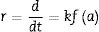SrZrO3-structured perovskite particles were prepared under hydrothermal conditions in a KOH (5M) solution using Zr-gel and SrSO4 mineral precursors. The treatments were conducted between 150 and 240°C for different reaction intervals (1–96h), and the KOH solution volume varied between 7.5 and 30mL. To evaluate the effect of the Zr-gel precursor, the treatments were preliminarily conducted with a coprecipitated pasty Zr-gel (Zr(OH)4·9.64H2O) and subsequently with a lyophilized Zr-gel Zr(OH)4 powder. Generally, SrZrO3 particles were produced by a single-step reaction following the simultaneous bulk dissolution of the Zr4+ gel precursor and the SrSO4 powder. However, in the preliminary experiments, a dehydration reaction of the pasty Zr-gel preceded the ultimate single-step reaction, resulting in complete SrZrO3 particle formation taking place over a longer interval of 96h at 240°C. In contrast, when using the dried Zr-gel powder, complete feedstock dissolution occurred more rapidly, producing SrZrO3 particles at 200°C over 48h. The SrZrO3 particle sizes varied significantly depending on whether the pasty gel or dried powder Zr precursor was used. Particles prepared with the pasty gel exhibited a bimodal size distribution with mean particle sizes of 25 and 65μm with pseudocubic and star-shaped cuboidal morphologies, respectively. In contrast, particle growth resulting from the rapid dissolution of solid powder feedstock produced cubic-shaped particles, monomodally distributed with an average particle size of 10μm. Furthermore, byproduct (SrCO3) formation occurred predominantly under earlier stages together with SrZrO3 particle irrespective of the 5M KOH filling volume; however, at a volume of 15mL spontaneously achieved in situ the SrCO3 dissolution at intermediate stages of reaction. This reaction pathway did not proceed at small (7.5mL) and large (30mL) volumes of the alkaline fluid. A kinetic study indicated that the activation energy required to produce the SrZrO3 cubic-shaped particles was low in both cases, i.e., 15.05 and 22.27kJmol−1 between the powder and pasty Zr4+ precursors, respectively.
Las partículas de SrZrO3 con estructura tipo perovskita se prepararon en condiciones hidrotermales en una solución de KOH (5M) usando precursores de Zr-gel y mineral de SrSO4. Los tratamientos se efectuaron entre 150 y 240°C en diferentes intervalos de reacción (1 a 96h), y el volumen de la solución de KOH varió entre 7,5 y 30ml. El efecto del Zr-gel precursor se evaluó mediante tratamientos que se realizaron con un Zr-gel coprecipitado con una consistencia pastosa (Zr(OH)4·9,64H2O) y otro en polvo de Zr(OH)4; este último se preparó mediante el liofilizado del Zr-gel pastoso. En general, las partículas de SrZrO3 se produjeron mediante una reacción de un solo paso, que involucra la disolución masiva de los precursores gel de Zr4+ y el polvo de SrSO4. Sin embargo, en los experimentos preliminares la reacción de deshidratación del Zr-gel con consistencia pastosa precedió a la reacción principal que ocurre en un solo paso, lo que prolongó la formación completa de partículas de SrZrO3 por un intervalo más largo (96h) a 240°C. En contraste, cuando se usó el Zr-gel en polvo, la disolución completa de la materia prima ocurrió rápidamente, produciendo partículas de SrZrO3 a 200°C por un intervalo de 48h. Los tamaños de partícula del SrZrO3 variaron significativamente dependiendo de la consistencia del Zr-gel empleado. Las partículas preparadas con el Zr-gel de consistencia pastosa mostraron una distribución bimodal de tamaño, con tamaños promedios de partícula de 25 y 65μm para las morfologías pseudocúbica y con forma de estrella, respectivamente. En contraste, el crecimiento de partículas promovido por la rápida disolución de la materia prima sólida en polvo produjo partículas de forma cúbica, distribuidas monomodalmente con un tamaño de partícula promedio de 10μm. Además, la formación del subproducto de reacción (SrCO3) ocurrió predominantemente por intervalos de reacción cortos y no depende del volumen de la solución alcalina empleada KOH 5M; sin embargo, a un volumen de 15ml, en este volumen se activó espontáneamente la disolución in situ del subproducto a tiempos intermedios de reacción. El estudio cinético demostró que la energía de activación requerida para producir las partículas de SrZrO3 fue baja en ambos casos, es decir, 15,05 y 20,27kJ mol−1 para los precursores de Zr4+ de consistencia en polvo y pastosa, respectivamente.
Strontium zirconate (SrZrO3) is a member of the perovskite group with a chemical formula of ABX3, and the crystalline structure stable at low temperature is orthorhombic with space group Pbnm[1]. Recently, SrZrO3 has attracted the attention of various research groups worldwide owing to its remarkable mechanical, thermal, chemical, and electric properties at elevated temperatures [1–6]. This material, among other perovskite oxides, exhibits a high dielectric constant, high breakdown strength [7], low leakage [7], and wide band gap [4]. Hence, this compound potentially has wide industrial applications in various areas, such as solid oxide fuel cells [5], hydrogen and oxygen sensors [2], catalysis [2,6], high gate dielectrics [8], and optical devices [4,9–11], among others. The physical and chemical properties might vary depending on crystalline structural defects or chemical composition deficiencies, which will vary with the production method [2,3,10]. Another potential application is in nuclear engineering, in which SrZrO3 can be used as an inert matrix to encapsulate plutonium fuel and other nuclear material wastes [4,5].
SrZrO3 powders have been prepared by conventional solid-state reaction processes employing SrCO3 and ZrO2 at high temperatures in the range of 1100–1300°C [2–5,11,12]. The powders produced under these conditions have some disadvantages, such as inhomogeneity, impurity contamination, and particle aggregation with variable particle size distributions. In contrast, low-temperature “soft” chemical processing techniques have demonstrated the production of SrZrO3 crystalline powders, and the processes recently applied include reverse micelle [12], Pechini [8], sol–gel [13,14], combustion [3] and alkaline flux methods [15]. These techniques enhance the preparation of particles with stoichiometric composition, homogeneous morphologies, and submicron particle sizes. Likewise, hydrothermal processing is an alternative technique capable of preparing a wide variety of perovskite materials [16–20], a few of which include SrZrO3[21,24]. Research work has focused on the hydrothermal production of fine SrZrO3 particles using highly concentrated (30–35M) alkaline solutions of NaOH [21] and KOH [22,23], employing inorganic nitrate or chloride precursors (Sr(NO3)2, Zr(NO3)4·5H2O, or ZrOCl2·8H2O). Reactions in such highly saturated alkaline media resulted in rapid zirconyl feedstock hydrolyzation, forming complex Zr(OH)5− ions and Sr(OH)+ formation. At 200°C and over a relatively short reaction period of 24h, the high molar saturation media triggered the crystallization of monodispersed SrZrO3 particles exhibiting cuboidal morphology and a unimodal particle (>0.3μm) size distribution [21,22,24]. The Ostwald Ripening mechanism subsequently resulted in SrZrO3 particle coarsening by dissolution of the fine SrZrO3 particles produced over reaction intervals of less than 24h. The SrZrO3 particle size was controlled by varying the KOH solution concentration, enabling, for example, micron-sized (1.5–2.0μm) cuboidal particles to be grown in an 8M KOH solution [22].
Recently, an alternative approach for producing alkaline metal earth perovskite compounds has been proposed using sulfate-based mineral ores containing Sr2+ or Ba2+ ions. The feasibility of using pure celestite (SrSO4) ore with a Ti(OH)4·4.5 H2O pasty gel has recently been investigated for synthesizing SrTiO3 particles under hydrothermal conditions [25]. Crystallization of fine SrTiO3 particles (>0.4μm) with a cubic morphology took place at 250°C over 24h, and this process occurred via a single-step reaction enhanced by a dissolution-crystallization mechanism. However, the SrSO4 dissolution and the reaction were hindered by the hydrolysis of Ti(OH)40 due to Ti gel dehydration, which occurred at an early stage in the treatment with a 5M KOH solution. The activation energy determined for the SrTiO3 hydrothermal processing was low (27.9kJmol−1) compared to that found for processing BaTiO3 particles obtained by chemical reactions in the liquid state [26]. Recently, low-grade barite-celestite (BC, Sr1−XBaXSO4) powder ore containing two elements to produce perovskite compounds, Sr2+ and Ba2+, was employed to prepare SrTiO3 powders [27]. The mineral ore usually consisted of 65wt% SrSO4 containing Ba as a major impurity and lesser amounts of Ca. Under hydrothermal conditions in a 5M KOH solution, cubic-shaped micron-sized SrTiO3 particles crystallized via a massive bulk dissolution-crystallization mechanism, which occurred when held at 250°C for 96h. Hydrothermal processing conditions have thus been shown to provide the chemical reaction environment for transforming low-cost mineral ores (Celestite or BC) without prior refinement to SrTiO3 particles free of byproducts (i.e., SrCO3) with the simultaneous release of the contaminants in the ore by hydrolysis in the hydrothermal fluid [27]. These processing features are likely to reduce the cost of this processing approach compared to the conventional hydrothermal treatment [21–24] that uses pure Sr2+ chemical reagents produced from the celestite ore. Therefore, the synthesis of different perovskite oxides, i.e., SrZrO3, by hydrothermal processing using mineral feedstocks is likely to be a significant opportunity in the preparation of functional ceramics.
A literature review suggests that no efforts have been made to synthesize SrZrO3 particles under alkaline hydrothermal conditions using a powdered mineral SrSO4 feedstock. Therefore, based on the chemical processing point of view, this research work comprises three scopes, which are described as follows: (1) To elucidate the Zr4+ feedstock consistency effect on the mineral SrSO4 dissolution in a 5M KOH solution. The experiments were conducted with a coprecipitated alkaline pasty Zr-gel structurally constituted of a large fraction of water molecules [24,27], and complementary experiments were carried out with a lyophilized dry alkaline Zr-gel powder. (2) To determine the chief chemical reaction equilibrium kinetic and activation energies over 150–240°C for reaction periods between 3–96h. The complementary scope aimed 3) To examine the effect of the alkaline KOH solution volume on the formation of reaction byproducts, namely SrCO3. The treatments were conducted using feedstock contents of 0.48g SrSO4 and 0.3874g dry Zr-gel, and the alkaline KOH (5M) solution volume varied between 7.5 and 30mL. These experiments aimed to determine the optimum volume to hinder byproduct formation (SrCO3) during SrZrO3 crystallization, which is the disadvantage of perovskite hydrothermal processing in a highly concentrated alkaline medium [21–24]. Furthermore, the SrZrO3 particle microstructural variations in morphology and particle size are discussed based on OH− saturation fluid differences, resulting in whether pasty Zr-gel and dried Zr-gel powder feedstocks were used.
