The synthesis of Fe3+, Mo4+ and Y3+ fully stabilized zirconia by the nitrate/urea combustion route and thermal stability in air was investigated. The solid solution obtained was characterized by X ray diffraction (XRD), scanning electron microscopy (SEM) and used the BET method for determining specific surface. The ceramic powders obtained were calcined at 1473K in air atmosphere in order to determine their thermal stability. The scanning electron microscopy (SEM) results showed a homogeneous grain surface, measuring several tens of micrometers across. The crystallographic study revealed that by this method it was successfully achieved zirconia doped with Fe3+, Mo4+ and Y3+ ions in the zirconia tetragonal monophase, even after calcinations.
Se investiga la síntesis de zirconia totalmente estabilizada, incorporando los iones Fe3+, Mo4+, Y3+ por la ruta de combustión nitrato/urea, así como su estabilidad térmica en aire. La solución sólida obtenida se caracterizó por difracción de rayos X, (DRX), microscopía electrónica de barrido (SEM) y se determinó la superficie específica por el método BET. Los polvos cerámicos obtenidos se calcinaron a 1473K en atmósfera de aire con el fin de determinar su estabilidad térmica. Los resultados de microscopía electrónica de barrido (SEM) mostraron granos con una superficie homogénea, de varias decenas de micrómetros de diámetro. El estudio cristalográfico reveló que por este método se logró con éxito obtener zirconia dopada con iones Fe3+, Mo4+, Y3+ en la monofase zirconia tetragonal, incluso después de la calcinación.
Zirconia powder (ZrO2) is a very important technological material with a wide variety of applications due to their excellent refractory properties, good chemical stability and high mechanical strength. Zirconia occurs in three different polymorphic forms: monoclinic m-ZrO2 (RT), tetragonal t-ZrO2 (≈1463K) and cubic phase c-ZrO2 (≈2643K). This polymorphism is accompanied with volume changes that restrict the use of pure zirconia powders for several applications [1]. Stabilized zirconia has many mechanical, electronic, biomedical and chemical applications. It is well know that some cations (e.g. Ca2+, Mg2+, Y3+ and some rare earth cations) from zirconia solid solutions stabilize the tetragonal or cubic phase. The influence of transition metals Co2+ and Fe3+ as stabilizers was studied early [2,3]. These works demonstrated that solubility in the stabilized solid solution begins to decrease at temperatures around 1148K and 898K respectively, causing the progressive transformation into the monoclinic zirconia phase. These relatively limited thermal stability, have led to introduce the additional incorporation of yttrium ions. The Y2O3–ZrO2 system has been studied extensively by Yashima et al. [4]. They identify two forms of the tetragonal phase that they call q′ a form close to the monoclinic form and q″ which is close to the cubic phase. In general, a content of between about 3 and 8mol% of Y2O3 in the zirconia (i.e. 5.8–14.87mol% YO1.5) stabilizes, more or less strongly, the tetragonal phase. Stabilization of cubic and tetragonal forms is gaining importance because of its excellent thermal stability, chemical resistance, mechanical properties and oxygen conductivity. The preparation of high-quality yttria-stabilized zirconia (YSZ) is important to the fabrication of high-performance solid-state ionic devices such as solid oxide fuel cells (SOFCs) and gas sensors. The incorporation Fe3+ ions in the zirconia lattice were investigated using several preparation methods. Davison et al. [5] studied the formation of solid solutions between α-Fe2O3 and ZrO2 using the nitrate decomposition and calcinations route. After calcinations in air at 873K, they found a tetragonal solid solution for 2.5 and 5at% Fe3+. For higher iron contents, hematite is also detected. Berry et al. [6,7] used a similar route and showed that the incorporation of Fe3+ ions within t-ZrO2 lattice at 773K stabilizes the cubic form and inhibits its transformation into the monoclinic form. The incorporation of Fe3+ and Mo2+ ions in the zirconia and its possible role in the stabilization of tetragonal or cubic structure is not mentioned in the literature.
