In this present study, synthetic tobermorites are prepared using bio-waste (snail shell) and municipal waste (container glasses) as lime and silica precursors respectively. Six batch compositions were formulated with varying combination of soda-lime glass and snail shell ash. The bodies were sintered at 950°C for a holding period of 2h in an electric muffle furnace. Analyses such as scanning electron microscopy (SEM/EDS), Fourier Transform Infra-red Spectroscopy (FT-IR), X-ray diffractometry (XRD) were used to assess the microstructure, functional groups and the phase composition of the prepared tobermorites respectively. The results of the morphology shows that the tobermorites possess irregular but spherical shaped grain with coated water films while the EDS shows the presence of Ca and Si with small amount of Al confirming tobermorite. The FT-IR indicates Ca–O–Si and Si–O–Si as main functional groups while the phase composition investigated by XRD indicate low intensity peaks of calcium silicate (CaSiO3).
En este estudio, las tobermoritas sintéticas se preparan utilizando biorresiduos (caparazón de caracol) y residuos municipales (vasos de contenedores) como precursores de cal y sílice, respectivamente. Se formularon seis composiciones discontinuas con una combinación variable de vidrio de cal sodada y ceniza de concha de caracol. Los cuerpos se sinterizaron a 950°C durante un período de retención de 2 h en un horno de mufla eléctrico. Se utilizaron análisis como microscopía electrónica de barrido (SEM/EDS), espectroscopía infrarroja por transformada de Fourier (FT-IR), difractometría de rayos X (XRD) para evaluar la microestructura, los grupos funcionales y la composición de fase de las tobermoritas preparadas, respectivamente. Los resultados de la morfología muestran que las tobermoritas poseen un grano irregular pero esférico con películas de agua recubiertas, mientras que el EDS muestra la presencia de Ca y Si con una pequeña cantidad de Al que confirma la tobermorita. El FT-IR indica Ca-O-Si y Si-O-Si como grupos funcionales principales, mientras que la composición de fase investigada por XRD indica picos de baja intensidad de silicato de calcio (CaSiO3).
Over the years, crucial economic and consideration for environment has geared several industries and researchers to develop and improve technologies targeted at drastically reducing wastes accumulation such as bio, agro, municipal and industrial wastes. In view of this, numerous efforts have been devoted on the utilization of these wastes which are known to be highly rich in vital chemical oxides such as silica (SiO2), lime (CaO) and alumina (Al2O3) to develop new and useful products [1–4].
Tobermorites are a family of naturally occurring hydrous calcium silicate minerals that exhibit selective alkali exchange when replaced with sodium and aluminum [5] and have been known to be an efficient sorbent for removal of divalent lead and cadmium ions in wastewater treatment [6]. However, due to rarity, several methods have been adopted for its preparation; which include sol-gel [7], hydrothermal [8] and sintering reaction [9]. Tobermorites have been prepared using various chemically pure materials as precursors but are known to be quite expensive [5,10]. However, in order to lower cost and energy, recent practices have adopted the use of wastes as precursors. Diatomite and rice husk ash has been used as silica sources [4,11] while egg shell and marble waste served as precursors for lime [3,12]. However, little or no detailed works have been investigated on the preparation of tobermorite from snail sell ash (bio waste) and waste soda-lime-silica glass (municipal waste) as precursors for calcium oxide and silica respectively.
In this regard, this present study aimed at preparation and characterization of synthetic tobermorite from waste glass (municipal waste) and snail shell ash (bio-waste) at varying compositions using sintering (solid state reaction) method.
