Hepatic angiosarcoma is a rare vascular malignancy that accounts for 2% of all hepatic primary tumours. The diagnosis is difficult, especially if the patient does not have history of exposure to carcinogens, which are considered as risk factors. The diagnosis is made by histopathology, but in a considerable percentage it can only be accomplished by autopsy. The performing of fine needle aspiration biopsy can lead to bleeding, with limitations in its use.
Clinical caseA 41 year-old male, with no history of exposure to carcinogens, who developed abdominal pain secondary to a haemoperitoneum due to tumour rupture, was diagnosed by imaging methods with a giant cavernous hepatic haemangioma. He was initially treated with embolisation, and later with a liver transplant. After six months he developed haemoptysis secondary to lung metastasis. The autopsy reported metastatic hepatic angiosarcoma.
DiscussionThis condition has been related to carcinogen exposure, with malignant transformation from a benign vascular neoplasia being proposed as a hypothesis. The differential diagnosis can be achieved with imaging studies such as CT scan, and the definitive diagnosis is made by histopathology with immunohistochemistry tests, with 35–100% being made in the autopsy.
ConclusionHepatic angiosarcoma is a malignant vascular neoplasia, the potential curative option is surgery with tumour free margins. Liver transplantation remains controversial because of its poor prognosis in the short term.
El angiosarcoma hepático es un tumor vascular raro y maligno el cual representa el 2% de todos los tumores hepáticos primarios. La orientación diagnóstica es difícil, en especial cuando el paciente no tiene antecedente de exposición a carcinógenos relacionados como factores de riesgo. El diagnóstico es histopatológico, y en la mayoría solo se logra con autopsia ya que la toma de biopsia por aspiración con aguja fina conlleva riesgo de sangrado lo que limita su utilización.
Caso clínicoMasculino de 41 años de edad el cual no tiene antecedentes de exposición a carcinógenos. Comienza con dolor abdominal secundario a hemoperitoneo por rotura tumoral y es diagnosticado por estudios de imagen con hemangioma hepático cavernoso gigante, tratado inicialmente con embolización y posteriormente con trasplante hepático. Seis meses después se presenta con hemoptisis por metástasis pulmonares. En la necropsia se reporta: angiosarcoma hepático metastásico.
DiscusiónEl angiosarcoma hepático se ha relacionado con la exposición a carcinógenos, y continúa siendo una hipótesis su transformación a partir de una neoplasia vascular benigna. En el diagnóstico diferencial son útiles los estudios de imágenes, como la tomografía contrastada. Sin embargo, el diagnóstico definitivo es histopatológico con pruebas inmunohistoquímicas, que en el 35-100% solo establece en la autopsia.
ConclusiónEs una neoplasia vascular maligna, donde la cirugía tiene un rol potencialmente curativo cuando se practica resección con márgenes libres. El trasplante hepático es controvertido ya que tiene pobre pronóstico a corto plazo.
In recent reports liver angiosarcoma has been associated with thorium dioxide, arsenic powder and vinyl chloride as risk factors. However, there have been cases where no triggering factor has been found.1–3
Liver angiosarcoma is difficult to differentiate from cavernous haemangioma in radiological studies as liver haemangioma are major lesions, heterogeneous in appearance which may have intratumoral haemorrhaging and necrosis, leading to diagnostic confusion with malignant vascular tumours.4 Whelan et al.5 reported that one of the standard imaging characteristics of liver angiosarcomas is peripheral nodular enhancement in the late arterial phase. 2-Deoxy-2-fluoro-d-glucose positron emission tomography (PET) is useful for differentiating benign vascular tumours from malignant ones, especially those which are considerable in size.4–6
Risk of spontaneous rupture and bleeding of the angiosarcoma is documented in approximately 15–27% and has an effect on prognosis, since the beginnings of tumour cells are created. Furthermore, the spontaneous rupture of a liver haemangioma is rare and its frequency is calculated as under 1%, generally in large tumours located on the lateral or underside of the liver; when there is a ruptured liver tumour malignant aetiology should first be suspected; transcatheter arterial embolisation is the initial treatment.9
Due to a poor prognosis of patients with liver angiosarcoma, resection surgery is the only definitive treatment and although complete resection is possible, recurrence is highly frequent. The average survival rate for the majority of patients is 11 months.10,11
The natural history of liver angiosarcoma is little known. However, when it is diagnosed it is often already at an advanced stage with an average diameter of 15–65mm. Performing a biopsy can represent a challenge due to its vascular nature.12
When a small tumour is discovered with haemangioma characteristics follow-up with imaging studies is required, since if rapid growth presents liver angiosarcoma must be considered as a possible diagnosis.12,13 Mortality rate varies between 70% and 100%; Immunohistochemical staining confirms diagnosis, usually with the expression of endothelial markers which included factor VIII, CD31, CD34, Ulex europaeus agglutin I and vascular endothelial growth factor.14
Clinical caseA 41 year old male, with no prior family history of note. He stated that he did not drink alcohol, smoke tobacco, or take drugs nor did he have any tattoos. He presented with no chronic degenerative diseases, nor suffered from any allergies. His blood group was O+.
