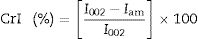To reduce the cost of obtaining bacterial cellulose, acidic by-products of the alcohol and dairy industries were used without any pretreatment or addition of other nitrogen sources. Studies have shown that the greatest accumulation of bacterial cellulose (6.19g/L) occurs on wheat thin stillage for 3 days of cultivation under dynamic conditions, which is almost 3 times higher than on standard Hestrin and Schramm medium (2.14g/L). The use of whey as a nutrient medium makes it possible to obtain 5.45g/L bacterial cellulose under similar conditions of cultivation. It is established that the pH of the medium during the growth of Gluconacetobacter sucrofermentans B-11267 depends on the feedstock used and its initial value. By culturing the bacterium on thin stillage and whey, there is a decrease in the acidity of the waste. It is shown that the infrared spectra of bacterial cellulose obtained in a variety of environments have a similar character, but we found differences in the micromorphology and crystallinity of the resulting biopolymer.
Bacterial cellulose (BC) attracts extensive attention due to its unique properties, such as its high degree of polymerization, high purity, good biocompatibility, biodegradability, high crystallinity and excellent mechanical properties.1–5 Its fiber has a high aspect ratio, with a fiber diameter of 20–100nm.6 As a result, it has a very high surface area per mass unit. This property of BC, in addition to its highly hydrophilic nature, results in very high liquid loading capacity. Therefore, BC is used extensively in many fields, including biomedical materials, drug delivery, tissue engineering, food industry, acoustic diaphragm, functional paper, optical displays, nanostructured biomaterials and biocomposites.7–16
Despite all the advantages of BC over polymers of plant origin, its production is a relatively expensive process, due primarily to the low productivity of known strains and the use of expensive culture media. During BC production, the culture medium represents approximately 30% of the total cost.7 Therefore, one important and challenging aspect of the fermentation process is identification of a new cost-effective culture medium that can facilitate the production of high yields within short periods of time.
Traditionally, glucose, fructose and glycerol were preferential carbon sources for cellulose production.17,18 To reduce the cost of BC biosynthesis, many researchers now suggest the use of the following media that include various waste products: wheat straw,19 spruce hydrolysate,20 wood hot water extracts,21 pineapple agroindustrial residues,22 fruit juices,23 rotten fruit,24 cotton-based waste textiles,25 molasses,26 waste from the dairy industry,24,27,28 wine fermentation waste broth,29–31 waste water of candied jujube-processing industry,32 waste and by-product streams from biodiesel and confectionery industries33 and acetone-butanol-ethanol fermentation wastewater.34
Thin stillage (TS) is a liquid byproduct that remains after microbial ethanol fermentation of carbohydrates by yeast and subsequent distillation of the fermented mash. TS contains salts, carbohydrates, proteins, and organic compounds. Wheat thin stillage (W-TS) is a by-product of ethanol production generated in large amounts in Russia. W-TS is rich in carbon sources such as glycerol, maltose and organic acids. It contains such sources of nitrogen nutrition as betaine and glycerophosphocholine,31 but its high acidity (pH 3.76–3.97)31 and high biological oxygen demand (up to 70g/L) make TS a disadvantage to distillery industries.
Researchers are currently studying the possibility of using stillage to increase the yield of BC in static conditions.29,30 Rice thin stillage (R-TS) – a wastewater from rice wine distillery that is rich in organic acids – was used to supplement the traditional HS medium for BC production. In the 50/50R-TS – HS medium, a BC concentration of 6.26g/L could be obtained after 7 days of static cultivation.30
Whey is a by-product of the manufacture of cheese or curd. It contains a large quantity of nutritionally rich components.35 BC formation on whey was also studied under static conditions.24,27,28 Carreira observed a low level of BC production (0.08g/L) on cheese whey.27 A low-cost medium based on the agricultural by-product soya bean whey was prepared and optimized for biocellulose production by Komagataeibacter sp. PAP1.28 According to their results, the optimal conditions for BC production were as follows: pH of 6.21 and ethanol concentration 1.61% (v/v). A maximum BC production of 4.10g/L was obtained on the seventh day of cultivation under static conditions.
Data for the study of BC production on thin stillage and whey without treatment and additional power sources in dynamic conditions are not available. The literature on the cultivation of strains of the species G. sucrofermentans on these wastes is also limited.
The aim of this study, therefore, was to investigate the production of bacterial cellulose by the strain Gluconacetobacter sucrofermentans B-11267 in agitated culture conditions using highly acidic by-products of the alcohol and dairy industries without any pretreatment or addition of other nitrogen sources.
Materials and methodsBacterial strain and culture conditionsThe bacterial culture of G. sucrofermentans B-11267 used in this study was isolated from Kombucha tea. 1mL of the suspension was withdrawn from the tea and added to a test tube with 9mL of 0.9% sodium chloride (w/v). Serial dilutions from 100 to 10−6 were prepared using sterilized saline solution. An aliquot of 0.1mL of each dilution was spread plated onto agar containing glucose (100g/L), yeast extract (10g/L), calcium carbonate (20g/L), and agar (15g/L). The plates were incubated at 28°C for 72h. The colonies with a clear zone around were selected and transferred to tube containing 10mL of Hestrin and Schramm (HS) medium. A cellulose positive strain was one that produced cellulose on the liquid medium.
For a more accurate identification of the isolated strain, base sequences were analyzed using the 16S rRNA complete sequencing method and their similarities were examined. 16S rRNA fragment was amplified by PCR from genomic DNA using 16S rRNA universal primers. The sequencing of amplified 16s rDNA fragments were performed in Research Institute for Genetics and Selection of Industrial Microorganisms (Moscow, Russia). A strain was deposited in the Russian National Collection of Industrial Microorganisms (VKPM) (Accession No. VKPM: B-11267).
For BC production, the following media were used: Hestrin and Schramm medium (HS) (g/L): glucose (20), peptone (5), yeast extract (5), citric acid (1.15), and disodium hydrogen phosphate (2.7), pH 6.0; cheese whey without pH adjustment, pH 4.96; thin stillage (TS) without pH adjustment, pH 3.95; TS with pH adjustment to pH 5.0 and pH 6.0. All culture media were autoclaved for 20min at 120°C.
The media were inoculated with 10% (v/v) inoculum. To prepare the inoculum, G. sucrofermentans B-11267 from an agar plate was transferred aseptically into a 250mL Erlenmeyer flask containing 100mL of culture medium and incubated on a shaker incubator (Model ES-20/60, BIOSAN, Latvia) at 28°C for 24h at 250rpm. BC was produced in 250mL Erlenmeyer flasks containing 100mL of culture medium on a shaker incubator at 28°C for 3 days at 250rpm. The experiments were conducted in triplicate, and the average of the results is reported.