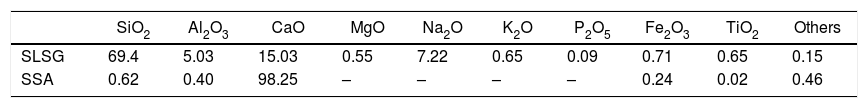
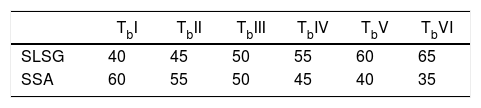
Material and methodsMaterialThe starting materials utilized in this work are waste soda-lime-silica glass (SLSG) of mix colors (green, amber and clear) and discarded snail shells (SS), which were obtained from municipal dumpsite. The SLSG is to serve as source of silica (SiO2) along with other needed oxides while the SS is a precursor for calcium oxide (CaO). The concept of adopting SLSG of mix colors is to minimize time and cost of sorting and the fact that color constituents cannot impaired the performance of the SLSG for the intended purpose [2]. The as-received waste glasses were taken through processing route in accordance with Owoeye et al. [2] to obtain a fine powder of 63μm. On the other hand, the as-received snail shells were initially washed thoroughly to remove adhered dirt and later dried in an electric oven at 110°C for 4h. The dried snail shells were then pyrolyzed at 950°C in a muffle furnace for a hold period of 4h to obtain a somewhat whitish snail shell ash (SSA) known to be highly rich in lime (CaO). The snail shell ash was then sieved to obtain a fine powder of 75μm. The chemical composition of the recycled SLSG is based on the work of Owoeye et al. [2] while that of SSA is according to Rimruthai et al. [13] as shown in Table 1. Fig. 1(a–d) indicates the representative diagram of the utilized materials.
A total of six (6) compositions comprising of varying weight percent mixtures of SLSG and SSA were prepared in this work as shown in Table 2. The bodies were thoroughly mixed respectively in a ball mill for several hours with addition of organic solvent as binder. The homogeneously mixed bodies were then uniaxially pressed respectively under a load of 10MPa. The pressed samples were initially allowed to dry at ambient temperature followed by oven drying at 110°C and later subjected to sintering in an electric muffle furnace at 950°C at a rate of 10°C/min for a holding period of 4h for proper solid state reaction to produce tobermorite. Table 2 indicates the sample designation for the prepared tobermorite with their varying amount of SLSG and SSA. Scanning electron microscopy with attached energy dispersive