The interest in studying zirconia-supported molybdenum oxide is mainly due to their performance as catalysts [8–12]. Powder synthesized by MoO3/ZrO2 with amounts of Mo varying from 1 to 12wt% prepared by incipient wetting, subsequently dried and calcined in air at 773K for 6h was reported by Bhaskar et al. [8]. Brown et al. incorporate molybdenum amounts corresponding to 2.5 and 10wt% MoO3/ZrO2, prepared by coprecipitation and calcination in static air at 1073K for 12h [9]. They reported that the addition of higher amounts of MoO3 increased the proportion of t-zirconia, but the powder was not fully stabilized. The incorporation of wt 11% Mo by impregnation, calcined in air at 873K for 3h, produces only t-ZrO2[10,11]. The Mo/ZrO2 and CoMo/ZrO2 catalysts were prepared by impregnation and calcination at 673°C by Kaluža et al. [12]. The authors achieve high hydrodesulphurization with the CoMo/ZrO2 solid solution. Recently, Samantary et al., demonstrated that t-ZrO2 could increase the efficiency of catalysis. They obtained fully t-ZrO2 powders by solution combustion method, using glycine as fuel, when 5 or 10wt% Mo was incorporated [13–15]. In the present paper, it is proposed to investigate this topic by preparing a stabilized Zr0.86Y0.1Fe0.02Mo0.02O1.94−δ solid solution using the nitrate/urea combustion route, which uses low cost reactants, produces gram-scale quantities of powders at laboratory scale and can be readily scaled up. The aim to elaborate stable solid solutions to high temperatures is to have a raw material in order to study the production of a composite Zirconia-Metal-CNT. In a previous work, has been reported the synthesis of composite powders containing well dispersed carbon nanotubes by selective reduction in H2±CH4 of oxide solid solutions between a nonreducible oxide such as Al2O3 or MgAl2O4 and one or more transition metal oxide(s). The reduction produces very small transition metal (Fe, Co, Ni and their alloys) nanoparticles at a temperature of usually >800°C. Quantities smaller to 0.01% of Mo and Fe as catalysts have been efficient to produce CNTs in situ solid solutions as MgO, SiO2, Al2O3 but never with ZrO2[16,17], due his polymorphism. In a second part of the study, the thermal stability in air of a t-Zr0.86Y0.1Fe0.02Mo0.02O1.94−δ solid solution has been investigated.
ExperimentalThe combustion method was adapted from that described by Patil [18,19] and applied to obtain a lot of solid solutions [2–4,18–26]. The ceramic powders ZrO2, Zr0.96Fe0.02Mo0.02O1.94−δ and Zr0.86Y0.1Fe0.02Mo0.02O1.94−δ were synthesized. However, for the sake of simplicity, the so-obtained powders will hereafter be denoted as ZrO2, ZF2M2 and ZYF2M2 respectively. The appropriate amounts of ZrO(NO3)2·4H2O, Y(NO3)3·6H2O, Fe(NO3)3·9H2O and (NH4)6Mo7O24·4H2O were dissolved in deionized water (20mL, 343K). The syntheses were made by combustion under the conditions developed above, that is to say, with a proportion of urea n=7, in order to produce 5g of solid solutions. All samples were synthesized in the same conditions. Combustion products were calcined in air at 873K for two hours to remove traces of carbon. The urea proportions were calculated by considering the total oxidant valences (VO) and reducing valences (VR) of the different species [18,27]. The total oxidant valences of the nitrates is VO=10.26. The reducing valence of urea is VR=6. The molar quantity of urea is first calculated so that the so-called stoichiometric ratio φ=mVR/VO is equal to unity, which gives m=1.71. Was studied early the synthesis of Fe3+-stabilized zirconia by the nitrate/urea combustion route [3]. These work determine the appropriate amount of urea is m=6–9 that allows to obtain a totally iron-stabilized nanocrystalline ZrO2. In this work m was fixed=7. However, as discussed by Zhang and Stangle [22], this method involves some approximations. Firstly, O2 from the air atmosphere is not taken into account, which affects VO. Secondly, the nitrogen-containing products are considered as being only N2 (thus, zero is taken as the valence of nitrogen element) despite that nitrogen oxides and/or NH3 can be formed in usually undetermined proportions. This affects both VO and VR. The container with the solution was placed in a furnace pre-heated at 873K, keeping the door of the furnace open. After water evaporation, a combustion reaction takes place according to a redox reaction between the nitrates and urea, producing an oxide powder (hereafter named as-prepared powder).