ExperimentalMaterialsCelestite ore (SrSO4) was obtained from a mining area in northeastern Coahuila state in Mexico. The granulated celestite sample was pulverized for a short period of 1h in a zirconia ball mill, and the powdered mineral was then sieved with a mesh screen 400 (<38μm particle size). SrSO4 powder X-ray analysis revealed that the mineral structure belongs to the orthorhombic system and had the following unit cell parameters: a=8.3677(7)Å, b=5.3555(4)Å, and c=6.8745(5)Å. In addition, wet chemical analyses of SrSO4 powders (1g) indicated that the main constituents in the mineral were 46.61wt% Sr, 1.33wt% Ba, and 52.05wt% SO42, which correspond to 96.8wt% SrSO4, 2.25wt% BaSO4, and the minor elements (0.73wt% CaO, 0.19wt% Fe2O3, 0.02wt% MnO, 0.007wt% Al2O3). The impurity levels determined are likely to promote the differences in the lattice parameters between the present sample and the reference sample SrSO4 (ICDD card 80-0523), and this reference ore contains a lower fraction of impurities.
The alkaline zirconium gel (Zr-gel), selected as a Zr4+ source, was prepared according to the procedure proposed elsewhere [25]. The pasty Zr-gel chemical formula was determined by thermogravimetric and differential scanning calorimetric analysis (DSC/TGA TA Instruments SDT Q600). The analysis was conducted at a heating rate of 5°C/min from 25 to 1150°C in an air atmosphere, and the sample weight loss indicated that the pasty Zr-gel chemical formula is Zr(OH)4·9.64H2O. Subsequently, other experiments were carried out with a dry Zr-gel powder source of Zr4+. The release of water molecules from the pasty Zr-gel took place via a dehydration reaction conducted at −50°C at a constant vacuum pressure of 3.0Pa for 48h in a freeze-drying apparatus (Labconco FreeZone 4.5), resulting in a structurally amorphous dried powder of Zr(OH)4. This dried Zr-gel powder will likely accelerate the precursor SrSO4 dissolution step to achieve rapid SrZrO3 crystallization.
Hydrothermal treatmentsPreliminary hydrothermal experiments were conducted with SrSO4 powder and pasty Zr-gel precursor amounts of 0.48±0.0006g and 3.1±0.001g, respectively. The cation concentration was 2.53mM and fit the ABX3 perovskite structured compound stoichiometric molar ratio Sr/Zr=1 [25]. These experiments aimed to examine the effect of the pasty Zr-gel dehydration reaction that precedes the ultimate chemical reaction triggering SrZrO3 particle crystallization. In contrast, the complementary experiments were carried out using a dry Zr-gel powder (0.3874±0.0003g) and the same SrSO4 weight; thus, the cation molar ratio Sr/Zr was 1. A KOH solution with a concentration of 5M and a volume of 15mL (21.4% autoclave filling ratio) were selected as standard parameters to determine the experimental conditions that led to complete feedstock dissolution. It is worth emphasizing that the effect of the [OH−] anion saturation on secondary phase crystallization, namely, SrCO3, has not been systematically studied. Hence, experiments were conducted with different volumes (7.5 and 35mL) of the 5M KOH solution using the dry Zr-gel feedstock (0.3874±0.0003g). The precursors were poured at the bottom of the Teflon (PTFE) liner together with the KOH solution. The PTFE liner was located inside the stainless-steel micro autoclave and sealed. The hydrothermal treatments were carried out in a forced air convection oven heated to a predetermined temperature (150–240°C) for various reaction periods (3–96h). After treatment, the reaction products were separated from the remaining mother liquor, rinsed five times with hot water at 80°C and dried overnight at 80°C.
Characterization of the reaction productsThe crystalline structural aspects of the reaction products were determined by the powder X-ray diffraction technique (PXRD; Rigaku Rotaflex Ultima IV diffractometer) operated with graphite-monochromatized Cu Kα radiation (λ=1.54056Å) at 40kV and 20mA. The diffraction patterns were collected in the 10–80° 2θ range at a 4°/min scanning speed in 2θ/θ scanning mode with a 0.02° step. Additionally, details associated with the Rietveld refinement characterization are described in the supplementary supporting information file (SSIF). The morphology and particle size observations were conducted using field emission scanning microscopy (FE-SEM JEOL, Prime-7800F). The observations were carried out in the secondary electron mode at different magnifications, and linear analyses of 50 particles on SEM images determined the SrZrO3 particle size. The SrZrO3 chemical composition was determined via wet chemical analyses using inductively coupled plasma-atomic emission spectrometry (ICP-AES) (Model Multitype I ICP-9000, Shimadzu, Japan). Further details related to these analyses are included in Section S3 of the SSIF.
The kinetics associated with the solid SrSO4 dissolution that triggers the crystallization of SrZrO3 were investigated by the shrinking core model involved in the solid–liquid system reported for leaching processes [28,29] and extended to the one-pot hydrothermal synthesis of SrTiO3[25]. According to this model, the SrSO4 powder dissolution rate (r) is controlled by the chemical reaction at the particle surface coupled with ionic diffusion through a solution boundary rim. Hence, the SrSO4 dissolution ratio (α) can be correlated to the reaction rate expression Eq. (1) as follows:
At any reaction interval, the amounts of SrSO4 remaining and SrZrO3 produced can be indicated by CiSrSO4 and CiSrZrO3, respectively. Therefore, the SrSO4 consumed at any point, α, can be calculated by the expression α=1−WiSrSO4 or, alternatively, α=WiSrZrO3. The consumption ratio (α) was obtained using the SrZrO3 content complement calculated by the Rietveld refinement algorithm applied to each sample X-ray diffraction pattern after treatment. The rate constant (k) was then determined from linear regression of the data plotted in the ln(1−α) vs. t plots and used to analyze various empirical kinetic laws according to the expression f(α)=(1−α)n, where n is a reaction order. This mathematical expression enables the calculation of empirical functions for matching the kinetics and consequently deducing the reaction mechanism at the solid–liquid interface [29,30]. The activation energies “Ea” related to the chief chemical reactions Eqs. (3) and (4), concerned with the pasty Zr-gel and dry Zr-gel usage, respectively, were determined with the Arrhenius equation using linear regression from the treatments conducted at different temperatures. The Ea is associated with the bulk SrSO4 powder dissolution in the alkaline media and simultaneous SrZrO3 powder crystallization whether pasty gel or dried powder Zr precursor was used.
Results and discussionThe methodology to carry out the transformation of celestite powders (<38μm) under hydrothermal conditions was aimed at evaluating the effect of the precursor chemical reactivity and kinetic factors on the SrZrO3 formation and product morphology. The 5M KOH solution selected is suitable to dissolve the mineral (SrSO4) under hydrothermal conditions [25], and this is supported by the thermodynamic data related to SrZrO3 formation published elsewhere [24]. These results depict the first attempt to determine the chemical pathway associated with SrZrO3 crystallization using SrSO4 mineral and Zr gel precursors. Table 1 summarizes the selection of the relevant experimental conditions including the newly crystallized SrZrO3 unit cell parameters and the amount (wt%) of all the crystalline phases in the reaction product.
Summary of the hydrothermal treatments carried out in a 5M KOH medium employing Zr4+ (pasty gel or dried powder) and SrSO4 mineral as precursors; all experiments were conducted at a 20% solvent filling ratio.
| Sample ID | Zr+4 precursor | Temperature (°C) | Time (h) | Crystalline phases content (wt%) | Lattice parameters | Cell volume (Å3) | Crystallite size (μm) Rietveld | Crystallite size (μm) SEM | Rwp (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SrZrO3 | SrSO4 | SrCO3 | a (Å)a | b (Å)a | c (Å)a | ||||||||
| SZR16 | Gel | 150 | 3 | 17.1 (0.2) | 78.9 (0.1) | 4.0 (0.2) | 5.8205 (6) | 5.8346 (2) | 8.2077 (4) | 278.74(0.37) | 11.47 (2.65) | – | 16.448 |
| SZR13 | Gel | 150 | 48 | 53.0 (0.8) | 43.0 (0.7) | 4.0 (0.2) | 5.8103 (2) | 5.8184 (6) | 8.2148 (15) | 277.72(0.06) | 12.30 (3.32) | 11.36 | 16.495 |
| SZR14 | Gel | 150 | 96 | 63.2 (0.5) | 34.8 (0.4) | 2.0 (0.1) | 5.8094 (8) | 5.8188 (8) | 8.2168 (4) | 277.76(0.05) | 15.86 (3.64) | – | 14.325 |
| SZR05 | Gel | 200 | 3 | 45.2 (0.7) | 51.5 (0.6) | 3.3 (0.2) | 5.8139 (3) | 5.8853 (3) | 8.2236 (7) | 281.38(0.15) | 12.76 (2.79) | – | 17.533 |
| SZR03 | Gel | 200 | 24 | 60.9 (0.7) | 36.9 (0.7) | 2.2 (0.1) | 5.8097 (8) | 5.8114 (10) | 8.2338 (5) | 277.98(0.06) | 14.80 (3.46) | – | 18.93 |
| SZR01 | Gel | 200 | 96 | 83.7 (0.2) | 15.6 (0.2) | 0.7 (0.7) | 5.8020 (7) | 5.8144 (6) | 8.2128 (4) | 277.06(0.05) | 10.93/25.3 (3.68) | 17.73/26.1 | 15.975 |
| SZR24 | Gel | 240 | 3 | 68.5 (0.5) | 29.3 (0.5) | 2.2 (0.1) | 5.8061 (6) | 5.8158 (6) | 8.2237 (11) | 277.69(0.05) | 12.84 (4.61) | 13.14 | 16.944 |
| SZR21 | Gel | 240 | 24 | 87.9 (0.3) | 10.3 (0.2) | 1.8 (0.1) | 5.8193 (8) | 5.7965 (8) | 8.2100 (4) | 276.94(0.05) | 13.46/47.2 (3.85) | 10.5/45.3 | 18.046 |
| SZR19 | Gel | 240 | 96 | 97.5 (0.3) | 2.5 (0.3) | – | 5.8089 (7) | 5.8080 (5) | 8.2145 (8) | 277.15(0.05) | 14.63/65.2 (3.42) | 15.5/65.5 | 17.963 |
| SZR48 | Powder | 150 | 3 | 51.3 (0.4) | 44.2 (0.6) | 4.5 (0.8) | 5.8185 (1) | 5.8174 (2) | 8.2601 (4) | 279.59(0.01) | 11.83 (3.18) | 12.3 | 10.737 |
| SZR44 | Powder | 150 | 48 | 90.5 (0.3) | 9.5 (0.3) | – | 5.8151 (6) | 5.8192 (9) | 8.2357 (5) | 278.69(0.099 | 10.28 (2.92) | 11.05 | 9.391 |
| SZR43 | Powder | 150 | 96 | 94.5 (0.2) | 5.5 (0.2) | – | 5.8172 (4) | 5.8184 (4) | 8.2301 (10) | 278.56(0.04) | 9.77 (2.33) | 10.2 | 10.089 |
| SZR36 | Powder | 200 | 3 | 76.2 (0.4) | 21.8 (0.3) | 2 (0.1) | 5.8277 (7) | 5.8136 (6) | 8.2159 (6) | 278.36(0.05) | 10.16 (2.21) | 10.1 | 11.08 |
| SZR33 | Powder | 200 | 24 | 93.3 (0.2) | 6.7 (0.2) | – | 5.8101 (8) | 5.8205 (9) | 8.2202 (4) | 277.98(0.06) | 9.81 (2.75) | 9.5 | 11.841 |
| SZR31 | Powder | 200 | 96 | 98.2 (0.1) | 1.8 (0.1) | – | 5.8100 (4) | 5.8163 (5) | 8.2230 (6) | 277.87 (0.04) | 8.95 (2.22) | 9.03 | 12.24 |
| SZR30 | Powder | 240 | 3 | 88.8 (0.4) | 7.9 (0.4) | 3.3 (0.1) | 5.8118 (4) | 5.8149 (4) | 8.2200 (7) | 277.79 (0.03) | 8.96 (2.34) | 9.42 | 13.837 |
| SZR27 | Powder | 240 | 24 | 100 (0.1) | – | – | 5.8105 (6) | 5.8165 (8) | 8.2161 (5) | 277.68 (0.05) | 8.85 (1.92) | 9.12 | 13.566 |
| SZR26 | Powder | 240 | 48 | 100 (0.1) | – | – | 5.8154 (9) | 5.8063 (7) | 8.2162 (2) | 277.43 (0.05) | 8.63 (1.19) | 8.84 | 15.134 |
| SZR68* | Powder | 240 | 3 | 71.8 (0.6) | 18.9 (0.6) | 9.3 (0.3) | 5.8110 (7) | 5.8202 (8) | 8.2187 (13) | 277.97 (0.06) | 9.42 (2.36) | – | 13.803 |
| SZR71* | Powder | 240 | 12 | 94.0 (0.9) | 7.9 (0.9) | 8.1 (0.2) | 5.8044 (15) | 5.8141 (9) | 8.2104 (21) | 277.08 (0.11) | 9.15 (2.64) | – | 15.141 |
| SZR73* | Powder | 240 | 24 | 93.1 (0.3) | 1.4 (0.8) | 5.5 (0.2) | 5.8044 (10) | 5.8185 (10) | 8.2117 (12) | 277.33 (0.08) | 8.73 (2.95) | – | 7.698 |
| SZR75** | Powder | 240 | 3 | 84.9 (0.1) | 9.0 (0.2) | 6.1 (0.3) | 5.8168 (6) | 5.8127 (3) | 8.2123 (7) | 277.67 (0.04) | 9.82 (2.07) | – | 7.931 |
| SZR78** | Powder | 240 | 12 | 89.6 (0.2) | 5.2 (0.2) | 5.2 (0.3) | 5.8083 (3) | 5.8139 (2) | 8.2205 (3) | 277.60 (0.02) | 9.36 (2.31) | – | 7.624 |
| SZR74** | Powder | 240 | 24 | 93.2 (0.1) | 3.4 (0.7) | 3.4 (0.2) | 5.8169 (2) | 5.8138 (1) | 8.2250 (2) | 278.15 (0.01) | 8.78 (2.76) | – | 7.342 |
| Strontium zirconate (SrZrO3)b | 5.786 | 5.815 | 8.196 | 275.75 | |||||||||
Note: *40% and **10% volume filling ratio of the autoclave vessel.