Purification of bacterial cellulose and analysis of BC yieldAfter incubation, BC was collected, washed thoroughly with distilled water to remove medium components, and treated with 1% sodium hydroxide (w/v) solution at 80°C for 1h to eliminate bacterial cells. Further, BC was rinsed extensively with 6% acetic acid (v/v) and then with distilled water until the pH of the water became neutral. The purified BC was dried to constant weight at 105°C. BC production has been reported as gram dry weight of cellulose per liter of the medium (g/L).
AFM micrographs of BCThe surface morphologies of BC were studied by contact atomic force microscopy (AFM) using an SPM 9600 (Shimadzu, Japan). BC samples were air dried on a glass slide in the form of a thin film. A silicon nitride cantilever with nominal radius of pyramidal tip 2nm was used. Scan rates ranged from 0.6 to 1.0Hz/s. Image resolution 256×256 points were set.
HPLCThe amounts of sugars and monocarboxylic acids were quantified by high-performance liquid chromatography (HPLC) during cultivation of G. sucrofermentans B-11267 in thin stillage (TS) and whey. The control was the initial concentration of sugars and monocarboxylic acids in the media. Prior to HPLC analysis, all samples and standards were mixed with acetonitrile (ACN) (3:1, v/v), then placed into sealed tubes and centrifuged at 13,000×g for 5min. Monosaccharides and monocarboxylic acids were analyzed on Shimadzu LC-20A Prominence with refractometric detection using RID10A and a computer controller. The SUPELCOSIL™ LC-NH2 HPLC column (4.6×150mm) was used, and the mobile phase was ACN: DH2O (distilled water) (3:1, v/v) with a flow rate of 0.5mL/min. The injection volume was 20μL, and the column temperature was 40°C.
FTIR spectra of BCBC was freeze-dried and crushed into powder form, mixed with potassium bromide, and pressed into a small tablet that was subjected to Fourier transform infrared (FT-IR) spectroscopy using a Fourier transform infrared spectrometry (FT-IR) IRPrestige-21 (Shimadzu, Japan) in absorption mode. For each sample, 32 scans at a 4cm−1 resolution at wave numbers ranging from 4000 to 400cm−1 were collected.
