection of ECM implants for repairing abdominal wall defects is based on several factors, including: the type of hospital, economical resources available, type of wall defect, associated clinical scenario (cost-effectiveness), the progressive development of ECM implants themselves and, more importantly, the preferences of the surgeon, the surgeon's level of understanding and experience with these materials and, obviously, the preferences of the patient after detailed, up-to-date information is given about the different options.
FundingThis paper has been partially funded by the Spanish Ministry for Science and Innovation, Instituto de Salud Carlos III (PI10/01431).
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: López Cano M, Armengol Carrasco M, Quiles Pérez MT, Arbós Vía MA. Implantes biológicos en la cirugía de las hernias de la pared abdominal. Cir Esp. 2013;91:217–223.