The typical X-ray diffraction patterns obtained by Rietveld refinements that allowed us to calculate the amount of each phase are portrayed in the plots shown in Figs. S3 and S6 (in SSIF). Generally, the calculated SrZrO3 particle lattice parameters did not exhibit a remarkable variation, and these are similar to those of the SrZrO3 single phase. The refinement analysis accuracy is acceptable; although a few “Rwp” coefficient values are over 10.0%, any value below it is considered acceptable accuracy. It should be noted that the samples prepared for long periods exhibited the lowest “Rwp” values below 10.0%. These values resulted because the refinement algorithm considers the SrZrO3 powder crystallinity, and the mean particle size determined by SEM observations. Furthermore, the nonsystematic variation revealed on the “Rwp” and the SrZrO3 particle lattice parameters (Table S5 in SSIF) is likely due to the full scanned 2θ range selected; only a few samples were analyzed between 15 and 130°, while the samples prepared at early and intermediate reaction intervals were only scanned for a short 2θ interval of 10–90°, causing the slight increase in Rwp.
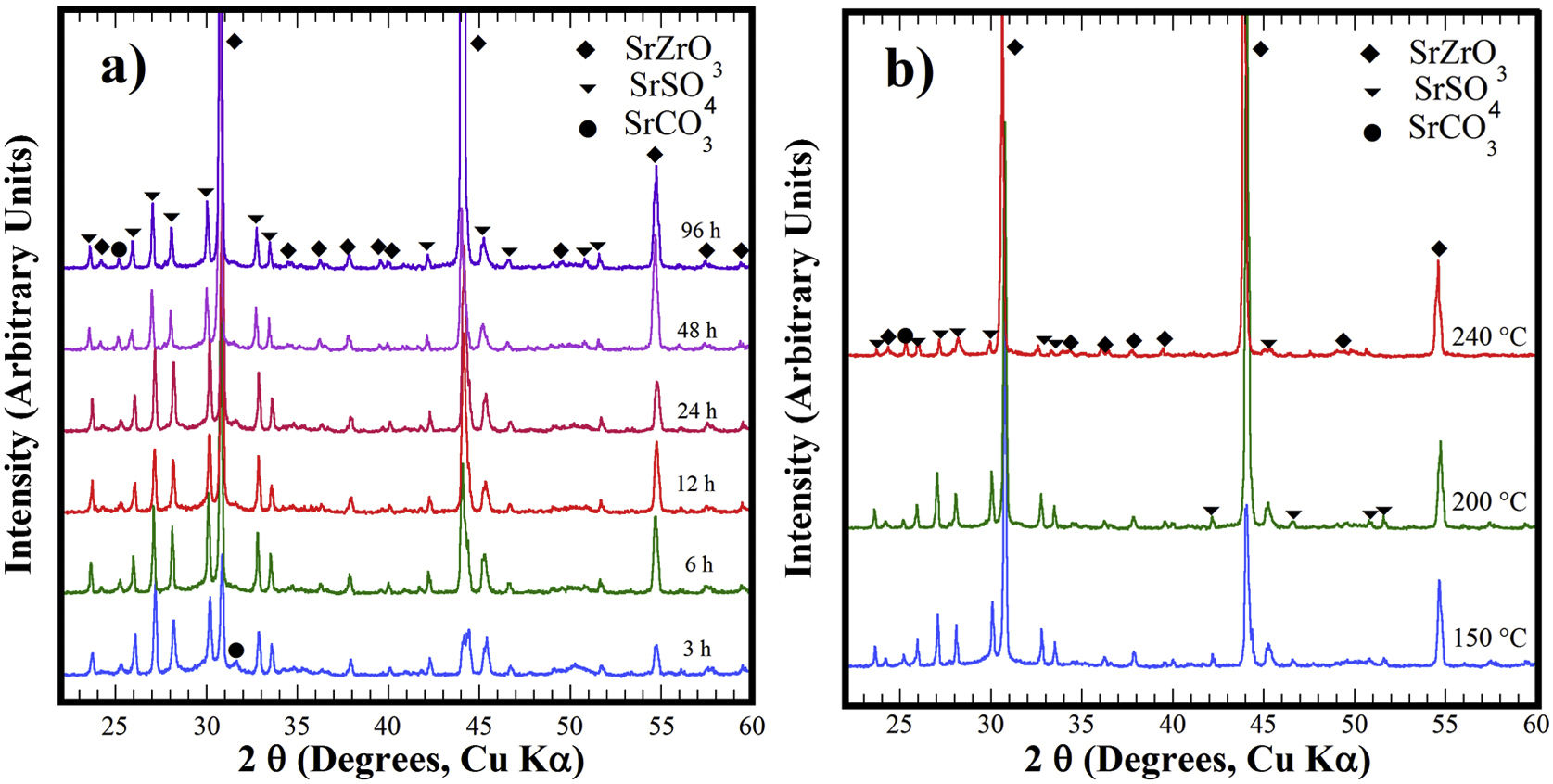
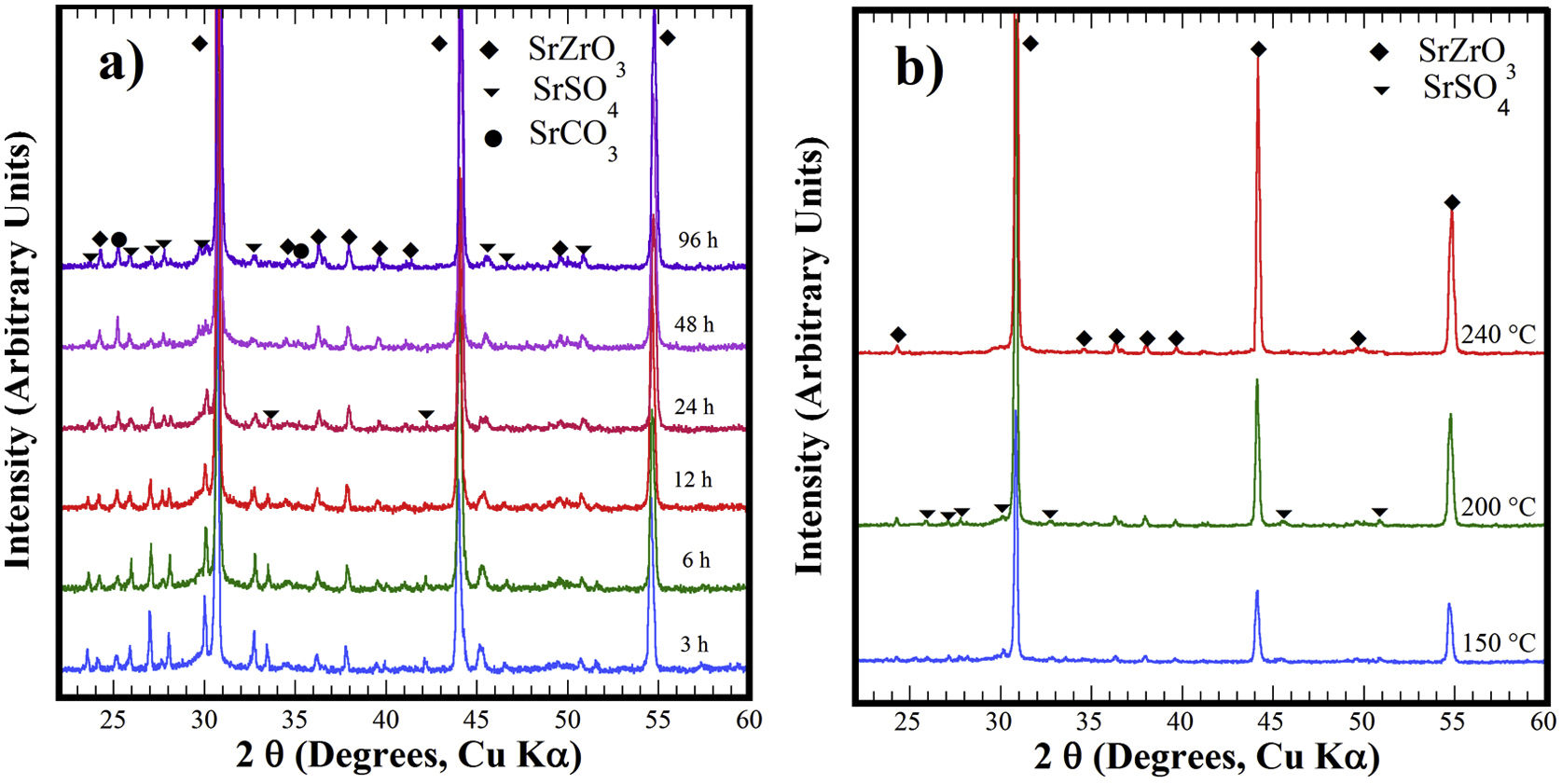
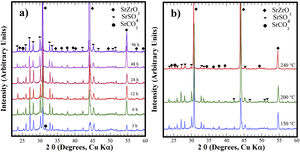
Structural aspects of the SrSO4 transformation into SrZrO3 under alkaline hydrothermal conditionsFig. 1 shows the PXRD patterns of the remaining products obtained using SrSO4 powders and the pasty Zr-gel. The treatments were conducted at 200°C for several intervals (Fig. 1a) and at different temperatures for 96h (Fig. 1b). Generally, the new SrZrO3 crystalline phase was revealed at a short reaction interval of 3h. In the 25°<2θ<32° range, the peaks indicating the gradual SrSO4 consumption are at 2θ<30°. The SrSO4 peak intensities decreased with increasing reaction period. In contrast, the new peak at 2θ∼30.86° constantly increased in intensity during intermediate and long treatment intervals. This peak corresponds to the main SrZrO3 peak with a (110) Miller index, with an orthorhombic structure and space group Pbnm (62) (ICDD card 70-0283). Likewise, the SrCO3 (ICDD card 05-0418) secondary phase and the SrSO4 precursor powder remained in the samples prepared at 200°C. Although the SrZrO3 content also increased over 200°C, the complete SrSO4 powder transformation to SrZrO3 did not occur under the reaction conditions selected. This inference is suggested by the small amount of SrSO4 ore revealed by the PXRD pattern of samples prepared for prolonged reaction intervals over 72h, as seen in Fig. 1b. Furthermore, the presence of the amorphous pasty Zr-gel trace revealed in the 28°>2θ<32° range was determined by the PXRD patterns of the samples prepared at temperatures lower than 200°C (Fig. S1 in the SSIF). These results suggest the low reactivity of the pasty Zr-gel below 200°C, which seemingly reduced the reaction kinetics, hindering the complete formation of SrZrO3 particles.