X-ray diffractionX-ray diffraction (XRD) measurement was carried out to analyze the change in crystallinity of the produced BC by an X-ray diffractometer Empyrean (PANalytical, Netherlands) in the filtered radiation of the copper anode (λ=0.15418nm, 40kV b 30mA) within an angular range (2θ) from 10 to 60°. Two-coordinate detector Pixcel 3D working in the mode of linear scanning (255 pixels on a strip) with the resolution of 0.013 degree/strip was also used. The samples were freeze-dried using FreeZone Freeze Dry System (Labconco, USA). The crystallinity index (CrI) was calculated from the ratio of the height of the 002 peak (I002, 2θ=22.5°) and the height of minimum (Iam) between the 002 and the 110 peaks (Iam, 2θ=18°) (Eq. (1)).
Statistical analysisAll presented data are averages of at least three runs of experiments, performed with three to six replicates of the mean. Standard deviations of the mean were calculated using Microsoft Excel 2013 (Microsoft Corporation, Redmond, USA). The obtained data were statistically analyzed by Student t-test: two-sample assuming equal variances. The differences were considered significant at the level of p<0.05.
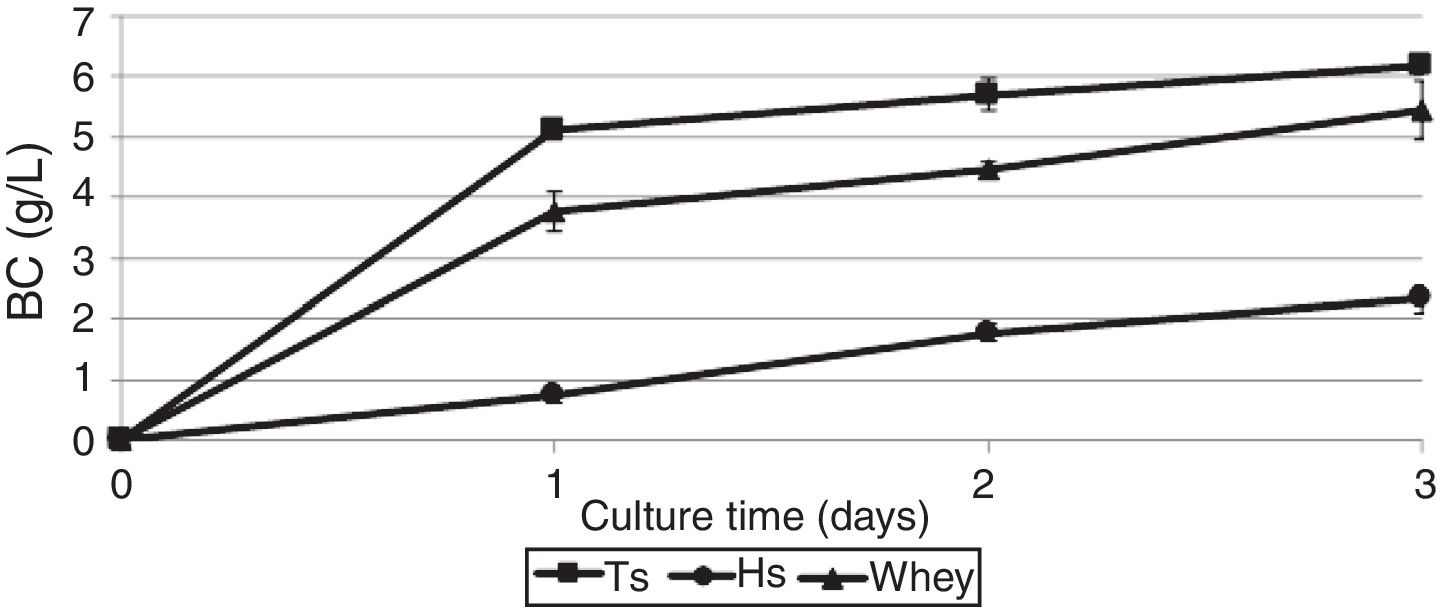
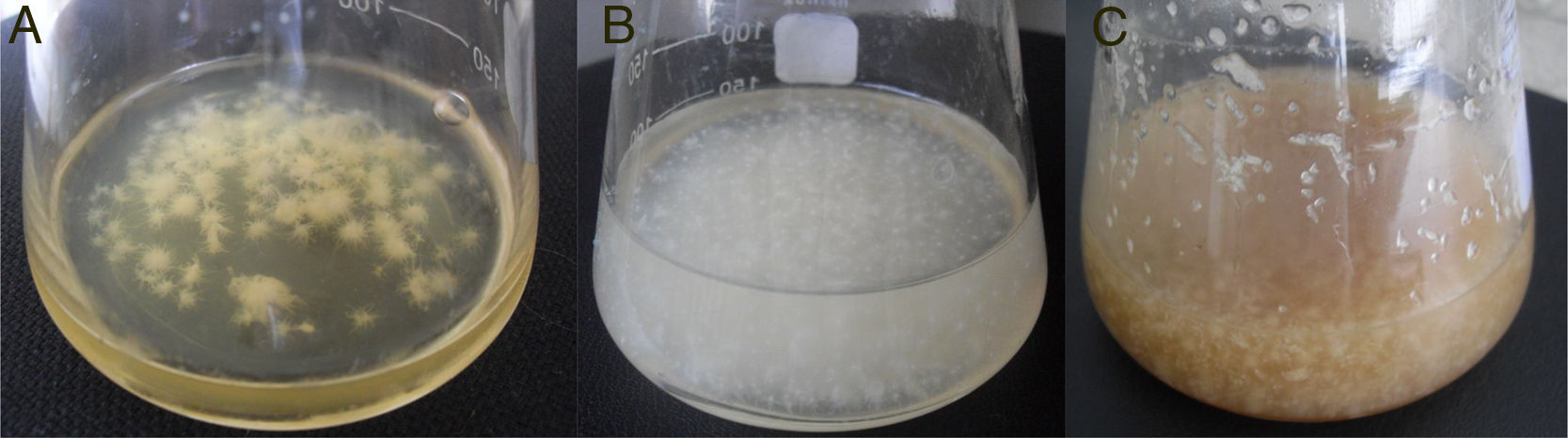
ResultsProduction of bacterial celluloseBC production by G. sucrofermentans B-11267 was investigated in agitated culture using thin stillage (TS) and whey without any pretreatment or addition of nutrient sources to reduce the manufacturing costs. HS medium was used for comparison.
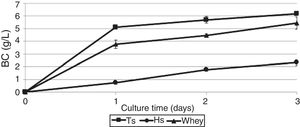
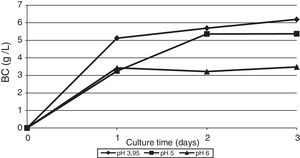
As shown in Fig. 1, the maximum BC yield (6.19±0.12g/L) was obtained after 3 days of cultivation on TS. This value was approximately 3 times higher than the yield on HS medium (2.14±0.02g/L). In the whey medium, a BC concentration of 5.45±0.09g/L could be obtained after 3 days of cultivation. The maximum rate of product formation on the waste occurs on the first day of growth of the bacterium.
For a deeper understanding of the growth of G. sucrofermentans B-11267 and BC production on the thin stillage and whey, we investigated the consumption of the primary sources of carbon that are part of these environments.
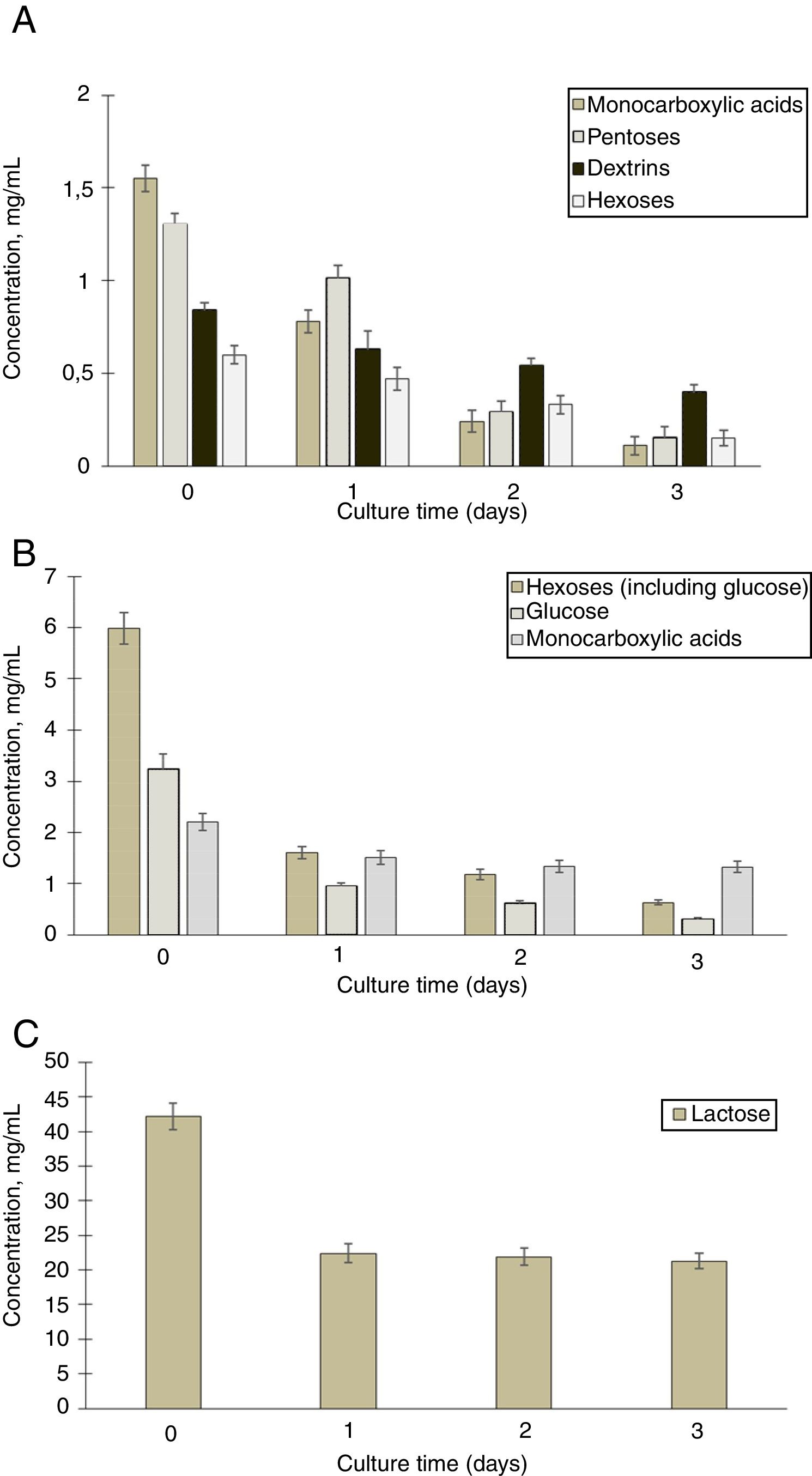
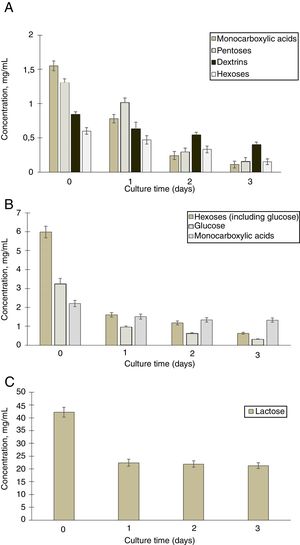
To detect the consumption of organic acids and sugars during cultivation of G. sucrofermentans B-11267 in TS and whey, HPLC analysis of samples was performed (Fig. 2A).