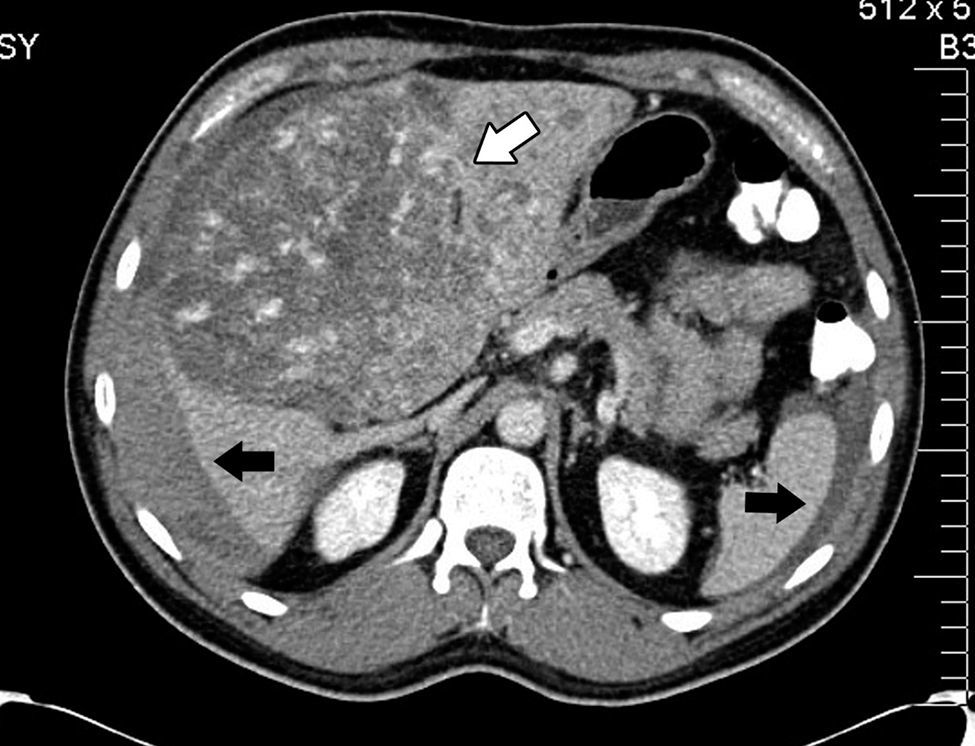
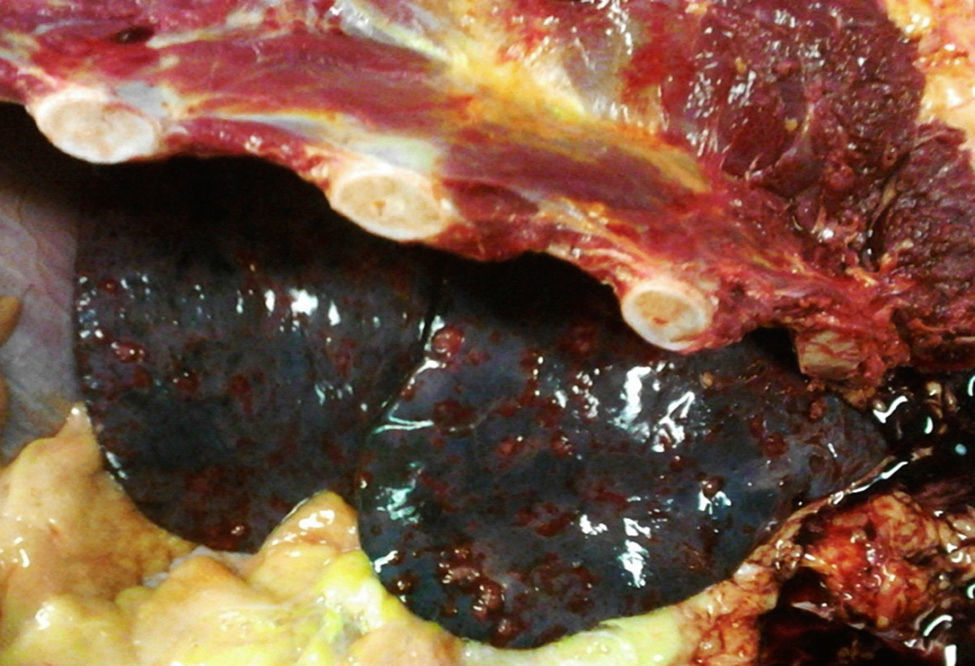
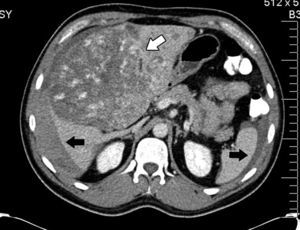
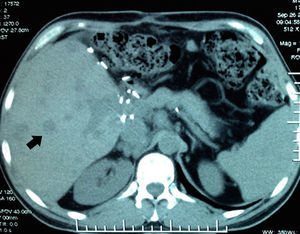
His condition began in March 2012 with sharp abdominal pain in the epigastrium which later spread to the whole abdomen. He presented with haemodynamic instability and therefore presented at the hospital in Houston, Texas, U.S.A. (his place of residence at the time), where massive intrabdominal bleeding was diagnosed secondary to cavernous haemangioma, bilobular in appearance (Fig. 1); angiographic embolisation was performed, with cessation of bleeding. When the patient had recovered an orthotopic liver transplant of the right lobe was performed, with blood type compatibility, with standard surgical