XRD patterns of reaction products obtained using celestite powder and the pasty Zr-gel, the treatments were carried under hydrothermal conditions in the 5M KOH alkaline media at (a) 200°C for different reaction times and (b) for 96h at different temperatures. Crystalline phases (♦) strontium zirconate (SrZrO3-ICDD card 70-0283), (▾) celestite (SrSO4-ICDD card 80-0523) and (●) strontianite (SrCO3-ICDD card 05-0418).
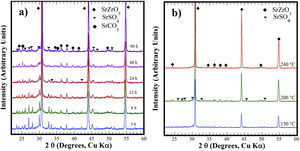
The hydrothermal treatments conducted using lyophilized Zr-gel revealed a similar reaction pattern to that observed for the treatments conducted with pasty Zr-gel, regarding the presence of dried Zr4+ powders (amorphous), which is attributed to the tiny broad lump in the 28°>2θ<32° range. This evidence revealed on the samples treated at temperatures below 200°C is shown in Fig. 2a and S1 (SSIF). However, the transformation of the SrSO4 powders rapidly occurred even for a short reaction interval of 3h at 200°C (Fig. 2a), and the SrSO4 content is seemingly lower than that remaining after the experiment conducted with the pasty Zr-gel under the same conditions (Fig. 1a). Indeed, this assumption is supported by the highly intense peak at the 2θ angle of 30.86°, which corresponds to a SrZrO3 content of 76.19wt% according to the refinement results. The SrZrO3 content gradually increased in the reaction product above the intermediate (48h) and long (96h) reaction intervals, as seen in Fig. 2a. Similar results were found by varying the reaction temperature, as seen in the powder PXRD patterns obtained after 96h at different temperatures (Fig. 2b). These results revealed a marked reduction in the SrSO4 peaks resulting in accelerated reaction progress by increasing the treatment temperature above 200°C.
XRD patterns of reaction products obtained using celestite and dried Zr-gel powders, the treatments were carried under hydrothermal conditions in the 5M KOH alkaline media at (a) 200°C for different reaction times and (b) for 96h at different temperatures. Crystalline phases (♦) strontium zirconate (SrZrO3-ICDD card 70-0283), (▾) celestite (SrSO4-ICDD card 80-0523) and (●) strontianite (SrCO3-ICDD card 05-0418).
At 240°C, the total dissolution of the celestite mineral particles (<38μm size) resulted in the SrZrO3 single-phase free of reaction byproducts. Additionally, the ultimate transformation of SrSO4 into SrZrO3 proceeded at a reaction temperature of 240°C for the shortest reaction interval of 24h (Fig. S2 SSIF). By inference, the present results indicate that the speed of the SrSO4 transformation process strongly depends on the Zr-gel precursor. The XRD powder diffraction results strongly suggest that the conversion proceeds in a single step by the chemical reaction Eq. (3), which only triggers the crystallization of the new SrZrO3 phase, and the reaction rate depends on the Zr-gel precursor condition at a given temperature under alkaline hydrothermal conditions.
The hydrothermally produced SrZrO3 powders are free of byproducts, and the chemical composition calculated by wet chemical analyses assisted by ICP is given in Table S6 (SSIF). The average content of the cationic constituents, Sr and Zr, was measured three times using the acidic diluted sample solution. These results indicated that the molar content of the powders produced by varying the temperature and reaction interval is similar to that of the stoichiometric Sr:Zr molar ratio of 1:1 for SrZrO3. This result was preferentially revealed on various samples prepared with the dried Zr-gel powder. The SrZrO3 formation proceeded by the single-step chief chemical equilibria is described in Eq. (3). It is worth emphasizing that the crystallization of SrZrO3 also proceeded by the pathway revealed for the analogous synthesis of SrTiO3 using SrSO4[25,27], and the release of major impurities, Ba2+, into the alkaline media took place because BaSO4 was hydrolyzed and remained as an ionic species for all treatments [27]. These results are supported by the PXRD analyses, which indicate that the particles produced only correspond to the perovskite structure of SrZrO3.
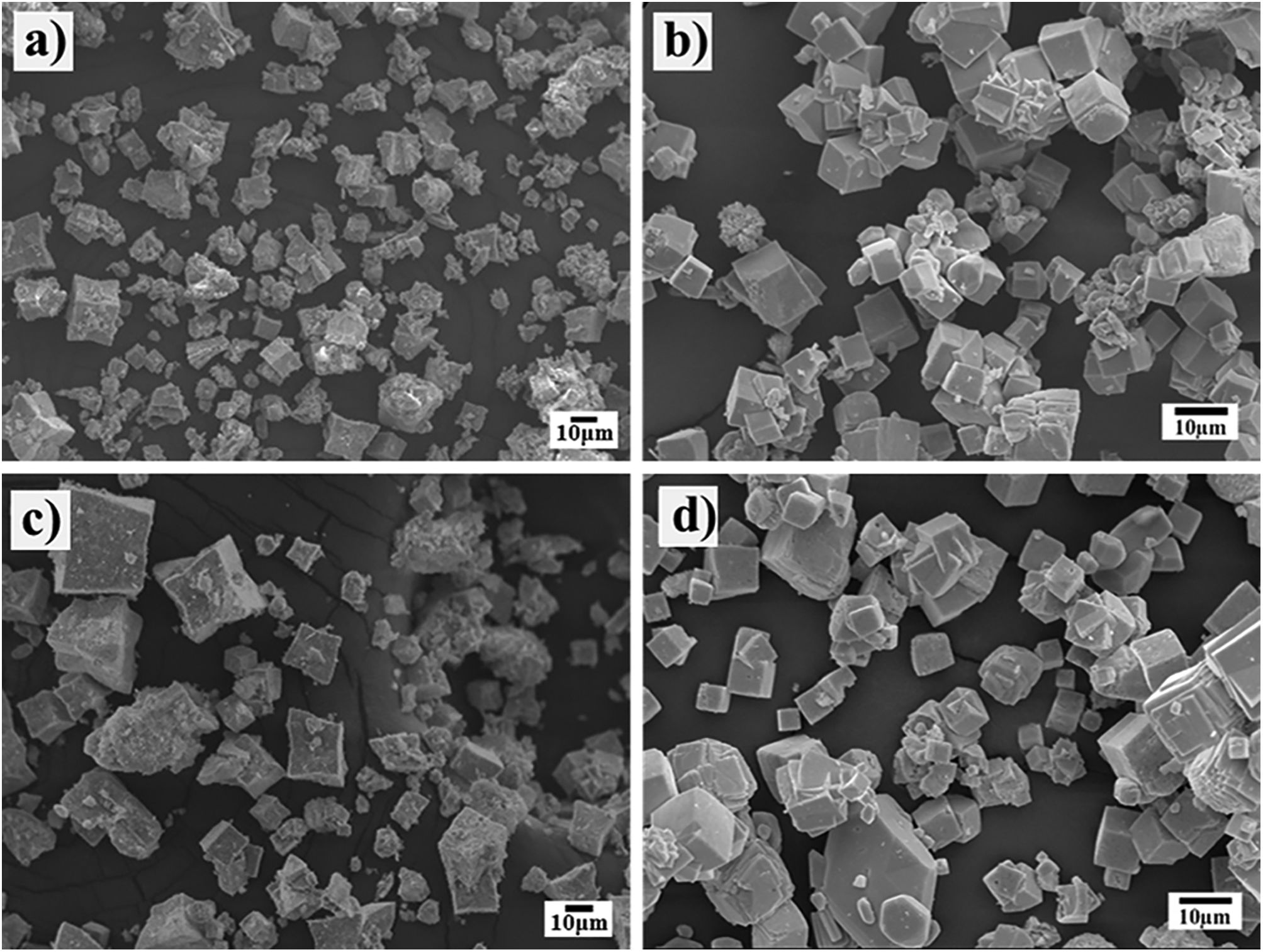
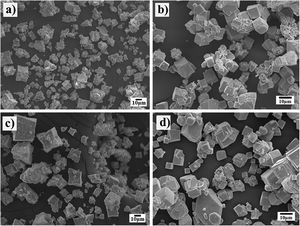
Morphological differences of the transformed SrZrO3 particles prepared using different Zr-gel precursorsThe powder morphology produced when the SrSO4 particles (<38μm) and the two Zr-gels, namely, the hydrated pasty and the dried powder, were treated at a temperature of 240°C for several reaction intervals is shown in Fig. 3. Generally, the powders produced after 3h with the pasty Zr-gel precursor exhibit a few new large particles (SrZrO3) of approximately 10μm in size with a peculiar basal start-like shape and a cuboidal habit. Furthermore, a considerable number of small particles below 5μm in size with an irregular (noneuhedral) morphology corresponding to the partially reacted SrSO4 particles were found in the reaction products (Fig. 3a). After 48h, the observations revealed that star-shaped particles grew to an average size of 15μm. Simultaneously, the formation of a noticeable number of pseudocubic-shaped SrZrO3 particles (nearly 5μm in size) occurred (Fig. 3c). The fine particles below 1μm in size exhibited remarkable agglomeration, possibly by inference, and these particles correspond to the remaining SrSO4 crystals, which are not visible in the micrograph. When the treatment was carried out at 96h, the progressive star-shaped particle growth proceeded preferentially along the crystallographic direction 〈110〉 of the cubic perovskite structure, resulting in the formation of numerous restricted but volumetrically significant large SrZrO3 star-shaped crystals. These particles exhibit a bimodal size distribution with averages of 25 and 65μm. Simultaneously, the fine SrZrO3 pseudocubic particles also slightly increased in size to approximately 10μm, as seen in Fig. 4c. This particle coarsening is likely provoked by the variation in the chemical conditions of the hydrothermal media caused by the pasty Zr-gel dehydration process, which is discussed in Section 3.3.