According to the data obtained after three days of bacterium cultivation in TS medium, there was intake of pentoses from 1.3±0.15mg/mL to 0.15±0.11mg/mL, hexoses from 0.6±0.13mg/mL to 0.15±0.10mg/mL, and dextrins from 0.84±0.12mg/mL to 0.4±0.10mg/mL. There was also a steady decrease in the concentration of monocarboxylic acids in culture from 1.55±0.15mg/mL to 0.11±0.10mg/mL. Therefore, within three days of cultivation of G. sucrofermentans B-11267 on the TS, almost full consumption of sugars and organic acids occurred.
We also investigated the consumption of sugars and organic acids in the process of cultivation of the bacterium on whey (Fig. 2B and C).
According to the presented data, the amount of lactose decreased from 42.12±1.91mg/mL to 22.4±1.33mg/mL during the first day of cultivation. Between one and three days, its concentration was almost stable. In addition, there was a decrease in the concentration of hexoses with increasing cultivation time of the bacterium. According to the presented data, after three days of bacterium cultivation, the amount of glucose decreased from 3.2±0.29mg/mL to 0.3±0.03mg/mL. Due to the presence of large amounts of available carbohydrates, the consumption of organic acids occurred to a lesser extent than in the previous example with stillage. During the first day of cultivation, their concentration decreased from 2.21±0.17mg/mL to 1.51±0.13mg/mL. The concentration of monocarboxylic acids was almost the same at a later stage.
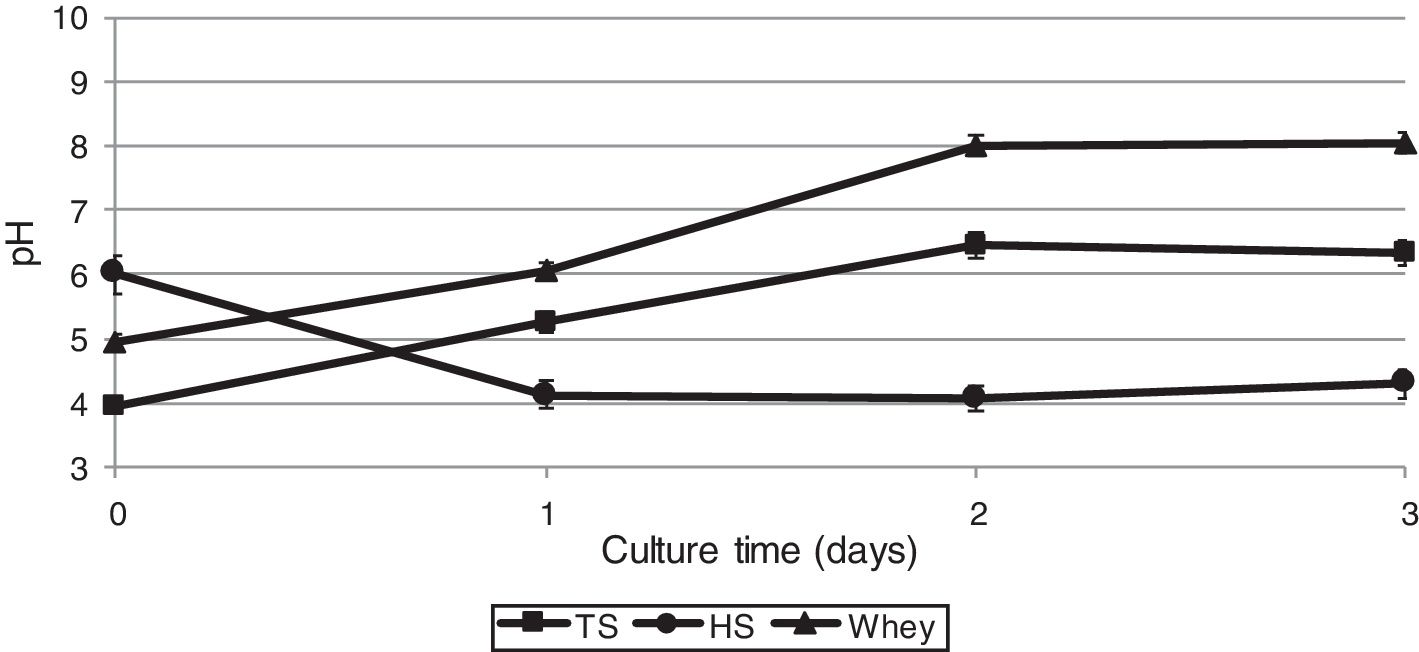
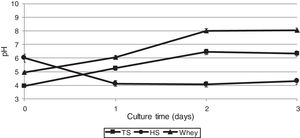
As biochemical reactions that occur in a culture medium are associated to a large extent with pH, we also studied the dynamics of changes in the acidity of the medium during the cultivation of G. sucrofermentans B-11267.