spectroscopy (Phenom Prox. SEM/EDS) was used to investigate the morphology and chemical composition of the synthesized tobermorite while Fourier transform infra-red spectrometry (spectrum 100 FT-IR Spectrometer, Perkin Elmer) was used to study the functional groups at wavenumber ranging from 500–4000cm−1. Phase identifications were determined by X-ray diffractometer using BRUKER AXS with D8 Advanced diffractometer Cu Kα radiation XRD in the range of 2θ angle from 5 to 70 scanning range. All the analytical procedures were carried out at room temperature (25°C).
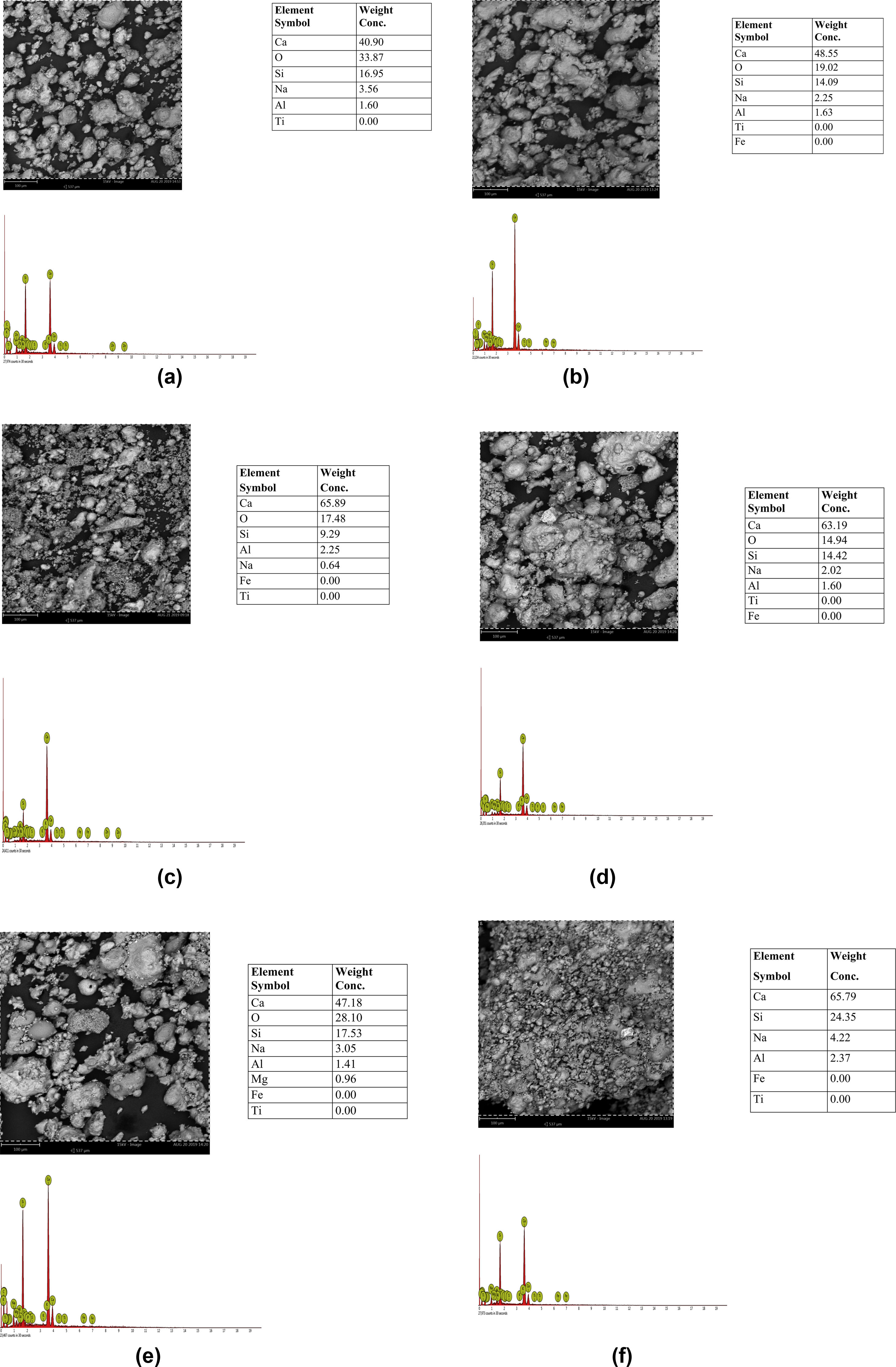
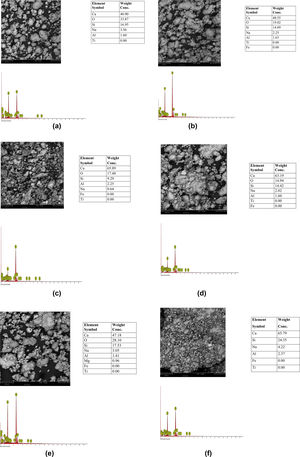
Results and discussionMorphological characteristics/chemical compositionThe results of the microstructure examination investigated by scanning electron microscopy with attached energy dispersive spectroscopy (SEM/EDS) on the synthesized tobermorite samples TbI–TbVI are shown respectively in Fig. 2(a–f). From the SE micrographs, it can be observed that all the synthesized tobermorite particles exhibited similar morphological characteristics. It is observed that all the synthesized tobermorite samples displayed somewhat irregular but spherical shaped particles which are mostly agglomerated. It can also be observed that a small amount of water indicated by a bubble-like film is wrapped on the agglomerated particles which give typical characteristics of calcium silicate hydrate (C-S-H) gel [14]. From the EDS spectra of all the synthesized tobermorites, it is observed that they all comprise mainly Ca and Si while a small amount of Al and Na is observed which might be attributed to the aluminum and sodium content present in the waste glass used. The presence of Ca, Si and Al confirms that the synthesized product is tobermorite containing two major phases of C-S-H and C-A-S-H (calcium aluminosilicate hydrate). These two major phases are indication that the synthesized tobermorite will be efficient for water treatment in the removal of heavy metals [15]. However, samples TbII and TbVI are regarded as the best tobermorite samples in this work due to their Ca/Si ratio, morphology and pore nature, thus serving as good sorbent for adsorption of heavy metals or filter medium.