In contrast, the treatments conducted with the dried Zr-gel powder, Zr(OH)4, at 240°C with 5M KOH for various reaction intervals (Fig. 3b and d) revealed that a considerable number of new SrZrO3 particles with a typical cuboidal morphology were produced in all the experiments conducted by varying the reaction interval. In these treatments, the transformation process proceeded faster than when the pasty Zr-gel was used. After 3h, the early precipitated SrZrO3 particles with a cuboidal morphology exhibited a homogeneous size distribution with a mean size of 8.0μm. The epitaxial particle growth triggered at intermediate stages promoted the formation of bulky cuboidal faceted agglomerates of ∼20.0μm, and by inferring from the PXRD results, the few irregularly shaped (noneuhedral) particles revealed in the SEM image corresponded to the residual SrSO4. In contrast, after 48h, when the complete dissolution of SrSO4 occurred, the particles had a unimodal particle size distribution, as seen in Fig. 3d.
According to these results, by inference, the early produced bulky agglomerates were dissolved in the alkaline fluid, resulting in the coarsening of some large agglomerates (∼25.0μm in size) promoted by (001) epitaxial faceted growth. Simultaneously, the crystallization of less numerous but volumetrically significant monodispersed fine cubic-shaped crystals with a size of ∼6.5μm occurred. The partial dissolution of the SrZrO3 particles produced at intermediate stages occurred preferentially over reaction intervals of 48h. Consequently, bulky SrZrO3 cuboidal particles grew via the Ostwald ripening mechanism. This phenomenon led to the formation of cubic-shaped particles with a sharp unimodal particle size distribution varying from 5.0 to 12.5μm, as seen in Fig. 4d.
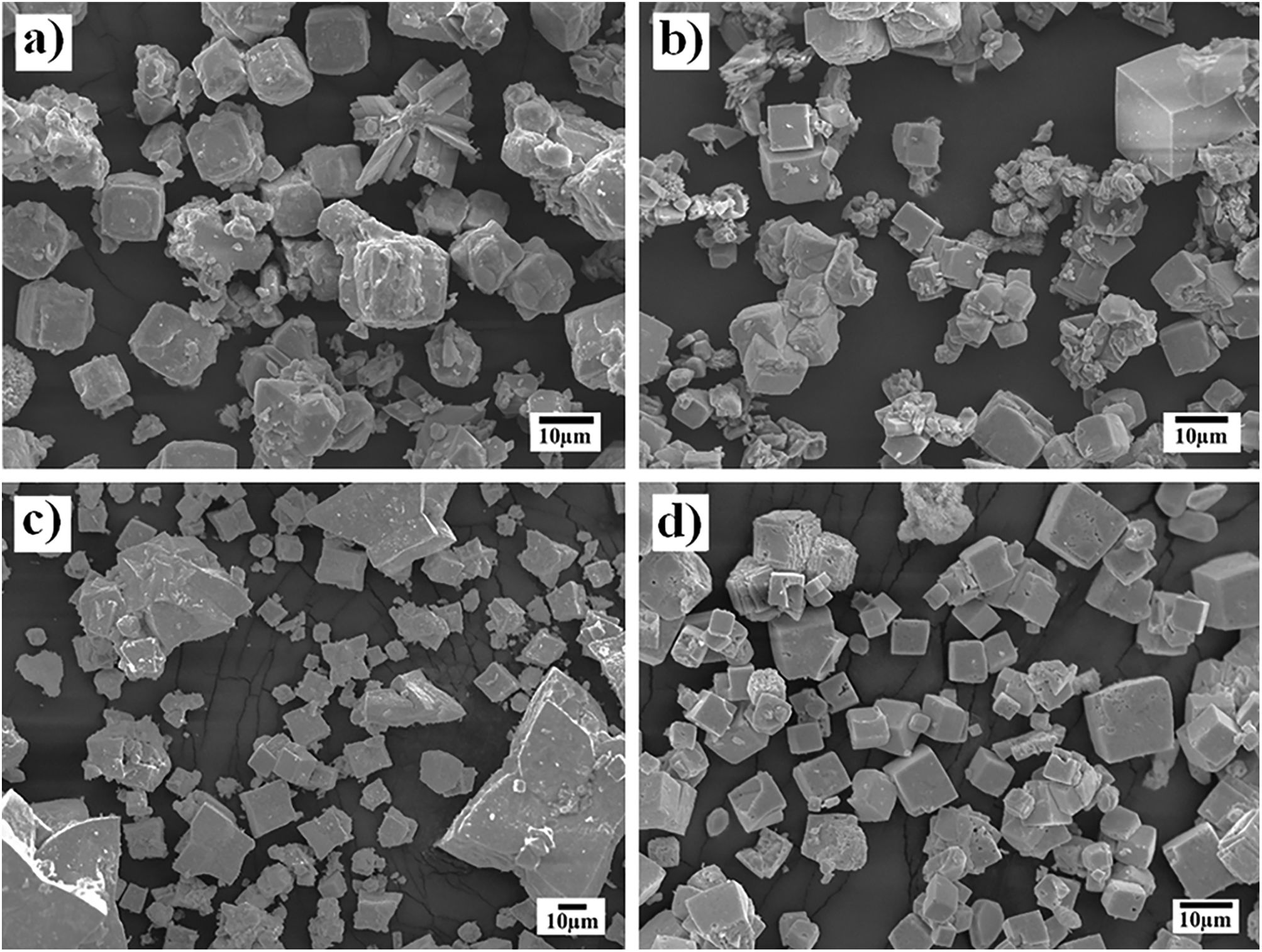
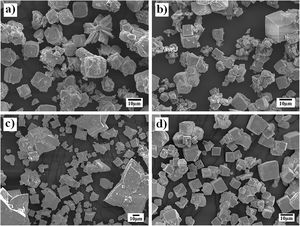
On the other hand, the SEM images (Fig. 4) indicate that a morphological variation takes place on the SrZrO3 particles and depends on whether pasty and dried Zr-gel precursors were used, as shown in the powders obtained in the 5M KOH solution for 96h at different reaction temperatures (Fig. 4). This behavior is analogous to that found in the experiments conducted at different reaction intervals. It is worth emphasizing that the growth of euhedral-shaped SrZrO3 particles slightly occurred at low temperatures (150°C) using both Zr-gel feedstocks (Fig. 4a and b). However, the increase in the treatment temperature up to 240°C produced a difference in the particle size and distribution of both SrZrO3 crystals prepared with the pasty Zr-gel (Zr(OH)4·9.64H2O, mostly star-shaped with bimodal distribution, Fig. 4c) and the dried Zr(OH)4 precursors (cubic-shaped crystals averaging 8.5μm in size with unimodal size distribution, Fig. 4d). In general, the results indicate that the Ostwald-ripening particle growth mechanism triggers SrZrO3 particle coarsening. This assumption is suggested by the fact that the small euhedral SrZrO3 particles with irregular morphology produced at lower temperatures (<240°C) were not observed in the SrZrO3 powders produced at 240°C for 96h (Fig. 4c and d). However, it can be inferred from the micrograph in Fig. 4c that SrZrO3 crystal coarsening at 240°C differed when the pasty Zr-gel was used as a Zr4+ precursor. The pasty Zr-gel dehydration provoked a reduction in the solute fluid supersaturation level because an in situ alkalinity reduction occurred by the released water molecules [25]. From our results, it is clear that a reduction in the OH− local concentration mitigates the OH− hydroxylation that preferentially takes place on the (001) facets that favor cubic crystal growth. Thermodynamically, the (001) facet exhibits the lowest interfacial energy and therefore allows OH− ion coverage because these ions stabilize on these surfaces faster than NO3−, as occurred on the particle growth of Co3O4 cubic particles [31]. This process is analogous to the formation of SrZrO3 particles and is supported by the TEM observation included in the SSIF. Therefore, under the fluid low supersaturation level provoked by the reduction in OH− content, solute nourishes the large bulky star-shaped agglomerates and the less numerous euhedral particles, which are assumed to grow via the epitaxially faceted coarsening enhanced by the dissolution-recrystallization process, as seen in Fig. 4c.
In addition, TEM images of the products produced for 3h at 240°C revealed a large amount of fine plate-like Zr(OH)4 particles (as suggested by the EDX compositional analyses) coexisting with submicron euhedral SrZrO3 star-shaped particles (see Figs. S7 and S8 in SSIF). These plate-shaped particles were formed by the dehydration reaction and do not correspond to ZrO2 because the peaks of this phase were detected by PXRD analyses on the sample prepared below 12h. Likewise, the micrographs revealed no evidence indicating that the amorphous Zr(OH)4 particle operates as a template to achieve a topotactic transformation of the Zr-gel feed into SrZrO3 because no SrZrO3 particles are visible at their surfaces. Hence, the results showed that the coupled transformation speed depends on the Zr-gel precursor condition and the reaction temperature. These experimental parameters triggered changes in the morphology of the SrZrO3 particles, as revealed in the FE-SEM and TEM observations and agree with the controlled SrZrO3 particle formation in highly concentrated KOH and NaOH (8M) solutions reported elsewhere [21–23].
Differences in the SrZrO3 transformation pathway and the control of secondary phasesAccording to the literature review, an analogous study to produce perovskite-structured materials, namely, SrTiO3 powders, has been recently conducted via the hydrothermal single-step reaction process, employing either celestite crystals or particulate feed material together with a Ti-gel (Ti(OH)4·4.5H2O) [25]. However, the celestite feedstock has not yet been considered for preparing other perovskite materials, such as SrZrO3, and the reaction mechanism provoked under hydrothermal conditions. In the present study, the transformation of the strontium source (polycrystalline SrSO4 ground<38μm) occurs via the chemical reactions proposed above, i.e., Eqs. (3) and (4), which are correlated to the employment of the pasty Zr-gel and the dried Zr-gel, respectively.