After 2 days of cultivating the bacterium on TS and whey, a pH increase was observed – from 3.95±0.02 to 6.45±0.01 and from 4.96±0.02 to 8.02±0.03, respectively (Fig. 3), which is most likely due to the consumption of organic acids of stillage and whey. The glucose culture (HS medium) gave the lowest pH of 4.0 on the 2nd day of cultivation, as the glucose dehydrogenase (GDH) of Gluconacetobacter could convert the glucose of the HS medium into gluconic acid, which also resulted in pH decrease.36
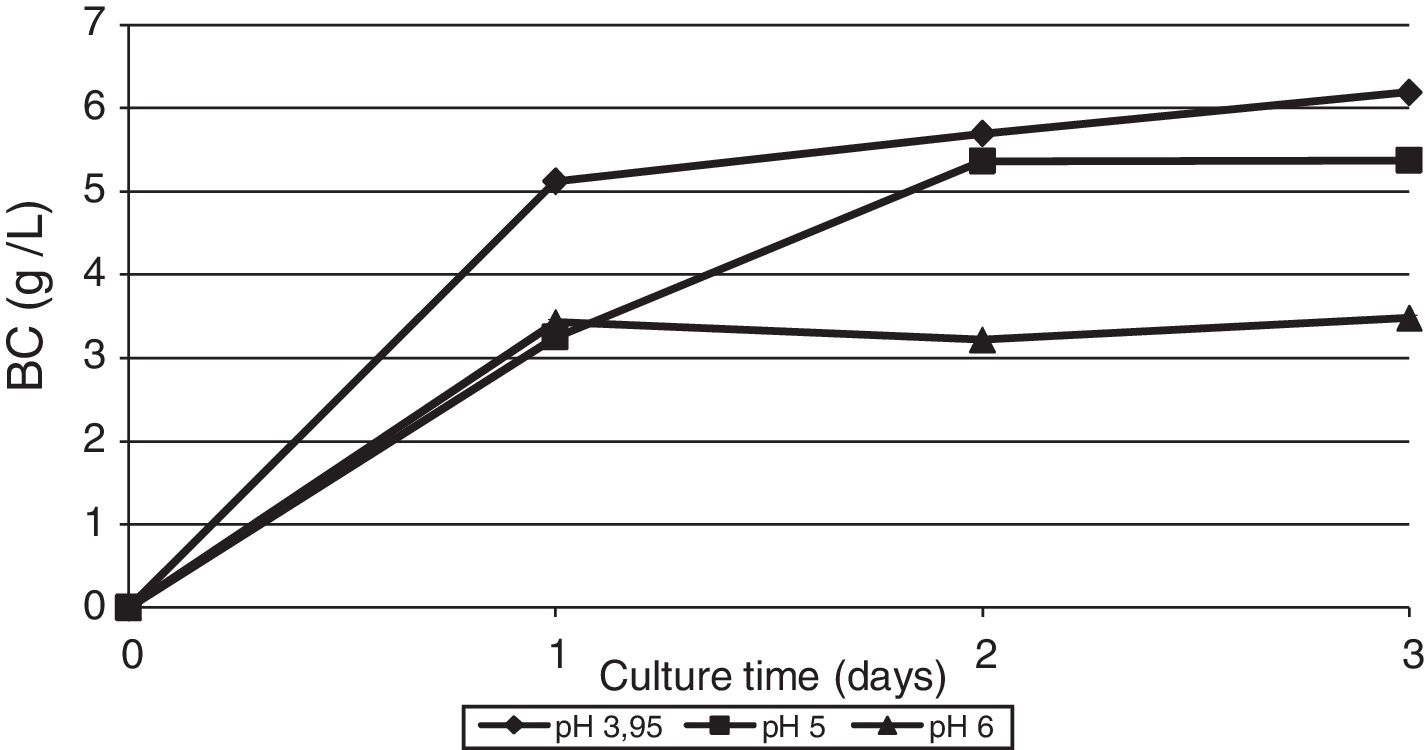
The effect of initial pH of the culture medium with thin stillage on cellulose production was investigated. When culturing the bacterium G. sucrofermentans B-11267 on TS, the highest BC production of 6.19g/L was observed at initial pH 3.95 (Fig. 4).
Structure of bacterial celluloseIt is known that the composition of the nutrient medium impacts not only performance but also the structure of cellulose.37
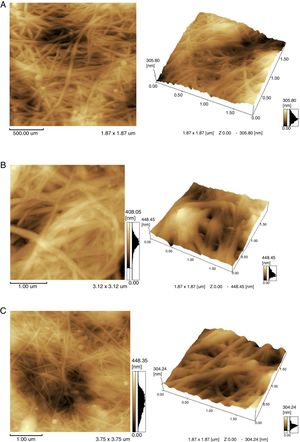
This paper shows that in the process of cultivating bacterium in dynamic conditions, agglomerates of cellulose with different shapes and sizes are formed (Fig. 5). It is found that the TS and whey, compared to standard HS medium, yield finer and homogeneous structures.