Batches of 80mg of the solid solutions powder were calcined by thermogravimetric (DTA, Netzsch 404S) equipment in flowing air (4.8Lh−1) at 1473K. A heating and cooling rate of 1473Kh−1 was applied (no dwell time at the target temperature). X-ray diffraction (XRD) patterns of all materials were recorded using Cu-Kα radiation (l=0.15418nm). The carbon content for the powders was determined by flash combustion with an accuracy of ±2%. The BET specific surface area of the materials was measured using N2 adsorption at liquid N2 temperature in a Micromeritics FlowSorb II 2300 apparatus (the reproducibility of the results is ±3%). The composite powders were studied using field-emission-gun scanning electron microscopy (FEG-SEM, Hitachi S4500, operated at 5kV or 8kV).
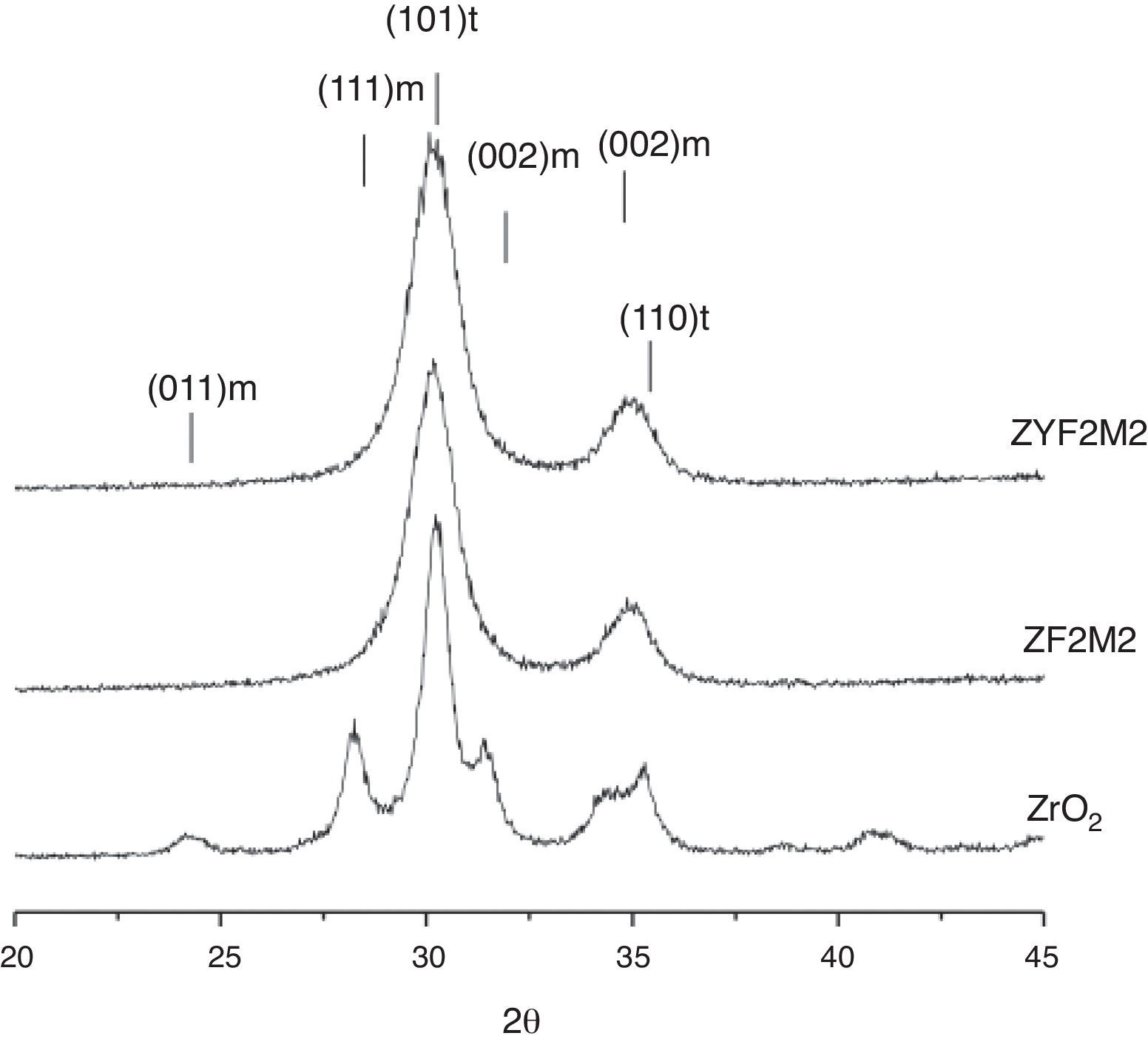
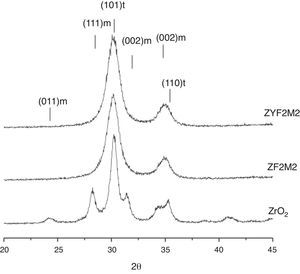
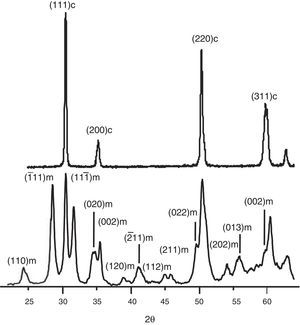
Results and discussionThe X-ray diffraction patterns of the solid solutions are shown in Fig. 1. The pure zirconia clearly reveals signals of t-ZrO2 and m-ZrO2. The others solid solutions are completely stabilized, only peaks of the tetragonal structure are shown. These observations indicate for stabilize the t-ZrO2 is necessary the presence of Y3+ or Fe3+/Mo4+ ions. We have shown in early works, the synthesis of Fe-ZrO2 powders by combustion method obtaining t-ZrO2 phase; this was determined by several characterization techniques, notably Mössbauer spectroscopy [3,22]. Nevertheless, is possible to distinguish the (101) and (110) peaks of ZF2M2 powders which appears to be slightly shifted to low 2θ angles compared with ZYF2M2 solid solution, this probably reveal that the corresponding inter-reticular distance is lightly higher. Additionally, for the stabilized solid solutions, is not detected any phase of molybdenum or iron oxide, so that could be considered that Y3+, Fe3+ and Mo4+ ions are in the zirconia lattice fully stabilized.
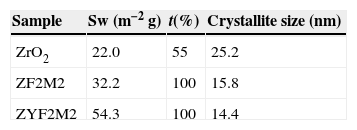
The DTA curve (not shown) revealed weak endothermic peaks at about 923, 1173 and 1423K (the latter extremely weak) during heating and a sharp exothermic peak at about 973K. Kiminami [23] reported weak and broad peaks during heating, indicating a low transformation rate, for a powder with 1mol% α-Fe2O3 and no observable peaks for 10mol%. It was verified that the prepared powder contain only trace amounts of carbon. The BET specific surface area Sw, and proportions of t-ZrO2 are shown in Table 1. The proportion of the tetragonal phase with respect to monoclinic phase was calculated by the Gravie-Nicholson method [28]. The BET specific area of the powders containing element yttrium, iron and molybdenum is the highest. Higher Sw could reflect a smaller grain size due to both stronger nature of the combustion process experimented for the solid solutions containing yttrium. Furthermore, when a low valency dopant cation, such as Y3+, Fe3+, is introduced into the ZrO2 lattice, oxygen vacancies are created for the charge balance and generation of positive holes with lattice defects. These lattice defects can be cause a small crystallite size. These data were compared to the XRD average crystallite size calculated by applying the Scherrer formula on the tetragonal (101) Bragg peak. One can note that the size evolution is also in good agreement with the Sw results. No attempt was made to calculate a size based on the Sw results because the hypothesis of non-agglomerated particles is not verified in the present powders.
Surface specific results for the solid solutions synthesized. Tetragonal proportion (t(%)) is the proportion of tetragonal phase evaluated from the XRD patterns (balance is monoclinic zirconia [29]). Average crystallite size determined from the XRD pattern using the FWHM of the tetragonal (101) Bragg peak.
| Sample | Sw (m−2g) | t(%) | Crystallite size (nm) |
|---|---|---|---|
| ZrO2 | 22.0 | 55 | 25.2 |
| ZF2M2 | 32.2 | 100 | 15.8 |
| ZYF2M2 | 54.3 | 100 | 14.4 |
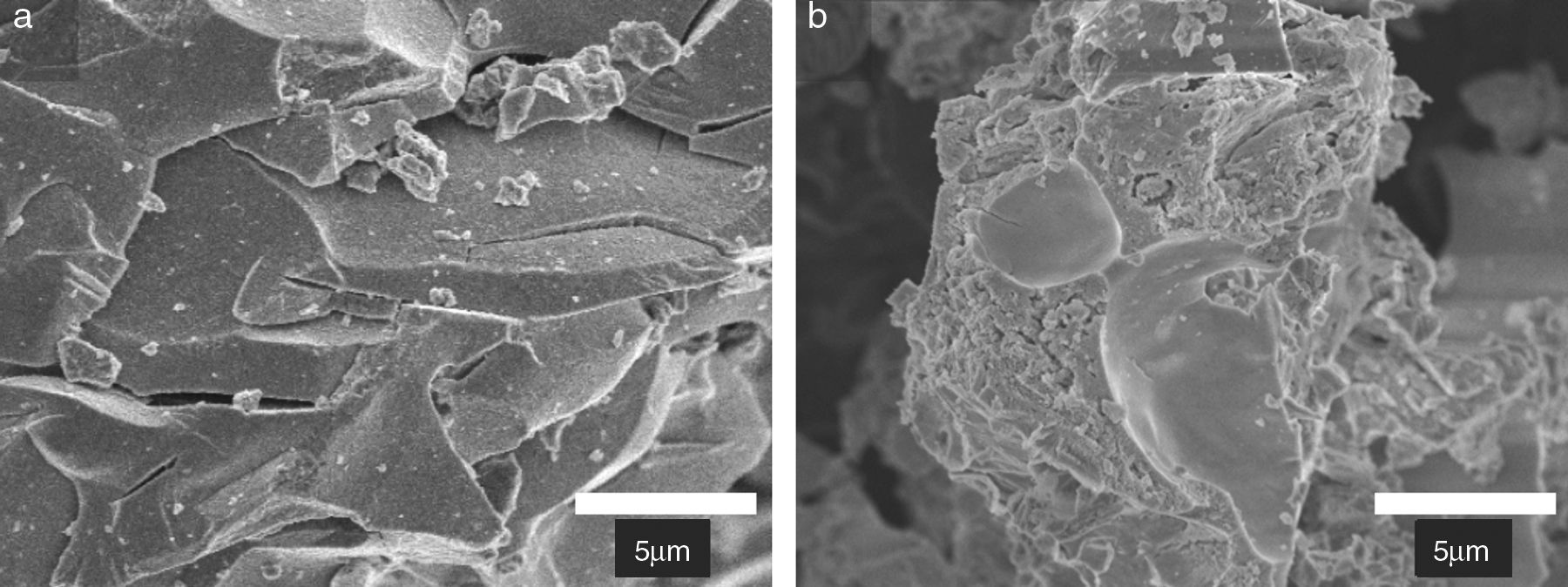
Typical low-magnification SEM images of the as-prepared powders are shown in Fig. 2. The powders consisting of large particles with a homogeneous surface, measuring several tens of micrometers across, as well as micro-or submicrometric particles: furthermore, the zirconia particles are quite porous, reflecting the escaping of gases during the combustion. Note that this morphology is evident for ZYF2M2 powders, Fig. 2(b), according with surface specific results.
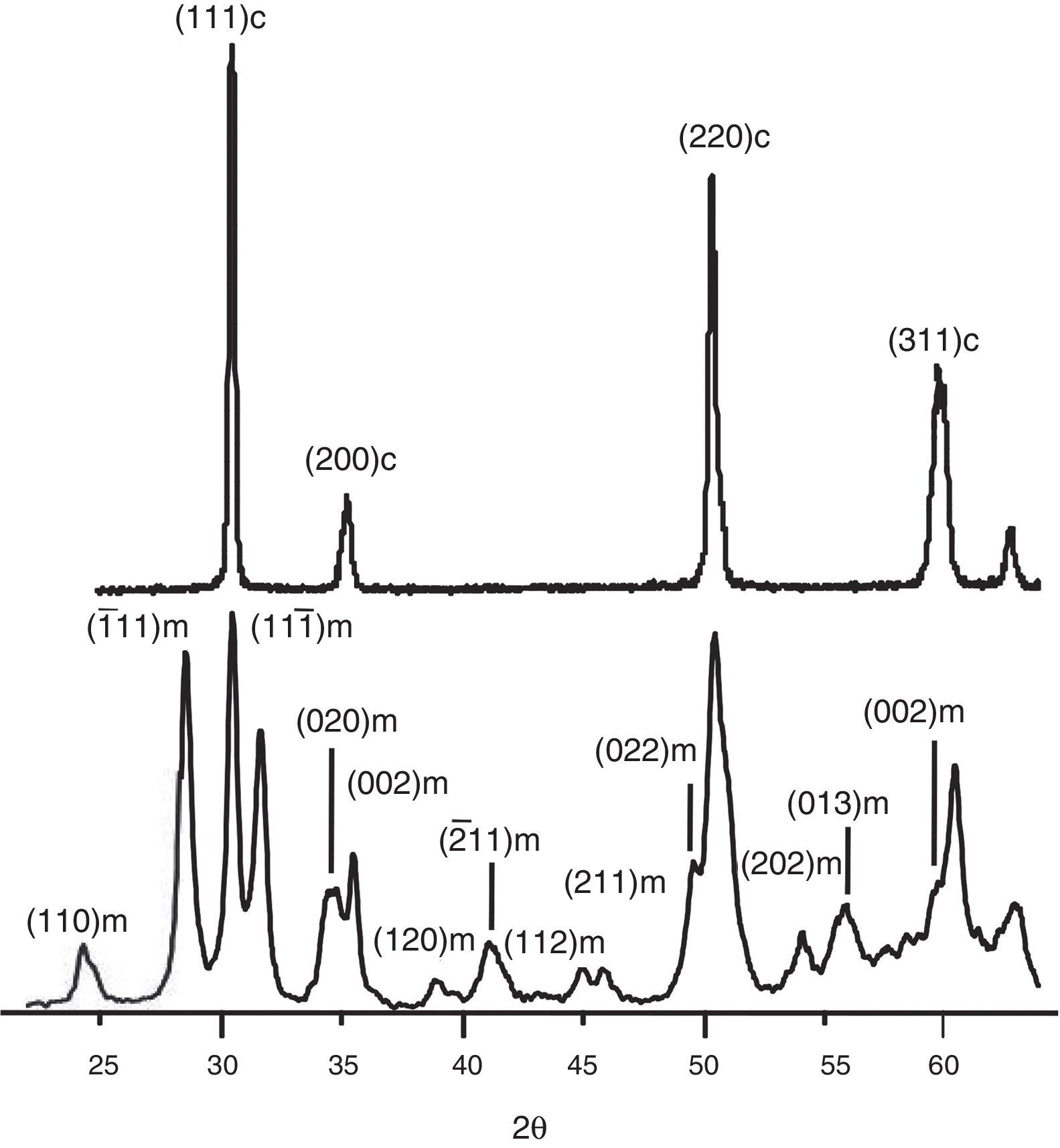
The XRD patterns of ZF2M2 and ZYF2M2 solid solutions after calcinations in air at 1473K are presented in Fig. 3. For the powder ZYF2M2, only the peak of fully tetragonal phase is detected. Also the (101) very fine and intense peak accounting for the tetragonal phase is more narrow, indicating some crystallite growth. In the case for ZF2M2 powders are clearly the transformations in t-ZrO2 and m-ZrO2 phases because the solubility decreases. These results are agreed with early studies. The Zr0.9Fe0.1O1.95 and Zr0.9Co0.1O1.9 were stabilized in the tetragonal phase by the combustion method using urea too. The study of their thermal stability, demonstrated to solubility in the stabilized solid solutions, show that it starts to decrease at temperatures about to 1148K and 898K respectively, provoking the progressive transformation into the monoclinic zirconia phase for both solid solutions. Free Mo3O or Fe2O3 could remain undetected on the XRD patterns because of a very small size.
ConclusionThe synthesis of the solid solutions Zr0.96Fe0.02Mo0.02O1.94−δ, and Zr0.86Y0.1Fe0.02Mo0.02O1.94−δ, fully stabilized in tetragonal form are reported for first time. The powders consisting of large grains with a homogeneous surface, measuring several tens of micrometers across. The BET on specific area of the powders contain element yttrium, iron and molybdenum is higher that for pure ZrO2. Higher Sw could reflect a smaller grain size due to both stronger natures of the combustions process experimented for the solid solutions containing yttrium, in agreement with the XRD results. The study of the thermal stability in air for ZF2M2 revealed that this solid solution is unstable at 1473K, this suggest that Mo4+ and Fe3+ ions leaving the solid solution provoking the mixture of t-ZrO2 and m-ZrO2 phases. The introduction of the element yttrium (ZYF2M2) provides a solid solution of zirconia stabilized in tetragonal form, even after calcinations at 1473K. Materials obtained could contribute to significantly improve the ionic conductivity of the solid state conductor. In addition, these results offer possibility that this solid solution can be exposed to CVD process to synthesize CNT-Metal ZrO2 composites, without phase changes of the zirconia.