reconstruction for the live donor in May 2012, in a private hospital in the city of after Guadalajara, Jalisco. Positive serology for CMV IgG for both the donor and the recipient was present. The patient was discharged 8 days after surgical procedure with immunosuppression therapy which included tacrolimus, mycophenolic acid and prednisone.
Abdominal axial CT scan with oral and intravenous contrast enhancement in arterial phase which shows liquid fluid in the abdominal cavity, predominantly perihepatic and perissplenic (black arrows) and a rounded tumour, heterogeneously contrasted, which occupies practically the entire liver (white arrow).
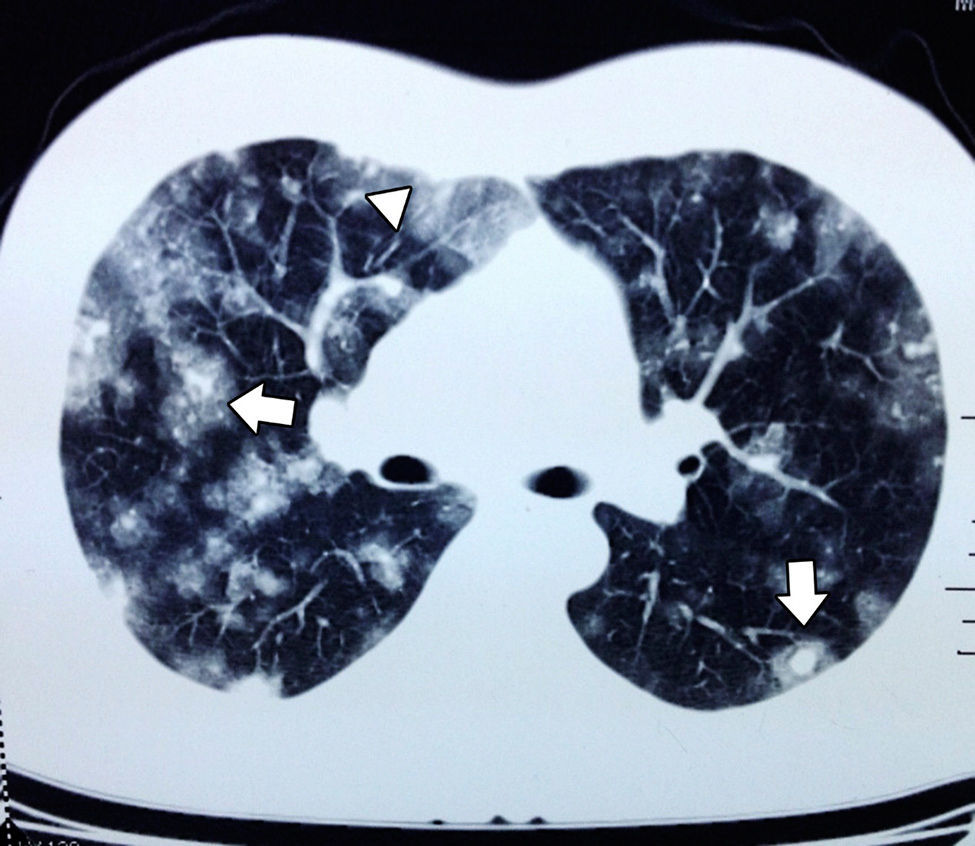
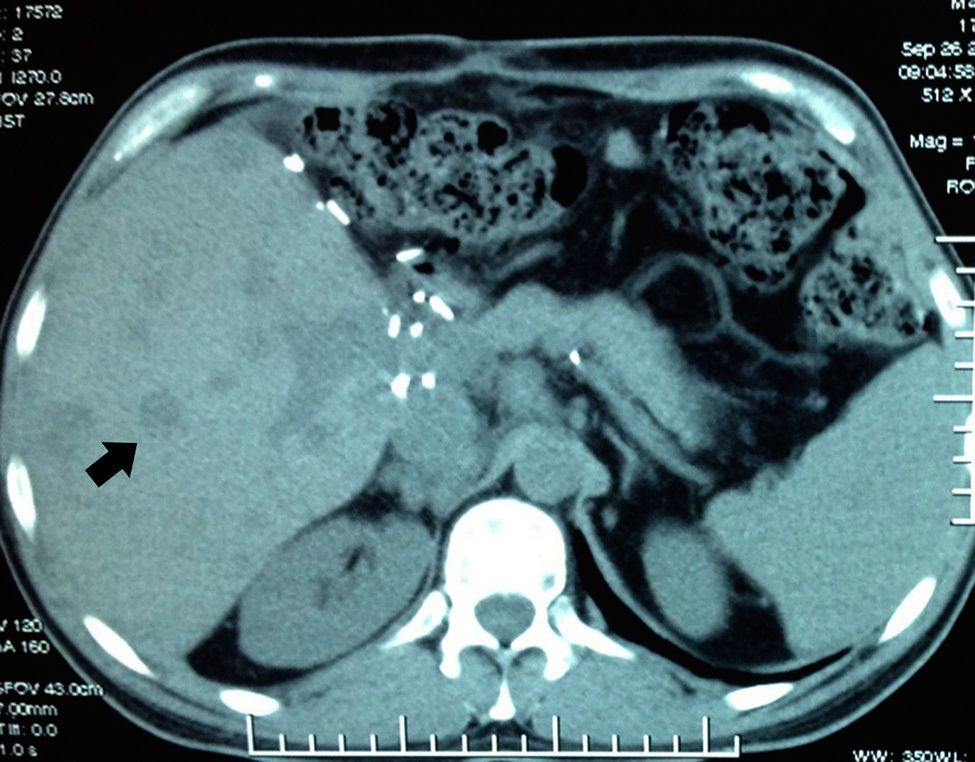
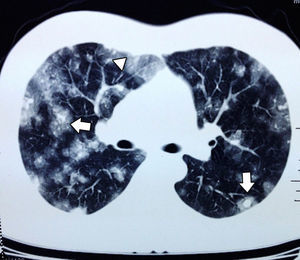
In September the patient presented with haemoptysis and dyspnoea, and was admitted to a private hospital, where a computed tomography (CT) chest scan was indicated (Fig. 2). A bronchoscopy was performed and cultures and biopsies were taken, which tested negative for malignancy, tuberculosis or fungal infection. Plain and contrasted CT scan of the abdomen was indicated which showed multiple, rounded lesions, hypodense in the liver graft (Fig. 3); CT scan-guided biopsy was performed which tested negative to malignancy.
The patient was admitted to our hospital in October 2012 to continue with a diagnostic and therapeutic approach; on admittance tegumentary paleness was noted, together with tachycardia, difficulty in breathing, active haemoptysis and asymptomatic abdomen.