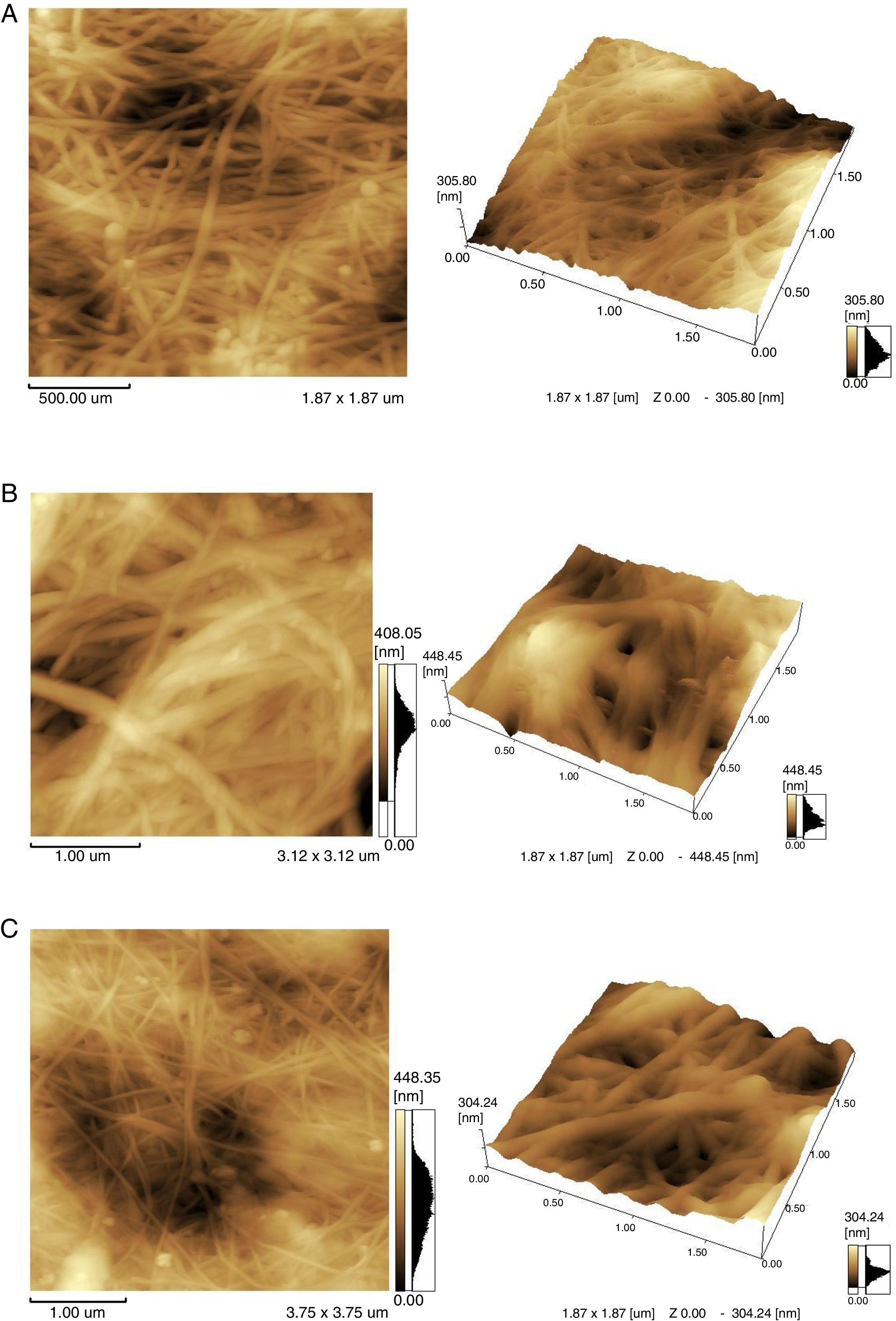
To study the microscopic details of the polymer, atomic force microscopy (AFM) was used. AFM imaging shows the biopolymer micro-morphology, topography and microscopic details of its surface. The outer part of the agglomerates was analyzed. The samples obtained in TS, whey and the classic environment HS were compared (Fig. 6).
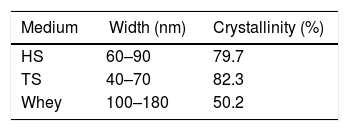
The morphology of BC samples from G. sucrofermentans B-11267 displayed nano-scale network structure (Fig. 6). BC generated in fermentation using wastewater had a more compact structure. There are also differences in the width of the microfibrils (Table 1). Narrow microfibrils of 40–70nm are formed on the TS, while more widely microfibrils of 100–180nm form on whey. The width of BC microfibrils formed on standard HS medium averaged 60–90nm.
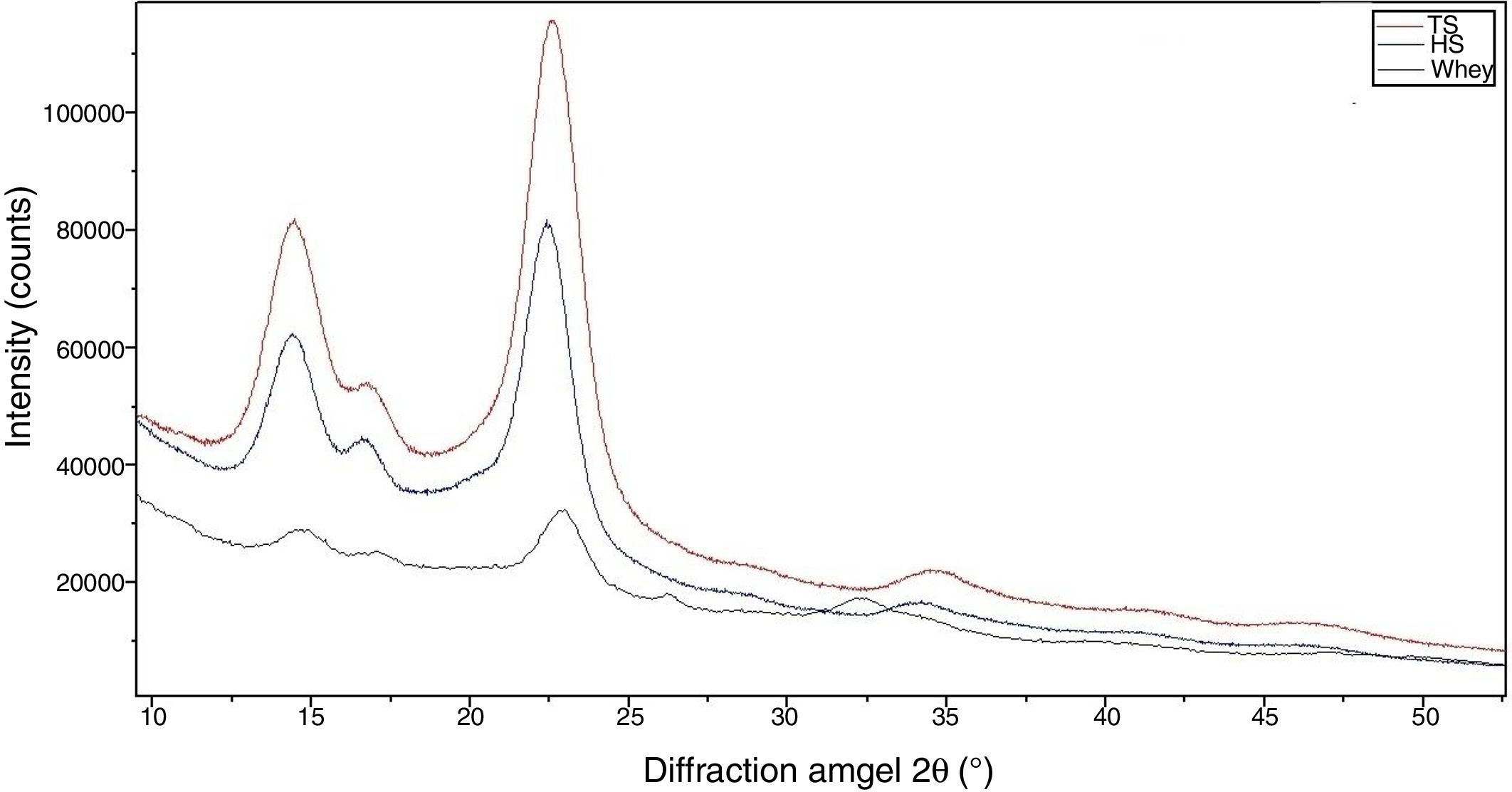
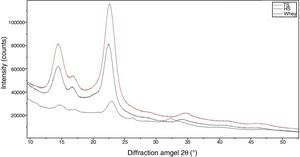
The morphological changes in BC can influence various properties and microstructures such as crystallinity. In order to evaluate the crystalline structure and the change in crystallinity of BC produced from different culture media, X-ray diffraction was used and the X-ray patterns of BC are exhibited in Fig. 7. The obtained X-ray patterns show cellulose with the same chemical structure but with different crystallinity degrees (Table 1).