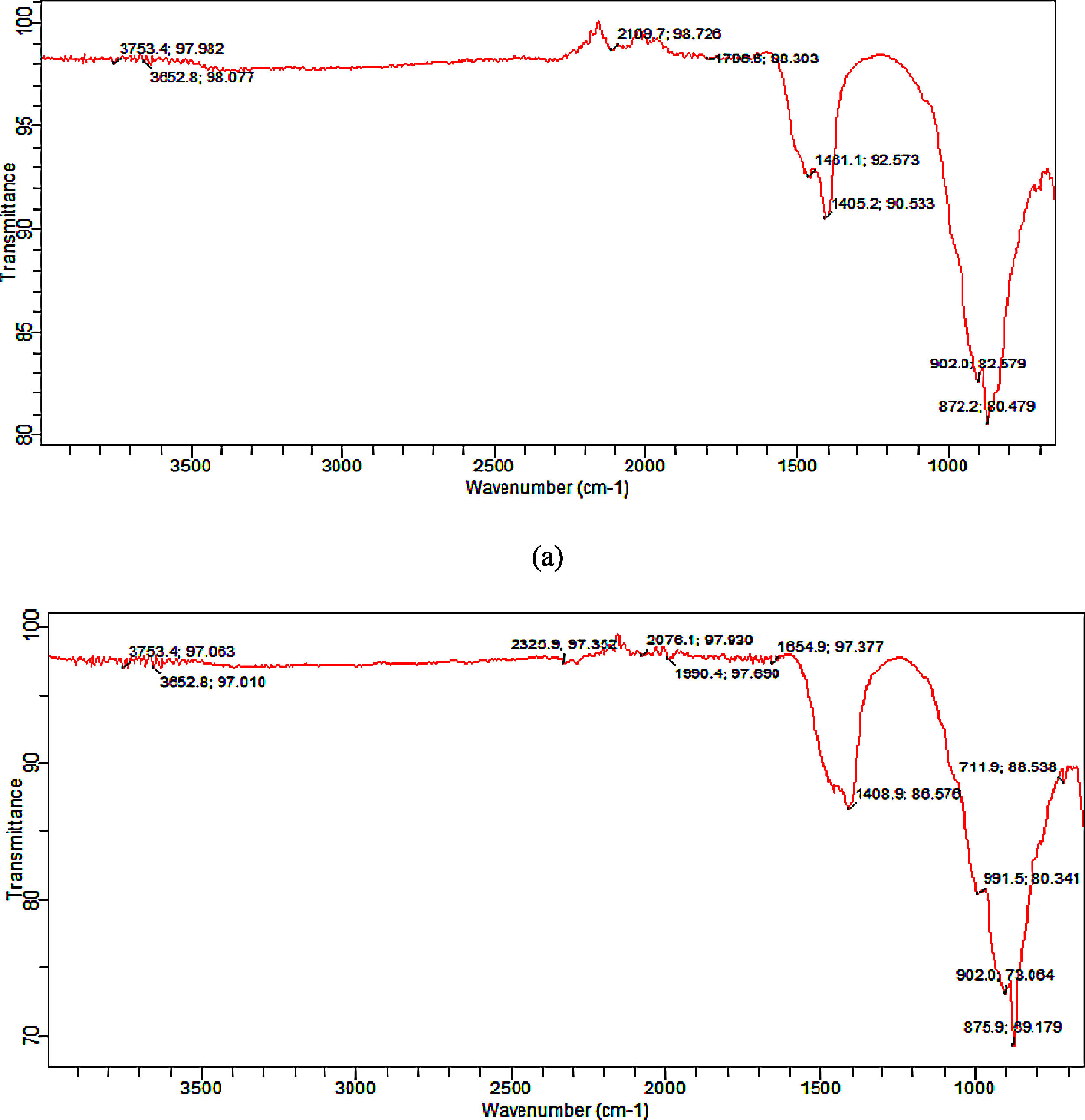
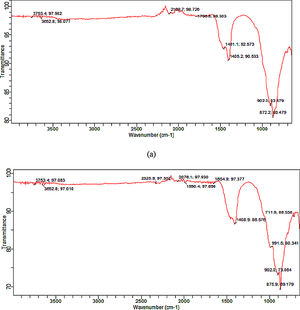
Functional group (synthesized tobermorite)Fig. 3(a) and (b) shows the representative diagram of the functional groups present in the synthesized tobermorite samples using FT-IR. Representative FT-IR diagram was used since all the synthesized tobermorites displayed similar functional peaks. However, sample TbII and TbVI exhibited broader functional peaks. The IR spectra were recorded by FTIR at wavenumber ranging from 500–4000cm−1. The transmission peaks observed between 3753.4–3652.8cm−1 might be attributed to the –OH group in calcium oxide as unreacted calcium oxide with water vapor [16]. However, the band in the range 1000–850cm−1 can be attributed to Ca–O–Si (calcium silicate) as stated by Meiszterics and Sinko [17]. The intense band between 872.2 and 902cm−1 might be due to Si–O–Si.
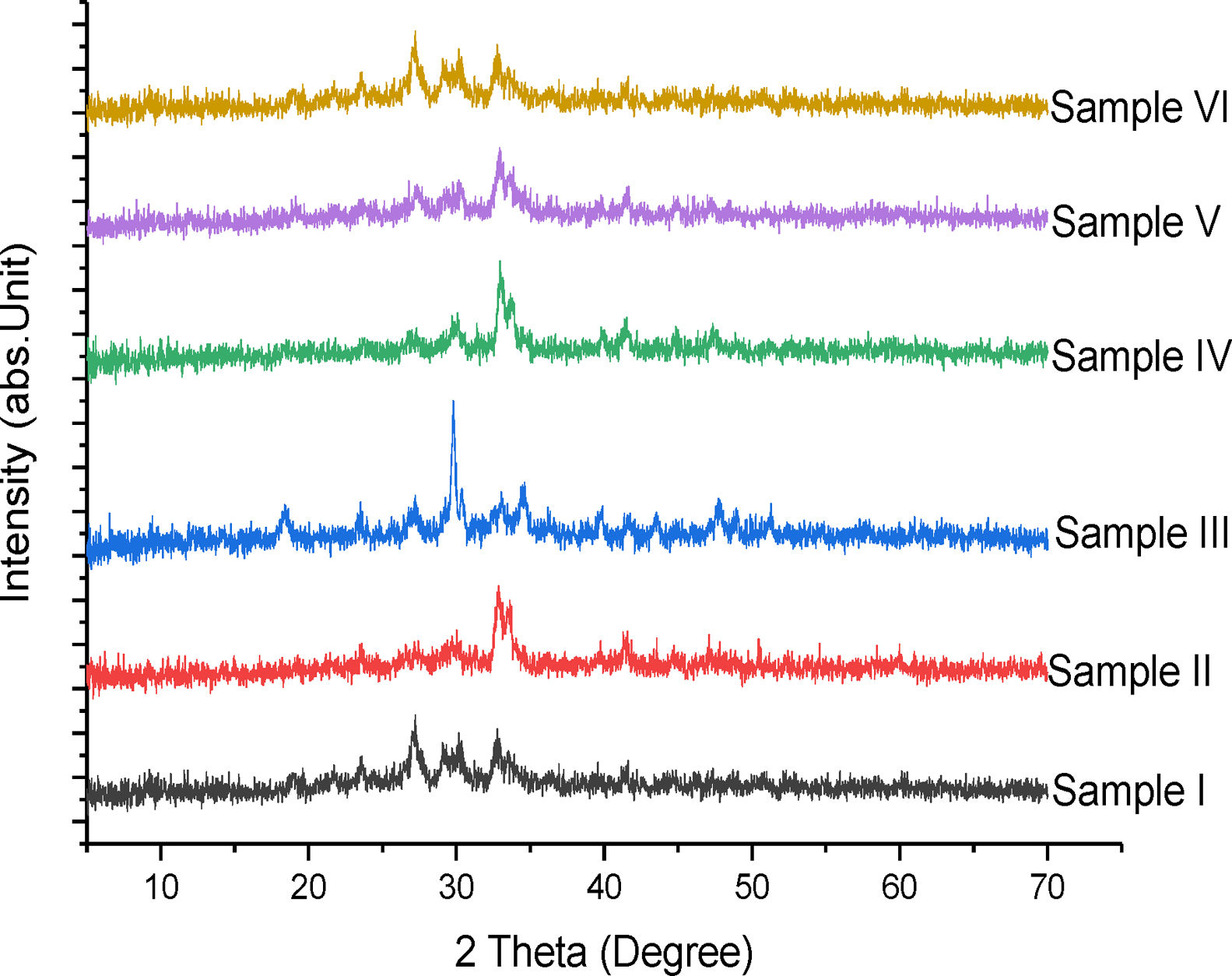
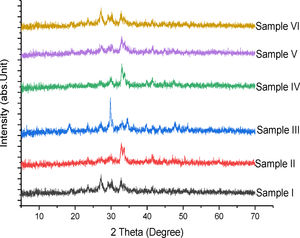