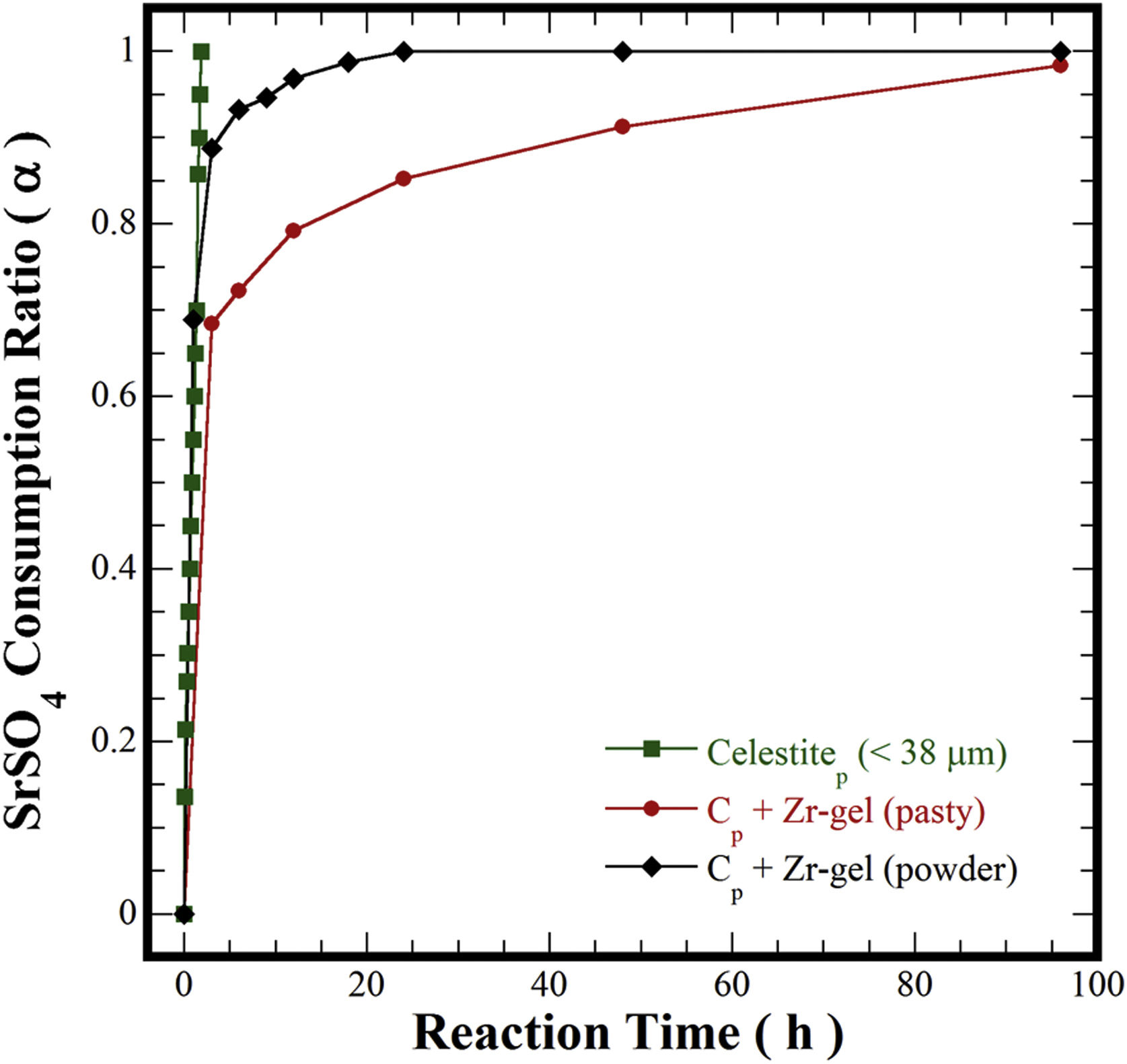
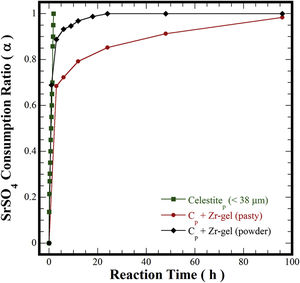
In pioneering studies focused on producing pure SrZrO3 powders under alkaline hydrothermal conditions [21–23,32], hydrothermal processing involved the treatment of a slurry prepared with Sr(NO3)2 and ZrO(NO3)2·5H2O reagents mixed with highly concentrated KOH or NaOH (8–30M) solutions. The SrZrO3 powder synthesis was achieved through a dissolution-crystallization mechanism, which took place at 200°C for a short reaction interval of 12h [22,24,32]. The highly alkaline media triggered the SrZrO3 crystallization conditions affected by the acid NO3− ions in the reaction system. Consequently, nanosized SrZrO3 cubic crystals preferentially formed in 30M KOH media at 200°C [24]. In our case, the reaction pathway was evaluated separately for each Zr-gel feedstock by measuring the SrSO4 dissolution capability in the hydrothermal system. These experiments were conducted at 240°C with a 5M KOH solution and are portrayed in Fig. 5. Generally, the total consumption of celestite powder (>38μm) feedstock occurred rapidly in the 5M KOH solution for 1.8h. However, the pasty Zr-gel markedly reduced the dissolution of SrSO4 over a fraction of 0.7, which was dissolved for 3h. Above 3h, a notorious reduction in the consumption of SrSO4 occurred up to 96h, and the maximum SrSO4 fraction consumed was 0.95. Although, the results indicated that the consumption of both precursors occurs by a single-step reaction according to Eq. (3). The dehydration is likely to take place at the interphase between the pasty Zr-gel and the surrounding fluid. This reaction rim is located at the bottom of the autoclave and remains static because no mass transfer occurred due to the downward motion of the convective fluid. The pasty Zr-gel dehydration proceeded according to Eq. (2), and this reaction is analogous to the one reported for the Ti(OH)4·4.5H2O precursor dehydration used to transform SrSO4 into SrTiO3[25].
In contrast, the preliminary dried Zr(OH)4 powder used boosted the SrSO4 consumption to a fraction of ∼0.9, resulting in the SrZrO3 and SrCO3 formation even for 3h. According to the results in Fig. 5, the alkalinity of the fluid did not vary; consequently, the massive dissolution of both nutrients (SrSO4 and Zr-gel) rapidly yielded the solute saturation of the stable chemical species Sr(OH)2 and Zr(OH)40[24]. Hence, spontaneous SrZrO3 crystallization was straight forward and was achieved by a single-step reaction, as shown in Eq. (4). This reaction is controlled by the dissolution rate of the solid species rather than the reaction with Zr(OH)4. It is worth mentioning that the slight reduction in the solid precursors might be caused by the presence of the SrCO3 contaminant that predominantly occurred between 1 and 12h of treatment. This inference is supported by the results discussed in Fig. 6 related to the effect of the solvent filling volume on the course of the SrCO3 byproduct elimination [33]. The ultimate SrZrO3 transformation at 240°C in 5M KOH occurred for a short reaction interval of 24h. Eventually, the primary unit operation conducted to process the pasty Zr-gel provides process optimization, leading to significant economic and environmental advantages for processing perovskite structured materials.
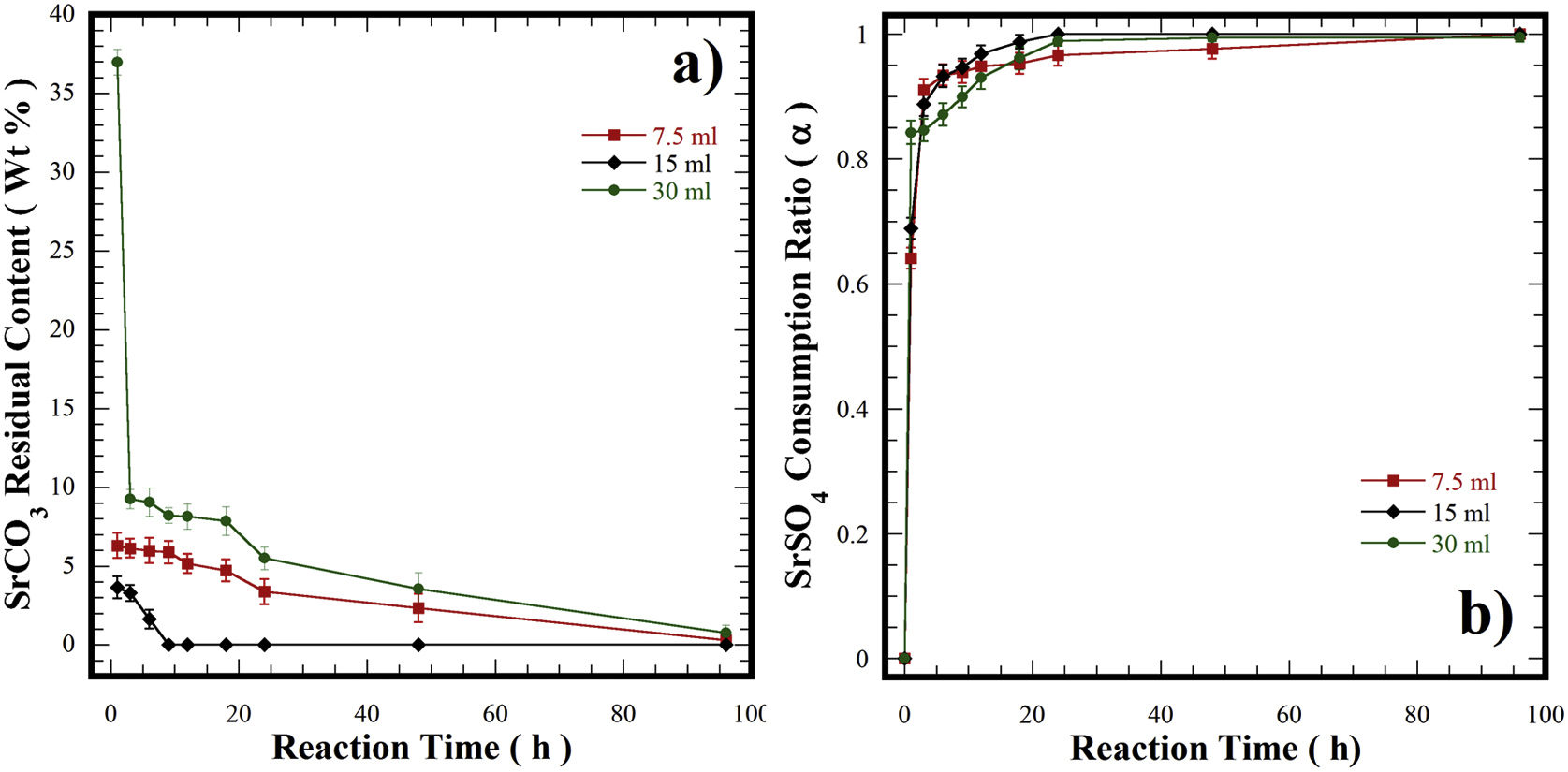
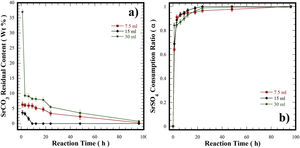
(a) Curves of residual content of SrCO3 at finished of hydrothermal treatments at 240°C for various reaction intervals. (b) Curves of the variation of the consumption of the precursor SrSO4 (α) under hydrothermal conditions at 240°C for various reaction intervals in a 5M of KOH solution and different filling volume of the autoclave ■ 10.7, ♦ 21.4 and ● 42.8%.