The diffraction diagrams of BC produced by all BC samples showed three main peaks at 2θ 14.4°, 16.8° and 22.5° (Fig. 7), corresponding to the crystallographic planes of (100), (010) and (110), respectively.38 The intensity of the 100 reflection is larger than that of the 010 one when the film is parallel to the X-ray beam and the effect is reversed in the perpendicular orientation. This reveals a strong uniplanarity that is due to the fact that the cellulose ribbons are preferentially oriented parallel to the film surface during drying.38
The crystallinity index values of BC from TS medium (82.3%) were slightly higher than that of BC from HS medium (79.7%), which is similar to that reported in a previous study for BC obtained through the same process.30 The crystallinity index of BC was reduced (50.2%) when grown in whey.
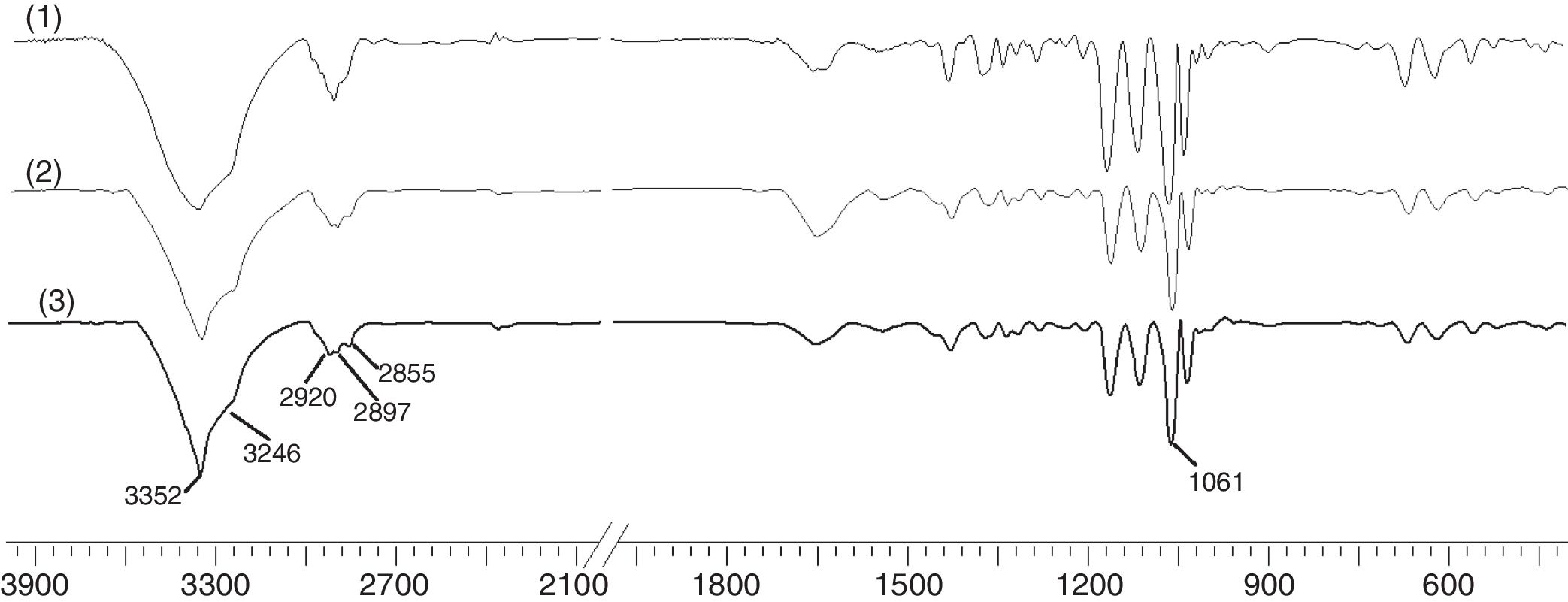
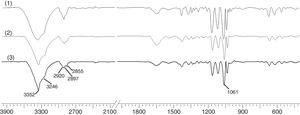
To study the chemical structure of BC, we analyzed FTIR spectra at wavelengths ranging from 400 to 4000cm−1. As shown in Fig. 8, the functional groups of BC samples obtained from fermentation using both wastewaters and HS medium were almost the same. The characteristic bands of cellulose (type I) appeared at 3352 and 3246cm−1 for the stretching vibration of hydroxyl groups (–OH), at 2897cm−1 for the asymmetric stretching vibration of methylene bridge (–CH2–), at 2855cm−1 for the symmetric stretching vibration of methyl (–CH3), and at 1061cm−1 for the C–O–C and C–O–H stretching vibrations of the sugar ring.34 The signals near 3240 and 750cm−1 were assigned to the triclinic Iα allomorph, and the signals near 3270 and 710cm−1 were assigned to the monoclinic Iβ form. Therefore, the synthesized cellulose contained both allomorphs.
DiscussionIn this study, the highest BC yield of 6.19g/L was observed using TS, which is almost 3 times higher than on standard HS medium (2.14g/L). The use of whey as a nutrient medium makes it possible to obtain 5.45g/L BC under similar conditions of cultivation. Fermentation on the TS and whey influenced the micro-morphology and crystallinity of BC but exhibited no effect on its chemical structure.
As previously reported, BC is made of subfibrils of 1.5nm assembled into nanofibrils of 2–4nm (but up 25nm) width composed of 10–250 single polymeric chains and 1–9nm length equivalent from 2000 up 18,000–20,000 glucose units organized into nanoribbons of 40–60nm width.8 In this study, the synthesized microfibrils are clearly ribbon-shaped with a width ranging between 40 and 90nm for HS medium and TS. Such observations agree with the typical morphology reported for BC.8 More widely microfibrils are formed on whey.