Phase compositionFig. 4 shows the superimposed diagram of the phase identification of the synthesized tobermorites (TbI–TbVI) respectively. It is observed that the synthesized tobermorites have somewhat close resemblance to those synthesized using soda-lime glass by Nichola et al. [18]. The low intensity peaks observed indicate the presence of CaSiO3[13], thus confirming the synthesized products to be tobermorite.
ConclusionThis research has successfully investigated preparation and characterization synthetic tobermorite (CaO–Al2O3–SiO2–H2O) from waste glass and snail shell ash using sintering method. The following conclusions were drawn based on the results obtained:
- •
Bio-waste (snail shell) and industrial waste (soda-lime glass scraps) can be successfully used as precursors for the synthesis of tobermorite instead of using chemically grade materials that are expensive.
- •
The morphology features indicate irregular and agglomerated but somewhat spherical grains coated with bubble-like film of water which is typical characteristics of calcium silicate hydrate (C-S-H) gel.
- •
The chemical composition by the EDS indicates the presence of Ca, Si and Al confirming that the synthesized product is tobermorite containing two major phases of C-S-H and C-A-S-H (calcium aluminosilicate hydrate). These two major phases are indication that the synthesized tobermorite.
- •
The FT-IR showed Ca–O–Si as the main functional group in the tobermorite while the XRD indicate low intensity peaks of calcium silicate (CaSiO3).
- •
For future work, the efficiency of the synthesized tobermorite products as adsorbent shall be evaluated.
The authors declare no conflict of interest.
The authors acknowledged the effort of TETfund Nigeria and Centre for Research, Innovation and Development of the Federal Polytechnic, Ado-Ekiti, Nigeria for the research grant given to finance this work.