The effect of the solvent-filling ratio that eventually affects the course of the transformation of SrSO4 into SrZrO3 was evaluated for three different 5M KOH solution volumes of 7.5 (10.7%), 15.0 (21.4%), and 30mL (42.8%) at 240°C (Fig. 6). The net amount of KOH changed with each filling ratio, while the solid feedstock (SrSO4 and Zr(OH)4) amounts were kept constant. This parameter alters the bulk molar SO42−/OH− ratio in the reaction vessel [33]. Generally, at early reaction times between 1h and 6h, the remaining SrCO3 content varied between 3.2wt% and 6.0wt% with the 21.4% (15mL) and 10.7% (7.5mL) filling ratios; however, a considerable amount of SrCO3 was produced (37.0wt%) when the largest 42.8% (30mL) filling ratio was used. A remarkable reduction in the byproduct content was triggered between 6 and 24h, causing a SrCO3 content decrease that proceeded slowly for the experiments carried out with the lowest and largest fluid filling ratios, thus, the minimum byproduct content produced was 3.4wt% and 5.5wt%, respectively. In contrast, SrCO3 formation did not occur within the intervals between 8h and 24h in the 21.4% fluid filling ratio (see Fig. 6a). Furthermore, at 30mL, the molar SO42−/OH− ratio was 0.08. In this solution, a large CO32− mass gradient resulting from the net KOH solution volume induced a high CO32− saturation state that triggered the simultaneous crystallization of SrCO3 and SrZrO3 particles. The remarkable SrSO4 consumption obtained in this solution was up to 93.0% (α=0.93) and took place between 8 and 24h, as seen in Fig. 6b. However, the SrCO3 particle dissolution did not proceed simultaneously in the alkaline media. Consequently, the dissolution hinders the ultimate single-step reaction progress of SrSO4 into SrZrO3 over intervals of 48h (Fig. 6b). Interestingly, a similar reaction trend also occurred in the KOH volume of 7.5mL. The molar ratio (SO42−/OH−) in the solution corresponds to 0.35, and the minimum residual SrCO3 content produced after treatment was 3.0wt%. The SO42− total content in the SrSO4 was kept constant in all the experiments. Under these conditions, the mass gradient produced (SO42−/OH−) in the fluid is likely to reduce the KOH solution dissolving capability for eliminating the byproduct SrCO3 particles (Fig. 6b). This inference agrees with the slight reduction in the amount of residual SrCO3 determined during the reaction interval between 1h and 24h (Fig. 6a) when the molar SO42−/OH− ratios reached 0.08 and 0.35.
The abovementioned results indicate that the filling volume of 15mL (21.4%) that provides an intermediate molar SO42−/OH− ratio (0.19) is the optimum fluid volume to maintain a mass gradient that does not reduce the fluid alkalinity to dissolve both the remaining SrSO4 and SrCO3. Under these conditions, the steady reaction state follows the chemical equilibrium in Eq. (4) to complete the SrSO4 transformation into SrZrO3. Hence, the critical factor that strongly affects the single-step reaction pathway to produce SrZrO3 is the molar SO42−/OH− ratio coupled with the dried Zr-gel precursor. The optimum volume and concentration of the KOH solution selected to carry the conversion process are likely to minimize the production cost and the environmental damage by the waste products. The main reaction byproduct (K2SO4–KOH solution) is expected to be economically treated by acid neutralization and evaporation to K2SO4·nH2O [34–36], which is widely used as fertilizer with an annual production of approximately 1.6 million tons [37–40].
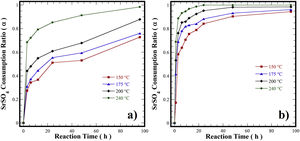
Differences in the kinetics related to the transformation of SrSO4 into SrZrO3 particlesThe kinetic process associated with the single-step reactions (Eqs. (3) and (4)) involve the consumption fraction (α) of the particulate SrSO4 and the Zr-gel source employed. This evaluation was conducted at a stoichiometric molar Sr:Zr ratio of 1:1 at four temperatures varying within the range of 150–240°C for various reaction intervals. The SrSO4 consumption fraction (α) with time under static hydrothermal conditions in 5M KOH for the two Zr-gel precursors studied (as-produced pasty gel and dehydrated Zr(OH)4 powder) is shown in Fig. 7.
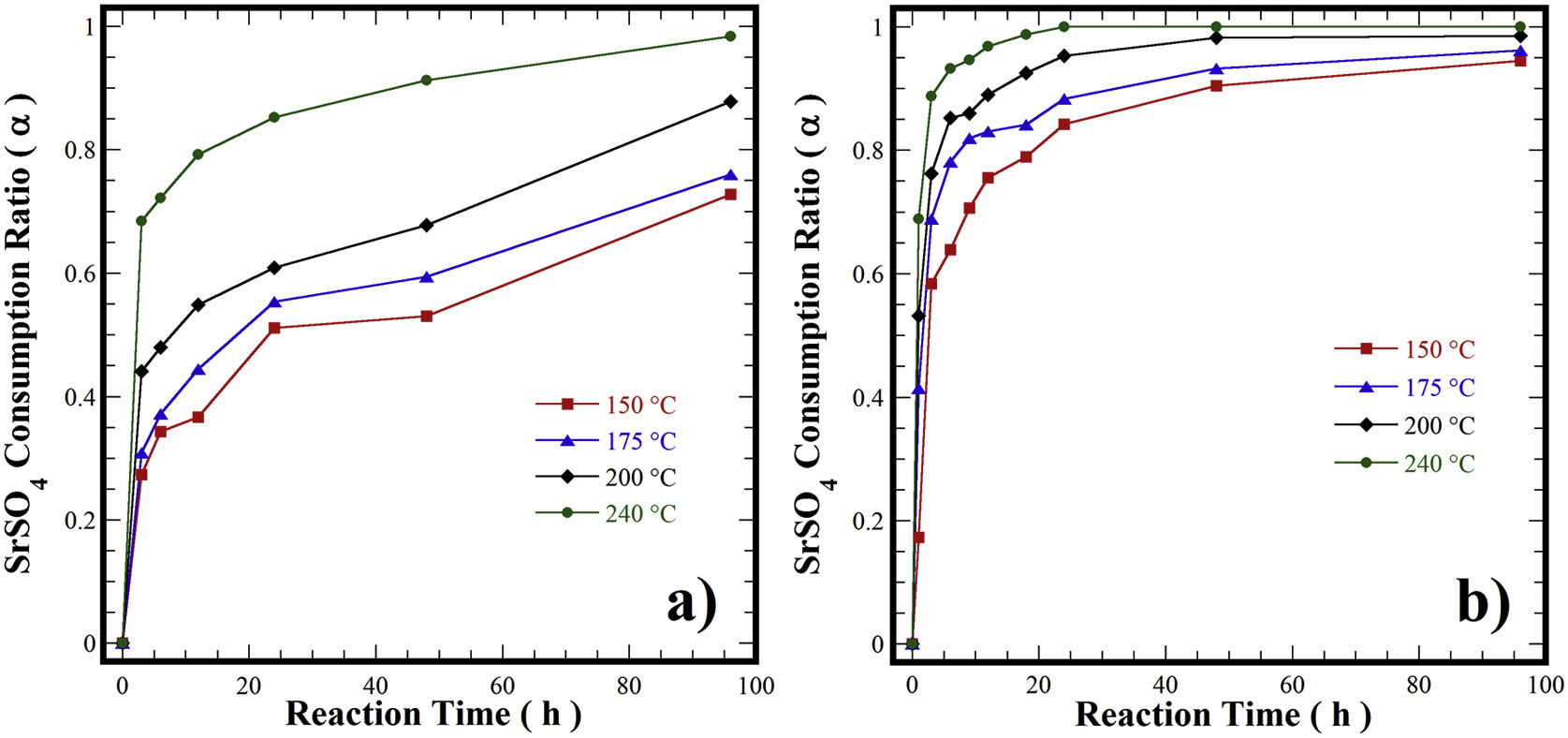
Curves of the ratio of SrSO4 consumption in the partial and totally transformation in the SrZrO3 compound under hydrothermal conditions, in three different temperatures (150, 175, 200 and 240°C) in an alkaline reaction medium 5M of KOH and using treatments were performed with an autoclave fill rate of 21.4% for all reaction intervals, respectively.precursors mineral of celestite and Zr(OH)4 in the form of (a) Zr-gel in pasty and (b) Zr-gel in powder.
In general, when the treatments were carried out using the pasty Zr-gel at different temperatures (Fig. 7a), the SrSO4 feed was slowly consumed at reaction temperatures below 200°C over 48h. The reaction reached 63% dissolution of SrSO4 (α=0.63) and increased up to 84% (α=0.84) for 96h. The increase in the reaction temperature at 240°C for 96h provoked a considerable increase in celestite consumption up to 96% (α=0.96), which was the maximum yield efficiency involved in the dissolution of SrSO4 mixed with the pasty Zr-gel, Zr(OH)4·9.64H2O. Nevertheless, the consumption kinetics were further improved when the hydrothermal treatments were conducted with the dried Zr(OH)4 powder. In this case, the amount of SrSO4 consumed even at 150°C was 83% (α=0.83). Interestingly, the complete mineral feed consumption into SrZrO3 occurred with yields of 100% (α=1.0) for a short reaction interval of 24h at the highest temperature (240°C), as seen in Fig. 7b. Hypothetically, the dry Zr(OH)4 gel contributes to the acceleration of the SrSO4 consumption in the reaction system because the dehydration process associated with the pasty gel did not take place in these treatments. The quantitative analyses outlined above indicated that the gel dehydration process causes a bulk molar variation in the solvent alkalinity, which might decrease the solvent's capability to provoke the complete dissolution of the SrSO4 feed to produce a 100% yield of SrZrO3. Hence, the highly reactive Zr(OH)4 gel rapidly dissolves and does not decrease the rate limiting bulk dissolution of the raw mineral ore, allowing a rapid SrZrO3 phase precipitation.
According to the literature survey, various classes of kinetic models have been derived for solid–liquid reaction systems occurring at low temperature and atmospheric pressure conditions [28,30]. Likewise, another traditional model has been applied to evaluate the kinetics of solid–liquid reactions, such as the Johnson–Mehl–Avrami model developed primarily to describe the solid-state reaction kinetics [41,42]. In the last decade, new models based on population balance approaches have been developed for reactions to produce perovskite-structured particles under hydrothermal conditions [16,34,43,44]. However, these models were excluded from the analysis due to the experimental conditions that triggered homogeneous nuclei formation and crystal growth in our case, which differed from those established to apply the population-based model. Hence, in the present work, we tested various models against the experimental SrSO4 consumption data.
The current experimental conditions investigated for producing functional SrZrO3 correlate with uniform system variables, such as the SrSO4 feed granulometry, temperature, molar SO42−/OH− ratio, and Zr4+ precursor consistency. These variables strongly affect the chemical interactions that might produce differences in dynamic/geometric factors, such as dissolution geometry and peculiar reaction rim formation, which we could not observe ab initio. Therefore, the results of each experiment conducted on the conditioning regime might depend on the interaction of these factors, and their relative importance allows for selecting a suitable quantitative kinetic model function. In the case of the study here, the steady state of the reaction involving solute (Sr2+ and Zr4+) homogeneous saturation in alkaline media for each given reaction temperature and type of Zr-gel precursor yields an optimum reaction rate for forming SrZrO3 particles while achieving homogeneous nucleation and continuous particle coarsening [25], which typically occurs in hydrothermal systems. Among the solid–liquid reaction models that are likely to match the reaction pathway described in Section 3.3 and were found to fit the experimental SrSO4 consumption kinetic data obtained using two different Zr-gel precursors under static hydrothermal conditions (Fig. 7), consider the first (F1, −ln(1–α)=kt) and second (F2, (1−α)−1=kt), the Jander diffusion-controlled function (D3, (1−(1−(1−α)1/3)2=kt)) and the Johnson–Mehl–Avrami function (JMA, ln[ln(1/1−α)]=kt). These kinetic functions were examined because the conversion kinetics related to the proposed solid–liquid reaction in each model are likely analogous to the alkaline hydrothermal processing of SrZrO3 particles from SrSO4 feedstock powders.