Laboratory tests on hospital admittance revealed: leukocytes 10.7thousand/μl, red blood cells 5.6g/dl, haematocrit 20.3%, platelets 124thousand/μl, Na 127mmol/l, prothrombin time 14.2/12seg, INR 1.12, lactate dehydrogenase 1753U/l, total bilirubin levels 1.5mg/dl, direct bilirubins 0.3mg/dl. 3 globular packs were transfused and sodium replacement was initiated.
Bronchoscopy was again performed, identifying: vocal folds with traces of blood, oedematous and hyperemic bronchial mucosa, with no identification of compactions or growths at any level; biopsies and cultures of secretions were taken (testing positive to Pseudomonas aeruginosa, Streptococcus viridans). A skin test was indicated for PPD and histoplasmin, which was not reactive. The patient tested non-reactive to HIV and hepatitis B and C.
The Doppler ultrasound of the graft reported: preservation of venous and arterial structures, with normal pulse, focal lesion at liver parenchymal level with permeability of vascular structures. The patient's general status continued to deteriorate, and he presented with massive haemoptysis (>600ml/24h). Laboratory tests showed: increase in leukocytes to 19.2thousands/μl, with improvement of red blood cell levels compared with baseline ones at 10.2g/dl; total bilirubin increase to 2.2mg/dl, direct bilirubin at 0.8mg/dl, and lactate dehydrogenase at 3371U/l, alpha-fetoprotein at 0.7ng/ml. Difficulty in breathing persisted; the patient received intubation, which deteriorated into cardiopulmonary arrest and did not respond to cardiopulmonary resuscitation manoeuvres. He died on the 6th day after admittance.
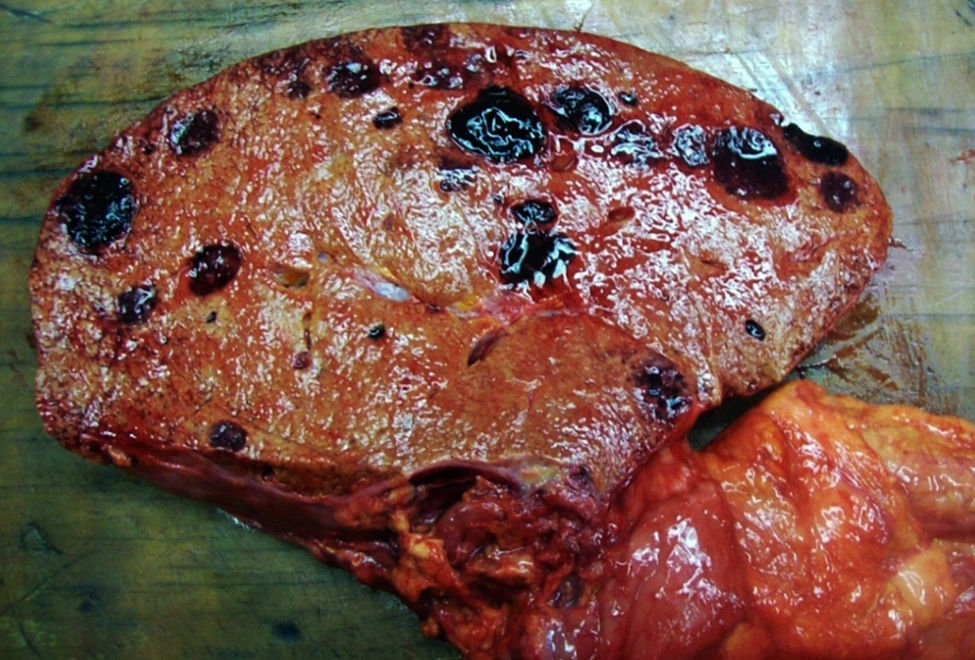
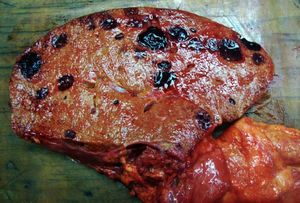
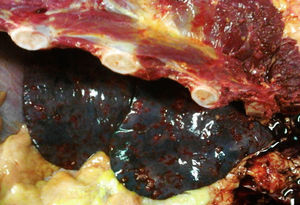
An autopsy was performed, where we observed rounded, vascularised tumours throughout the liver graft, involving the hepatic artery and portal vein (Fig. 4), in pulmonary parenchyma, multiple metastases of small tumours, with bleeding (Fig. 5). Metastasis was also present in the spleen, suprarenal glands and pancreas.
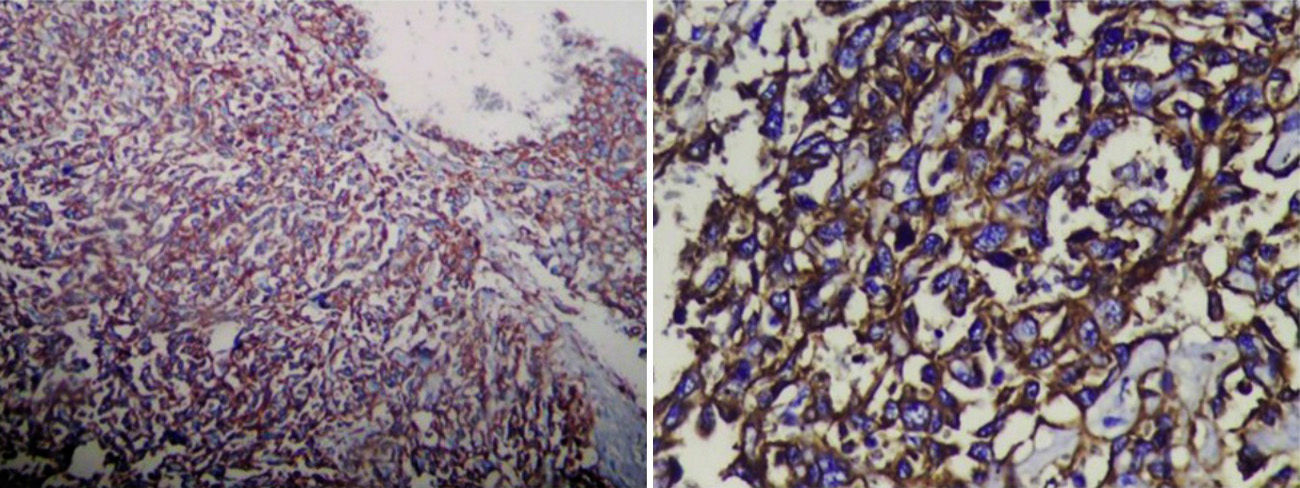
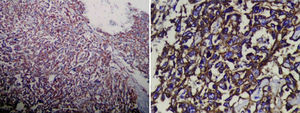
Histologically, the spindle cells were grouped into strands and invaded the vascular cavities, with several areas of necrosis and atypical nuclei. Immunohistological staining tested positive for CD31 and CD34, thus confirming the diagnosis of liver angiosarcoma (Fig. 6).
DiscussionLiver angiosarcoma is a rare malignant tumour which represents under 2% of primary liver tumours.1 Falk et al.2 reported a series of 168 cases between 1964 and 1974, of which 37 (22%) were associated with vinyl chloride but in the majority of cases no aetiological factors were found. Exposure to vinyl chloride is associated with mutations of genes K-ras and p53. The latency period between exposure and disease appearance may be between 9 and 35 years.3,4
Other carcinogens such as thorium dioxide and arsenic have been associated with its aetiology; however, exposure to these chemicals is rare and in the majority of cases there is no defined aetiology, there are reports of cases where an association was found with Schistosoma japonica, exposure to cyclophosphamide and the prolonged use of anabolic androgenic steroids.5 The hypothesis of a malignant transformation from cavernous liver haemangioma has been developed since 1991, with a case of a solitary encapsulated liver tumour, which showed a cavernous haemangioma surrounded by an angiosarcoma. There have been several reported cases of angiosarcoma developing from vascular malformations/haemangiomas, all of the patients being in their 60s and 70s. A study in 2006 reported that 83% of angiosarcomas had suffered the loss of one allele in chromosome 17p13 (p53), 66% in 13q14 (RB), 50% 11p13 (WT-1); in comparison, 60% of the haemangiomas had a loss of alleles in 17p13, 13q14, but only 20% in 11p13;6 however, association is not yet clear between benign vascular lesions and angiosarcoma.
Angiosarcoma is characterised by rapid growth, the majority of patients appear to be suffering from chronic liver disease. Abdominal pain, weakness, fatigue and weight loss are frequent symptoms with hepatosplenomegalia, ascites, jaundice and anaemia being the major clinical symptoms.6 Spontaneous rupture of liver angiosarcoma is reported in 15–27% of cases.7
Benign tumours such as cavernous haemangioma carry a low risk of rupture, under 1%, and malignant neoplasia should be suspected when there is a tumour rupture.5 When the clinical presentation is accompanied by haemoptysis pulmonary metastases should be the suspected diagnosis.8
There are no specific tumour markers for this type of tumour; Alpha-fetoprotein and CA 19-9 are generally at normal of slightly elevated levels. In imaging studies (tomography or magnetic resonance) liver angiosarcomas appear as multiple nodules, dominant masses or a lesion with diffuse infiltration.9
Tumour diagnosis is difficult, particularly if the patient has no history of any exposure to specific carcinogens; it is easy to confuse this type of neoplasias with cavernous haemangioma in imaging study findings.9–11 In CT scans some lesions usually have increased uptake which reflect the increase of the lesion vascularity, others are hypodense or normal. Imaging studies which mimic haemangiomas are usually attributed to image interpretation in a single (late) phase and with assessment established in relation to the hepatic parenchyma and not with vascular structures, such as the aorta or hepatic artery. The image of a solitary lesion is uncommon for angiosarcoma and the presence of multiple lesions is not typical of haemangioma.9–12
Angiosarcoma presents with early metastasis to other organs such as the lung, spleen or bone.10 In plain chest X-rays there may be diffuse infiltrations. The most common findings in CT chests scans indicated for angiosarcoma metastasis are multiple solid tumours. When they present with haemorrhaging they result in a ground-glass opacity imaging known as a halo sign and this occurs in 32% of patients. To a lower extent cystic changes may be found in the thin walls (13%) which when distributed in the pleural cavities increase the incidence of pneumothorax or haemothorax.12
The performing of fine needle aspiration biopsy makes diagnosis difficult and may lead to bleeding.12
Definitive diagnosis is anatomopathological and in 35–100% of cases is reached during autopsy.9–13 Mortality rates vary between 70% and 100%. Assessment must be made by an expert pathologist, since presentation varies from well differentiated angiosarcomas which form vascular channels that may mimic a benign tumour,13 to poorly differentiated ones where there is a presence of endothelial tumour cells which are epitheloid, rounded, or spindle-like in appearance and have prominent pleomorphic and hyperchromatic nuclei, with vascular space which are cavernous in appearance.14
Immunohistochemical staining is useful for confirming diagnosis, and usually expresses endothelial markers which include VIII, CD31, CD34, agglutin I Ulex europaeus and vascular endothelial growth factor.14
Differential diagnosis must be established with visceral Kaposi sarcoma, haemorrhagic hepatocellular carcinoma, vascular leiomiosarcoma, malignant epitheliod haemangioendothelimoa, diffuse metastatic processes and hepatic peliosis.15
Survival rates for patient with hepatic angiosarcoma are poor: the mean rate is 6 months with no treatment, and even with treatment there is a 3% 2-year patient survival rate. Standard treatment for liver angiosarcoma is surgical resection,16 although in under 20% of cases tumours are localised and resectionable. Liver transplant is controversial as treatment due to high recurrence and poor prognostic factors.17 In the majority of cases the tumours are not resectionable due to their size and the presence of metastasis. Liver angiosarcoma is resistant to radiotherapy. The majority of patients with metastatic angiosarcoma are treated with chemotherapy; however, no chemotherapy regimens have been established; several of the most commonly used agents are: doxorubicin, ifosfamide, gemcitabine, taxanes, imatinib, interferon, cisplatin, cyclophosphamide and irinotecan.18,19
ConclusionLiver angiosarcoma is a rare tumour which is difficult to diagnose and requires thorough analysis with the use of a series of imaging studies and a high index of disease suspicion. The only curative treatment option is early resection with negative resection margins and no metastasis. Moreover, liver transplant for this type of tumours is controversial due to the high rate of recurrence.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Huerta-Orozco LD, Leonher-Ruezga KL, Ramírez-González LR, Hermosillo-Sandoval JM, Sandoval-Alvarado JJ, Morán-Galaviz RE. Angiosarcoma hepático y trasplante hepático: reporte de caso y revisión de bibliografía. Cir Cir. 2015;83:510–515.