Furthermore, the crystallinity index of the BC from TS is higher than that from other media. This reveals that TS can affect the aggregation and crystallization of BC microfibrils and increase the crystallinity index.
Stillage contains organic and inorganic compounds, some of which may be valuable fermentation coproducts.31 Ratanapariyanuch et al. used HPLC to analyze the components of TS.31 They found that the major components in wheat thin stillage were dextrin (8.47–11.65g/L), glycerol (2.39–7.87g/L), lactic acid (5.07–7.41g/L), acetic acid (0.56–2.72g/L), succinic acid (0.63–0.93g/L), ethanol (0.23–1.31g/L), maltotriose (0.14–1.10g/L), maltose monohydrate (0.03–1.05g/L), glycerophosphorylcholine (0.91–1.11g/L) and betaine (0.8–1.03g/L).31
Whey contains lactose (approximately 70% of all dry substances). In addition to lactose, other carbohydrates, such as glucose, galactose, lactulose, and arabinose, are present in whey in small amounts. The high biological value of whey is due to proteins (mainly albumins and globulins), amino acids, vitamins, and organic acids (lactic, citric).35
It is known that the choice of carbon source and its quantity is one of the main factors in influencing the BC formation.5,17,18
According to research literature findings, organic acids have a positive effect on BC biosynthesis.36 Thus, the efficiency of its formation is enhanced when the medium contains acetic acid, which reduces the formation of gluconic acid.37
Acetic acid is one of metabolized products in the metabolic network of G. xylinus.37 It can be transformed into acetyl-CoA and metabolized in tricarboxylic acid (TCA) cycle to generate ATP. In addition, acetic acid can be used in the pathway of gluconeogenesis, increasing glucose availability.39
There are findings that state the positive influence of lactic acid on the synthesis of the BC.40 Lactate stimulates the growth of cells at an early stage of culture development.40 It is assumed that it acts as an accelerator of the Krebs cycle, resulting in the increased formation of cellulose and cell growth.40
The significant effect of succinic acid on BC production enhancement has been reported previously.29,30 In addition to succinic acid, other organic acids in TS such as gluconic acid, citric acid and malic acid are also beneficial for BC production enhancement.29,30 These organic acids are all located in the pathway of TCA cycle.
According to literature, the pH of the culture medium is a critical factor for BC productivity.41–43 Previous studies demonstrated that the range of pH value for BC production was approximately 4–7 and the optimum pH for BC production varies with the bacterium strains, but had usually been attributed to be within a neutral to slightly acidic pH range.5,41,43,44
A reasonable question is therefore if similar conditions favor BC production in the presented system. TS used in this research was acidic (pH 3.95) due to the presence of organic acids. This result was consistent with the work of Ratanapariyanuch et al., who reported that the range of pH of W-TS was 3.76–3.97.31
When using TS, the maximum BC production of G. sucrofermentans B-11267 reached 6.19g/L when initial pH was 3.95. The availability of organic acids in the waste that contribute to its high acidity also has a positive impact on the formation of biopolymer. The initial pH for maximum cellulose production was distinctively low compared to the pH typically used in incubation with bacteria of this genus. Overall, the strain G. sucrofermentans B-11267 is acid-resistant and can be used sustainable in fermentation processes for BC production using acidic food industry by-products.
Several attempts have been made to harvest or engineer strains that are resilient to low pH media during BC biosynthesis. Castro et al. reported that a bacterial strain isolated from the fermentation of Colombian homemade vinegar, Gluconacetobacter medellensis, is highly tolerant to low pH: an optimum yield of 4.5g/L was achieved at pH 3.5, which is generally too low for other bacterial species to function.38 Kim et al. reported that cellulose producing bacterial strain Gluconacetobacter sp. gel_SEA623-2 isolated from citrus fruit juice fungus is also highly tolerant to low pH. In order to investigate the effect of initial pH on BC production, SEA623-2 was cultivated in the biocellulose production medium with different pH levels (2.5–5) under static culture. The optimum pH for BC production was 3.5.45
Recently, it has been reported that BC can be synthesized under alkaline environment.44 A bacterial strain isolated from fermented fruit juice, Komagataeibacter intermedius FST213-1, can produce BC within pH 4–9 and exhibit maximum BC production (1.2g/L) at pH 8 in short-term (4-day) cultivation.44
Changes in pH can indicate the biochemical reactions occurring in the culture.43 BC synthesis is an energy-dependent process, and therefore, in HS medium with only glucose as energy source, the oxidation of glucose to gluconate prevails over the synthesis of BC. The formation of gluconic acid subtracts the carbon skeleton (glucose) from the BC production.46 Furthermore, the development of gluconate significantly decreases the pH of the medium and this is an additional limitation to the BC production.
Culturing the bacterium G. sucrofermentans B-11267 on TS and whey, a pH increase was observed, which is most likely due to the consumption of organic acids of stillage and whey. The concentration of monocarboxylic acids in TS medium after 3 days of cultivation was negligible, suggesting that they were metabolized via TCA cycle or that the formed oxaloacetate was decarboxylated into pyruvate that is used by the gluconeogenesis pathway to produce glucose, as previously stated for K. xylinus.39
This result was consistent with the work of Hwang et al., who stated that during the cell growth/cellulose production phase, cellulose production was accompanied by cell growth and consumption of the gluconic acid.47 At 35h, the cell mass and cellulose concentration were maximal and the pH of the culture broth increased from the lowest value of 4.3 to 6.5. In the stationary phase, however, gluconic acid continued to be consumed without further production of cellulose, and the pH of the culture broth increased continuously from 6.5 to 7.0. It was considered from this result that the culture medium must contain a certain level of gluconic acid for cellulose production. However, further study will be needed to clarify this point.
ConclusionOn the basis of the obtained results, it can be concluded that acidic by-products of the alcohol and dairy industries, such as thin wheat stillage and whey, are a promising substrate for obtaining BC. These low-cost by-products can significantly contribute to the reduction of production costs. Furthermore, BC can be produced by employing thin wheat stillage to achieve high yields and quality of the product. Remarkably, the strain G. sucrofermentans B-11267 investigated here yields a large amount of BC in low pH media, indicating its tolerance to acidic environments while optimally producing cellulose. Such situation is highly desirable in industrially relevant fermentation processes for BC production with the added advantage that it limits microbial contamination. Thus, the results of this study present an excellent viewpoint to produce a nanomaterial with different possible applications by using acidic food industry by-products.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Dr K.N. Nishchev and Dr V.M. Kyashkin for help in AFM and XRD studies.