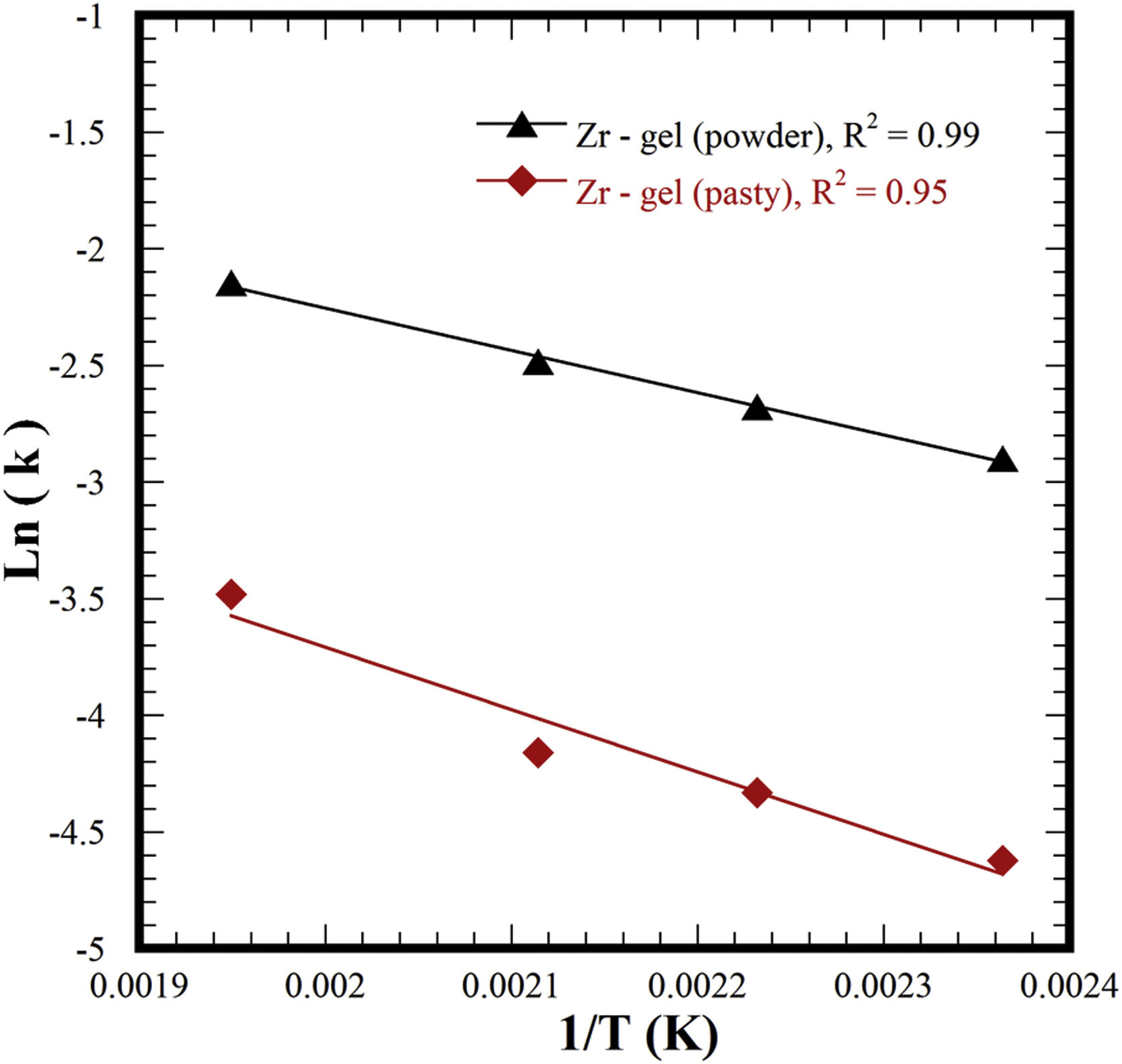
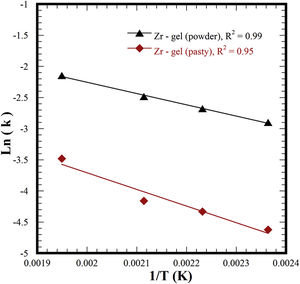
It is worth emphasizing that the experimental kinetic data for the two different Zr-gel precursors accurately matched the first-order kinetic function (F1) because of the high correlation coefficient R2 values calculated in both cases, i.e., 0.96–0.99 for the paste Zr-gel and 0.97–0.99 for the dried Zr(OH)4 powder. Likewise, the ionic diffusion-controlled function of Jander D3 (0.95 and 0.99) and F2 (0.90 and 0.95) exhibited high R2 values. These functions assume that a massive ionic diffusion process controls the SrZrO3 crystallization at the reaction interface, but this reaction mechanism was interrupted because the ionic species massive diffusion did not proceed. Under steady-state hydrothermal reaction conditions, ionic transport did not occur in our treatments because these were conducted without agitation and vessel thermal gradients. According to these results, we determined that the single-step hydrothermal transformation of SrZrO3 particles is a chemical reaction-controlled process, which is analogous to the hydrothermal alkaline synthesis of SrTiO3 particles using a SrSO4 feed precursor [25]. Interestingly, faster SrSO4 particle dissolution occurred with the dried Zr-gel powder feed, triggering the rapid, local homogeneous formation of nuclei and particle coarsening without mass transfer in the liquid. The calculated constant rates for each set of experiments were employed to determine the activation energy (Ea) required to transform the feeding SrSO4 and Zr-gel precursors into SrZrO3 powders. The Ea value determined from the Arrhenius plot for the experimental set conducted with the pasty Zr-gel (Zr(OH)4·9.64H2O) was 22.27kJmol−1 (see Fig. 8). However, a decrease in the Ea value occurred by using the dried Zr(OH)4 to carry out the treatments; the minimum Ea value associated with Eq. (4) was 15.05kJmol−1. The Ea values calculated for the SrSO4 transformation into SrZrO3 using two Zr-gel precursors are similar to those of analogous functional materials produced via the single-step mineral transformation reaction, i.e., SrTiO3 (27.9kJmol−1) [25] and SrWO4 (27.2kJmol−1) [33]; these compounds were prepared with a 5M KOH solution under the same hydrothermal conditions used here.
The literature survey suggests that the efficiency of mineral transformation processing under hydrothermal conditions might proceed by triggering mass ionic transport via mechanical stirring. Indeed, this inference was experimentally proven elsewhere [45], at a rotation speed of 20rpm, and the activation energy required for the hydrothermal transformation of SrSO4 into SrWO4 was reduced to 18.3kJmol−1; this low Ea value is related to the chief single-step reaction [45] and correlates the feasibility for boosting the hydrothermal process efficiency; this Ea value is nearly comparable with that calculated for the conversion of SrSO4 into SrZrO3. However, in this study, the process efficiency was promoted by separately dehydrating the pasty Zr(OH)4·9.64H2O gel rather than carrying out the hydrothermal treatments under stirring. The dry Zr(OH)4 powder accelerates the efficiency of the entire chemical process to synthesize the SrZrO3 using the mineral powder. Hence, the noticeable Ea reduction resulted from the water molecules released from the Zr-gel in the preliminary dehydration step. The in situ dehydration reaction, however, cannot be avoided when the pasty metal gel was the feedstock for the hydrothermal treatment [25]. This reaction limits the transformation rate, causing an increase in the SrZrO3 conversion activation energy and the entire process efficiency. Hence, the present approach provides the first experimental evidence for the optimum processing of a perovskite compound powder, which took place in a mild alkaline hydrothermal media compared to those employed in previous studies. In addition, this approach opens the possibility of preparing other functional compounds based on the single-step hydrothermal reaction methodology. Based on the results, we surmise that the present chemical processing has the potential to be applied for large-scale industrial preparation because the process is economically attractive due to its low energy consumption, which is associated with the low activation energy required to produce the powder feeding transformation.
ConclusionsThe hydrothermal processing investigated for preparing the SrZrO3 compound was successfully carried out using the low-grade SrSO4 ore and two Zr-gel precursors. The transformation processing of celestite (SrSO4) powder (<38μm) was further accelerated using a dried Zr(OH)4 powder; this enables complete transformation via a single-step reaction, resulting in rapid SrZrO3 powder formation at 240°C for a short reaction interval of 24h. In contrast, the SrZrO3 formation proceeded slowly using the hydrated alkaline Zr-gel precursor, and the maximum SrZrO3 yield obtained for 96h at 240°C was 97.5% (α=0.97). The synthesis of SrZrO3 particles was triggered by a massive dissolution–crystallization mechanism of the ionic species, which was affected by the Zr-gel reactivity rather than the SrSO4 feedstock powder. The growth of SrZrO3 particles with bimodal distribution (25.0 and 65.0μm average sizes) and flower-like morphology occurred by a decrease in the alkalinity resulting from the water release from the pasty Zr-gel. In contrast, monodispersed (25μm on average) SrZrO3 cuboidal particle formation was rapidly triggered by the dried Zr-gel powder. In both cases, the SrZrO3 particle growth took place by the Ostwald Ripening mechanism, which resulted in epitaxial faceted coarsening provoked by the dissolution–crystallization of SrZrO3 small particles.
In addition, the formation of SrCO3 that retards the transformation reaction of feedstocks to produce SrZrO3 depends on the bulk OH− concentration, this phenomenon predominantly occurs during early reaction intervals (1–3h) irrespective of the fluid volume employed. However, at an autoclave filling volume of 15mL (21.4%) with a molar SO42−/OH− ratio of 0.19, the byproduct dissolution occurred rapidly during intermediate intervals over 6h resulting on the solely formation of SrZrO3 particles. This process was markedly hindered on treatments conducted at low (7.5mL) and large (30mL) 5M KOH volumes supplying molar SO42−/OH− ratios of 0.38 and 0.1, respectively, because the CO32− mass gradient is favorable to stabilize the SrCO3 byproduct when these volumes were employed. Furthermore, the kinetics analysis for the proposed reaction systems revealed that the activation energy for the single–step transformation of SrZrO3 powder from the precursor SrSO4 mineral is low, 15.05kJmol−1, and it increases to 22.27kJmol−1 because the dehydration reaction preceding the ultimate chemical reaction slowed down the SrZrO3 crystallization. Hence, these results indicate the potential application of the hydrothermal processing method derived in this work for processing other functional inorganic perovskite compounds.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This study was supported by the federal research budget of the Centre for Research and Advanced Studies of the NPI (C-3000). J.C.R.A, Z.M.V., and J.L.R.G. are indebted to CONACYT-SNI. J.R.Q.G. is indebted to the National Council for Science and Technology of Mexico for its financial support as a PhD scholarship. Many thanks are also given to PhD T. Matsuzaki from the Center of Advanced Marine Core Research, Kochi University, Japan, for his assistance with the Field Emission Scanning Electron Microscopy observations.
The Supporting Information is available free of charge at https://j.bsecv.org/doi/.
Complementary X-ray diffraction patterns of various reaction conditions; Rietveld refinement plots; FE-SEM images of particles produced at various reaction stages; HRTEM and SAED images and microprobe mapping observations; chemical compositional analyses of SrZrO3 particles, (PDF).
The following are the supplementary data to this article: