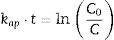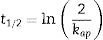Photocatalytic processes are an efficient and important technique to mineralize organic contaminants in aqueous effluents. However, it is paramount that there is a way to recover the catalyst after degradation. Based on this problem, this research seeks to evaluate the photocatalytic properties of TiO2 under porous ceramics support for the degradation of Rhodamine B (RhB). TiO2 was synthesized by sol-gel, dried at 100°C and calcined at 400°C. The morphological, optical and structural proprieties of the particles were characterized. The XRD patterns of samples calcined at 400°C showed only the anatase phase, confirmed by Raman. Not heat-treated xerogel was amorphous. The agglomerates are composed of fine particles, in the nanometric scale of 15nm. The bandgap of the powder is 3.21eV, and the surface area is 60.1m2g−1. To evaluate its photocatalytic activity, the anatase TiO2 was supported in a porous ceramic substrate by a dip-coating process. The heterogeneous photocatalysis showed excellent results, with the degradation of up to 83% of RhB. It was possible obtained with successful an efficient technique for the treatment of wastewater with Anatase nanoparticles supported in the ceramic support obtained from of reuse of the residues.
Los procesos fotocatalíticos son técnicas eficientes para la mineralización de contaminantes orgánicos en efluentes acuosos. Sin embargo, es fundamental que haya una forma de recuperar el catalizador después de la degradación. En base a este problema, esta investigación busca evaluar las propiedades fotocatalíticas del dióxido de titanio (TiO2) bajo el soporte de cerámica porosa para la degradación de la rodamina B (RhB). El TiO2 se sintetizó mediante sol-gel, se secó a 100°C y se calcinó a 400°C. Se caracterizaron las propiedades morfológicas, ópticas y estructurales de las partículas. Los patrones DRX de las muestras calcinadas a 400°C mostraron solo la fase anatasa, confirmada por Raman. El xerogel no tratado térmicamente era amorfo. Los aglomerados están compuestos de partículas finas, en la escala de 15nm. El intervalo de banda es de 3,21eV, y el área de superficie es de 60,1m2.g−1. Para evaluar la actividad fotocatalítica, se recubrió un sustrato cerámico poroso mediante el proceso de recubrimiento por inmersión. La fotocatálisis heterogénea mostró excelentes resultados, con la degradación de hasta el 83% de la RhB. Fue posible obtener con éxito una técnica eficiente para el tratamiento de aguas residuales con nanopartículas de anatasa soportadas en el soporte cerámico obtenido de la reutilización de los residuos.
Nowadays the management of wastes generated by industrial operations represents a challenge for a sustainable world. The management of wastes may comprise steps of collection, transportation, treatment, recycling, and landfill disposal after reducing the volume. An issue of concern from the environmental perspective is the water pollution caused by organic dyes. Wastewater containing dyes represents a serious environmental problem because of the high toxicity and possible accumulation of these dyes in the environment due to their carcinogenic and mutagenic effect [1].
Furthermore, dyes in textile mill effluents, in agates dyeing industries and dye industry of paper, may induce antagonistic and synergistic toxicological effects in aquatic life and human health. Rhodamine is one of the most common dyes used in this process [2,3]. The non-biodegradable nature of organic dyes and their high color intensity can also reduce aquatic diversity by blocking the passage of sunlight through the water, especially in the process of photosynthesis and oxygenation of the water spring that receives this effluents [2,4,5]. Besides, these organic dyes are commonly employed as a model molecule in degradation tests [6,7].
Advanced oxidation processes (AOPs) have been mainly the most engaged approach for the reduction of dyes in environmental matrices [5]. The AOPs are based on the in situ generations of strong oxidants, mainly hydroxyl radicals (OH
) for the oxidation of organic compounds, capable of mineralizing organic matter to non-toxic forms such as CO2 and H2O [8]. Hydroxyl radicals formation can occur in some ways, especially by combining UV radiation with ozone (O3/UV), hydrogen peroxide (H2O2/UV), fenton and photo-fenton reactions (H2O2/Fe/UV), ultrasound, electrochemical oxidation (individual anodes), and heterogeneous photocatalysis [9].The heterogeneous photocatalysis technique has been widely studied as a method of destruction of organic and inorganic pollutants [8,10–14]. The process involves redox reactions induced by radiation on the surface of mineral semiconductors used as catalysts [11].
One of the most utilized photocatalysts is the titanium dioxide (TiO2), it is a polymorphous material and may present in the rutile, anatase and brookite phases [15]. The anatase phase, most commonly used, shows tetragonal crystalline structure, and is formed at low temperatures, around 450°C [16]. The excellent performance of TiO2 in heterogeneous photocatalysis processes is due it is insoluble in water, non-toxic, photostable and chemically stable over a wide range of pH [17]. Various routes can synthesize TiO2: sol-gel; condensation to inert gas; plasma evaporation; hydrothermal microwave-assisted; ultrasonic spray pyrolysis; hydrothermal techniques; and chemical vapor deposition, among others [8,18,19,17,20]. The sol-gel method is widely used in the production of oxide nanoparticles, given its low-cost and environmentally friendly character, coupled to the fact that it allows for the fine-tuning of several synthesis parameters [21].
For an effective heterogeneous photocatalysis, the photocatalysts can be used suspended in the solution or supported on some substrate. Reactors with suspended particles are more common for research in the laboratory [22]. The main advantage of these reactors is the high specific surface area of suspended catalyst particles, which contributes to the higher degradation rate of pollutants. However, for practical applications in large-scale, reactors with immobile TiO2 are preferred, because in this way the operation can be carried out continuously, and separation of catalyst particles at the end of the process is not required. Also, the supported photocatalytic carriers can be reused for several cycles, since in systems with suspended catalysts the particles can hinder the passage of light into the solution or further sediment, thus not being available for the process [23].
Like suspended photocatalysts, immobilized photocatalysts also have some downsides. In this case, the available reaction surface is reduced decreasing the degradation rate [24]. Because of that, the cycle of photocatalytic treatment has to be prolonged, lowering the daily capacity of treatment. In this respect, porous supports, especially clay-based, are very interesting because they are highly available in the earth's crust, cheap, and show mechanical, thermal and chemical stability [25]. Clays provide TiO2 with the high surface area, porosity, high number of surface active sites, improving the efficiency of the photocatalysis process [12–14].
In this context, the present work seeks to evaluate the potential of discoloration of Rhodamine B, in the process of heterogeneous photocatalysis using nanostructured titanium dioxide supported on porous ceramic material. For this study, the TiO2 was produced by sol-gel synthesis, and characterized by structural, morphological and optical properties. Then, the TiO2 was deposited by dip coating on the porous ceramic support and has shown promising results in the photocatalytic process.
ExperimentalThe synthesis of nanostructured TiO2 was performed by the sol-gel method [21]. The reagents were isopropyl alcohol 70% (Sigma Aldrich/Germany), titanium tetraisopropoxide (TIPT IV) 97% (Sigma Aldrich/Germany) and ultra-pure type 2 water (MilliQ, 18MΩcm) in 3.27;1.62;1 relation (vol/vol). Initially, the isopropyl alcohol was mixed with TIPT IV. The mixture was homogenized on a heating plate (BioMixer, 78HW-1) at 60°C for 1h, after which water was added to the system. After that, the temperature was raised to 100°C, and the mixture was stirred for another 2h until a gel was obtained. Finally, the TiO2 was dried (Marte, MB 150/6) at 100°C for 1h until the formation of a xerogel and heat-treated in a muffle furnace (Jung, 9613) at 2°Cmin−1 and a plateau of 2h at 400°C. The crystallinity of the synthesized TiO2 samples was determined by X-Ray Diffraction (XRD) using a Philips X’Pert MDP diffractometer equipped with a Cu Kα radiation source (λ=1.5418Å), 40kV, 40mA, step of 0.02°/s, dwell time of 2s per step and 2θ between 10 and 90° 2θ angle range. The surface area was determined using Brunauer–Emmett–Teller (BET) analysis, with the support of an Autosorb Quantachrome Instrument Nova 1000 device. The diffuse reflectance was done with the Agilent Cary 5000 UV–Vis–NIR universal measurement spectrophotometer in wavelength between 190nm and 1200nm. The Scanning Electron Microscopy (SEM) technique was used for the morphological characterization of the powder and ceramics substrate. The analysis was carried out in a Carl Zeiss, model EVO MA10 at 10kV. The Raman experiments were carried out on Renishaw inVia™ Spectrometer System for Raman spectral analysis, using a 532nm laser.
The TiO2 synthesized was supported in a porous ceramic (60×20×5mm) produced by the study group previously [26]. This porous ceramic is composed of 60% red clay and 40% of residues (20% glass powder, and 20% yerba mate), heat-treated at 1100°C. Several formulations were studied; tests of linear retraction, water absorption, apparent porosity, and mechanical strength were performed. This configuration was select due it presents values of residue aggregation of 40% and yet exhibits satisfactory properties when compared to the material without the addition of residues, like apparent porosity (26.19±0.25%) and water absorption (17.69±0.43%). Furthermore, good mechanical strength was obtained (7.93±0.26MPa) [26].
Two suspensions were obtained from catalyst and the deposition was evaluated: Suspension (a) with 5g of TiO2 in 100mL of ultra-pure water (Milli-Q) [27], and suspension (b): 5g de TiO2 and 10mL of ultra-pure water (Milli-Q) in 90mL of alcohol [28]. The suspensions were homogenized on a magnetic stirrer (BioMixer/78HW-1) for 24h until complete dispersion of the catalyst in the solution. After homogenization, the catalyst was deposited on ceramic material by a home-made dip-coating technique. Fifty immersions were performed in each ceramic substrate by 1min. To obtain the exact value of the catalyst involved in the reaction, only one side of the substrate received the material; the others were isolated.
After impregnation, the porous ceramics were dried (Marte, MB150/6) at 100°C for 60min and then thermally treated in a muffle (Jung, 9613), with a heating rate of 2°Cmin−1 at 400°C in 60min. The amount of photocatalyst supported in the porous ceramic was obtained by the difference in their mass before and after the impregnation process.
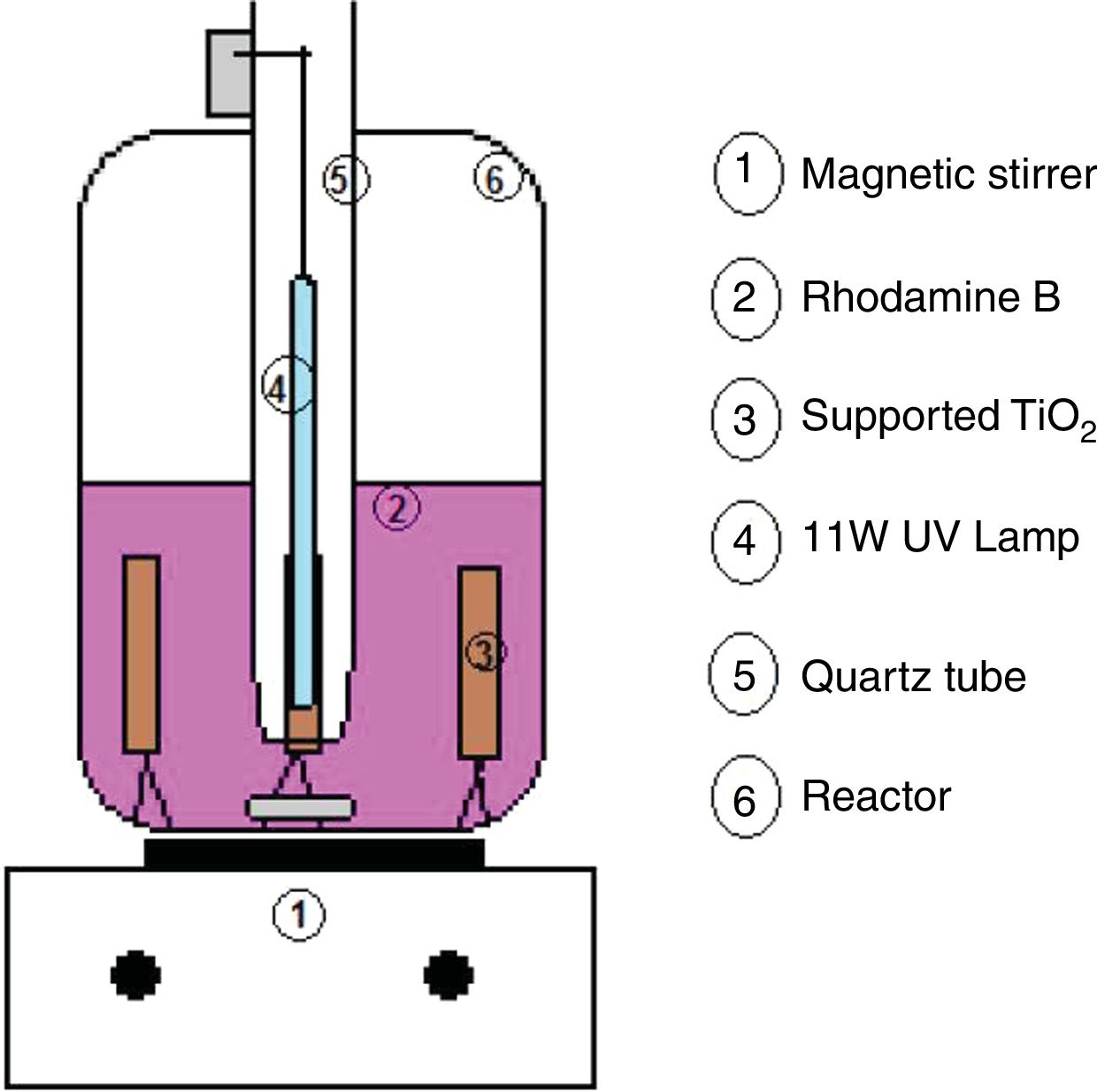
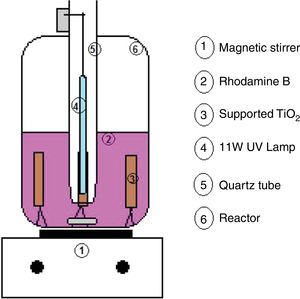
For each analysis, 500mL of Rhodamine B solution was used to simulate the effluent. A magnetic stirrer (BioMixer/78HW-1) was used, to maintain stirring during the tests. Fig. 1 shows the diagram of the reactor used. A low irradiation source was used (an 11W UV lamp/Osram), isolated from the solution by a quartz tube. Due to its low power, it does not change the temperature of the solution, and then the tests were performed at room temperature (20°C). Four substrates were placed per test, totaling 0.28g of TiO2, and application factor of 560mg of TiO2 per liter of effluent, affixed in a circular metal holder, with the catalyst containing face to the lamp.
Simulating a real effluent, a concentration of 5mgL−1 of Rhodamine B in deionized water was used. To relate the level of Rhodamine B, during the photocatalysis tests with its absorbance reading, a calibration curve of Rhodamine B was elaborated, where the results of absorbance were analyzed in a spectrophotometer (V-1100) with wavelength (λmax) for Rhodamine B at 550nm [29]. The calibration curve for Rhodamine B presented linear regression equation y=0.0044x−0.3406 and a coefficient of determination (R2) of 0.9981, showing good linearity.
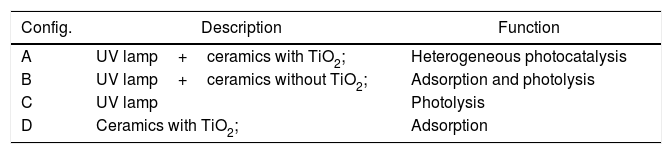
The heterogeneous photocatalysis degradation tests were performed for 120min with aliquot withdrawals every 10min for absorbance analysis in the first 60min. After this, an aliquot was withdrawals at 90 and 120min for each assay. It was proposed four configurations to evaluate the influence of heterogeneous photocatalysis, photolysis, and adsorption on effluent degradation, according to Table 1:
The kinetics of photocatalysis reactions best fit the Langmuir–Hinshelwood Model (Eq. (1)), with a pseudo-first-order degradation rate. In addition to the kinetic constant, it is possible to calculate the half-life time (Eq. (2)), a parameter that expresses the time required for the concentration of the solution in question to decrease to half of its initial value [30].
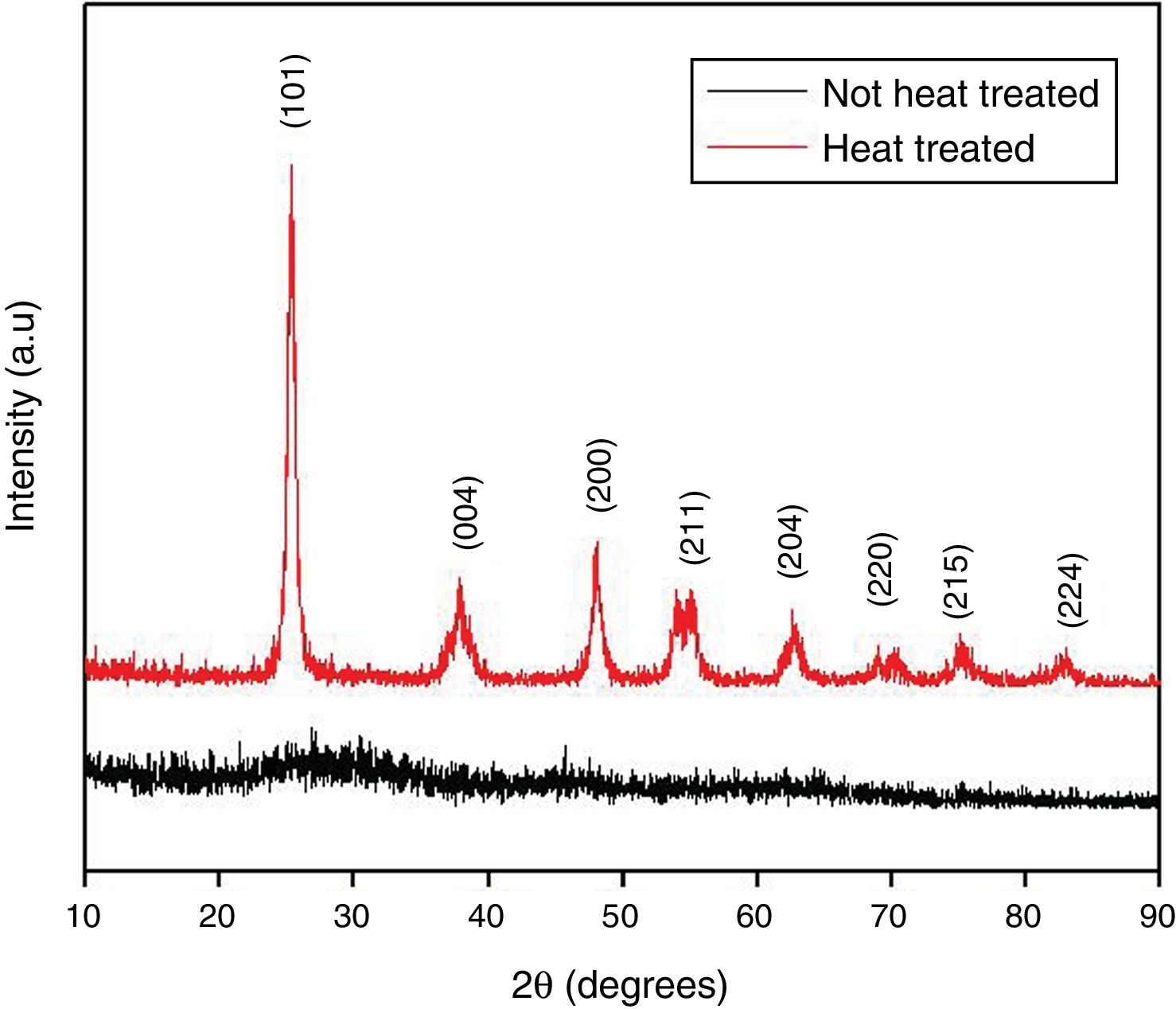
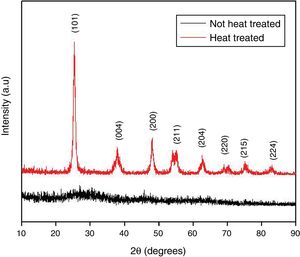
where kap is the apparent kinetic constant of the reaction (min−1); t is the elapsed time (min); C0 is the initial concentration; C is the concentration at time t; t1/2 is the half-life time (min).Results and discussionTiO2 characterizationsTo evaluate the structure of obtained materials, XRD analysis was performed. Fig. 2 displays the diffractograms of the xerogel (Not heat treated) and the samples thermally treated at 400°C (Heat treated). As expected, the xerogel has demonstrated an amorphous structure, which can be accomplished from the broad characteristic diffraction peak between 2θ ∼ 20° and 30°. On the other hand, all the diffraction peaks of the sample heat-treated at 400°C are indexed to be representative of the pure anatase phase (JCPDS 01-071-1166) with tetragonal structure indicating the successful preparation of the TiO2via the proposed synthesis pathways. Furthermore, this diffractogram presents a marked broadening of the reflections, which means crystallite sizes on a nanometric scale. The application of the Scherrer equation to the experimental curves yields lower limits of average crystallite size of 15nm thereby confirming the formation of nanostructured TiO2 with structure anatase.
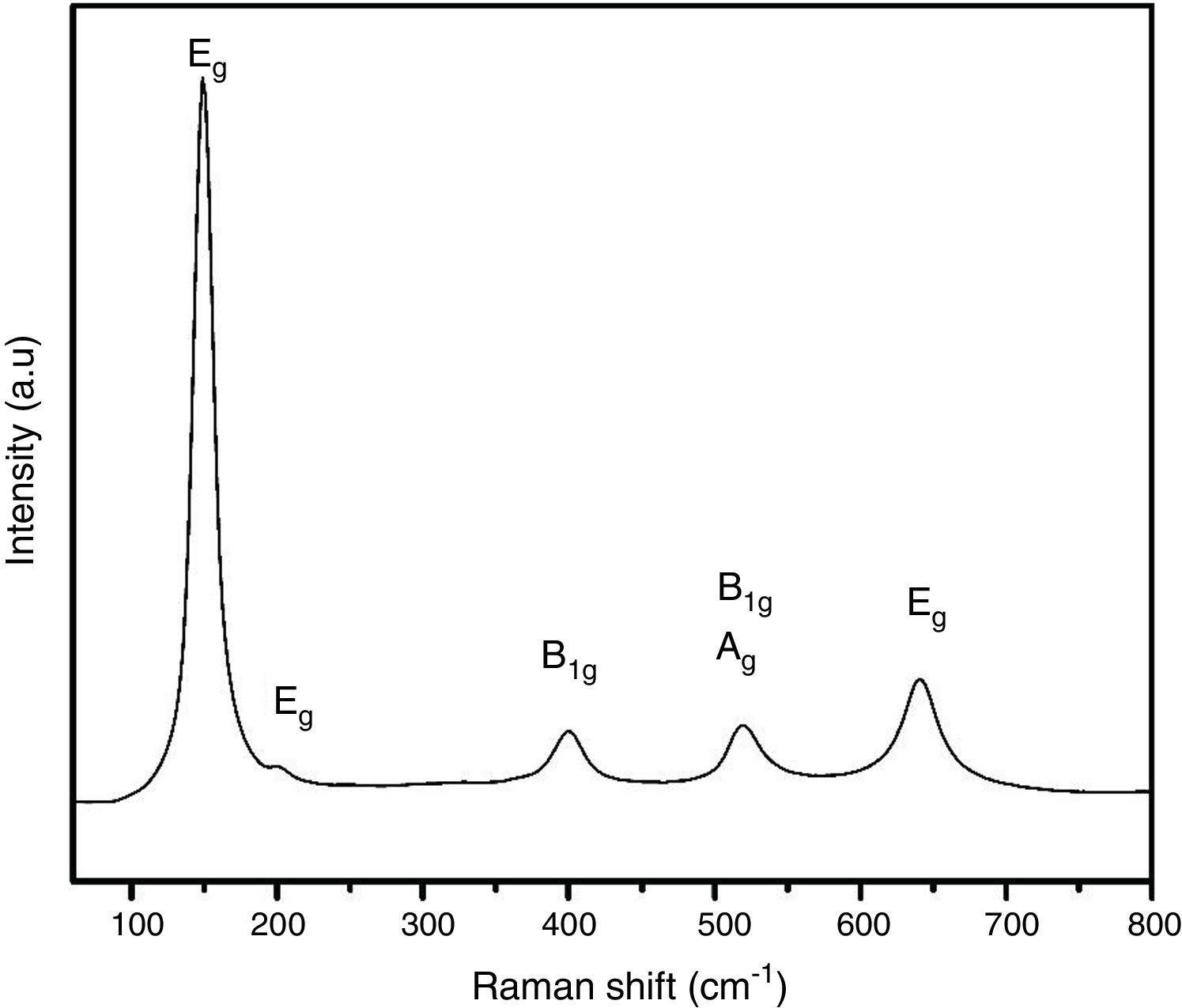
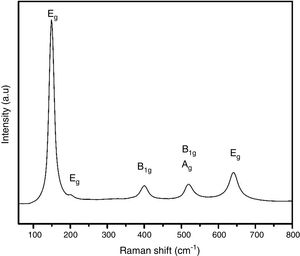
The crystal structure of thermally treated at 400°C sample can be confirmed by Raman spectroscopy studies and it is shown in Fig. 3. Anatase has six Raman active modes: Eg(1) (149cm−1), Eg(2) (199cm−1), B1g(1) (399cm−1), A1g/B1g(2) (overlapped at 519cm−1), and Eg(3) (641cm−1) [31]. The presence of the Eg(2) peak, which is usually very hard to detect, evidences the high degree of crystallinity of the samples.
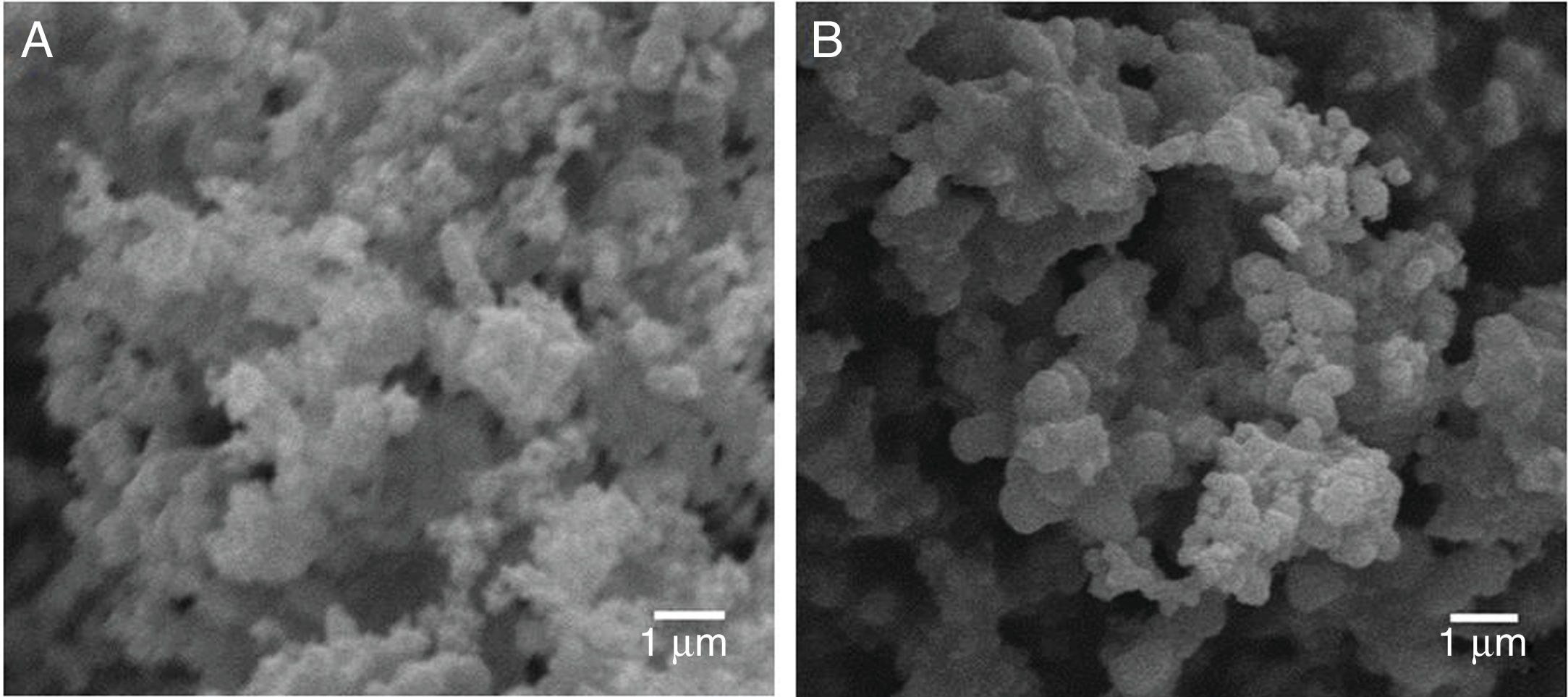
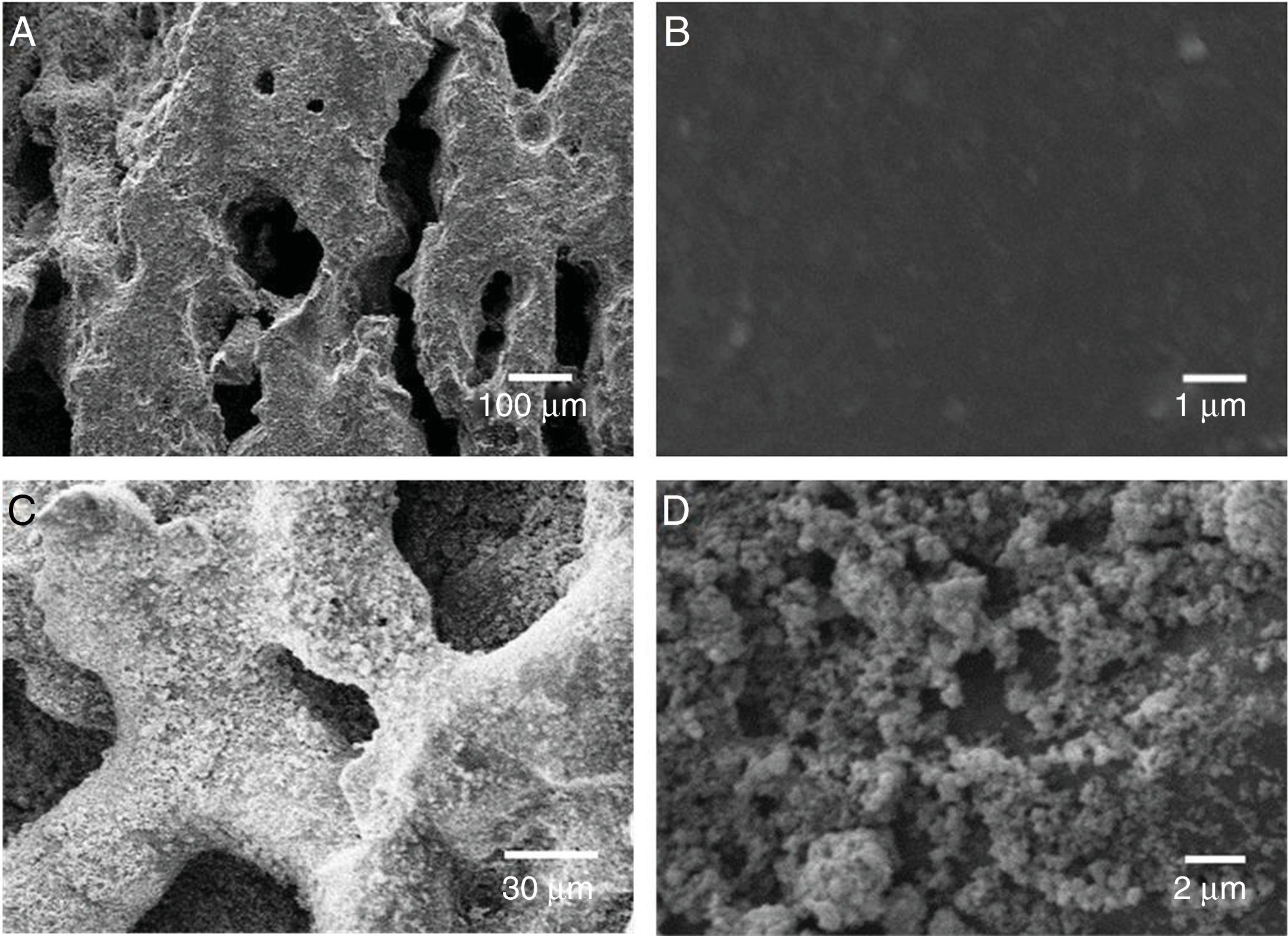
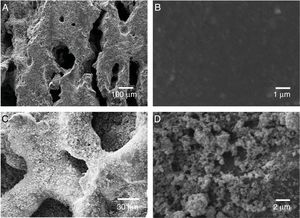
To evaluate the obtained morphologies, SEM studies were performed. In Fig. 4 it is observed the formation of agglomerates in both the TiO2 xerogel and the anatase (sample thermally treated TiO2 sample at 400°C). The agglomerates are composed of fine particles, in the nanometric scale in the case of the sample heat-treated at 400°C. The nanoparticles are highly reactive, which promotes the formation of agglomerates. The surface area of the particles has been evaluated by BET and is 60.106m2g−1, which can be considered very high if compared with anatase prepared by the sol-gel route [18].
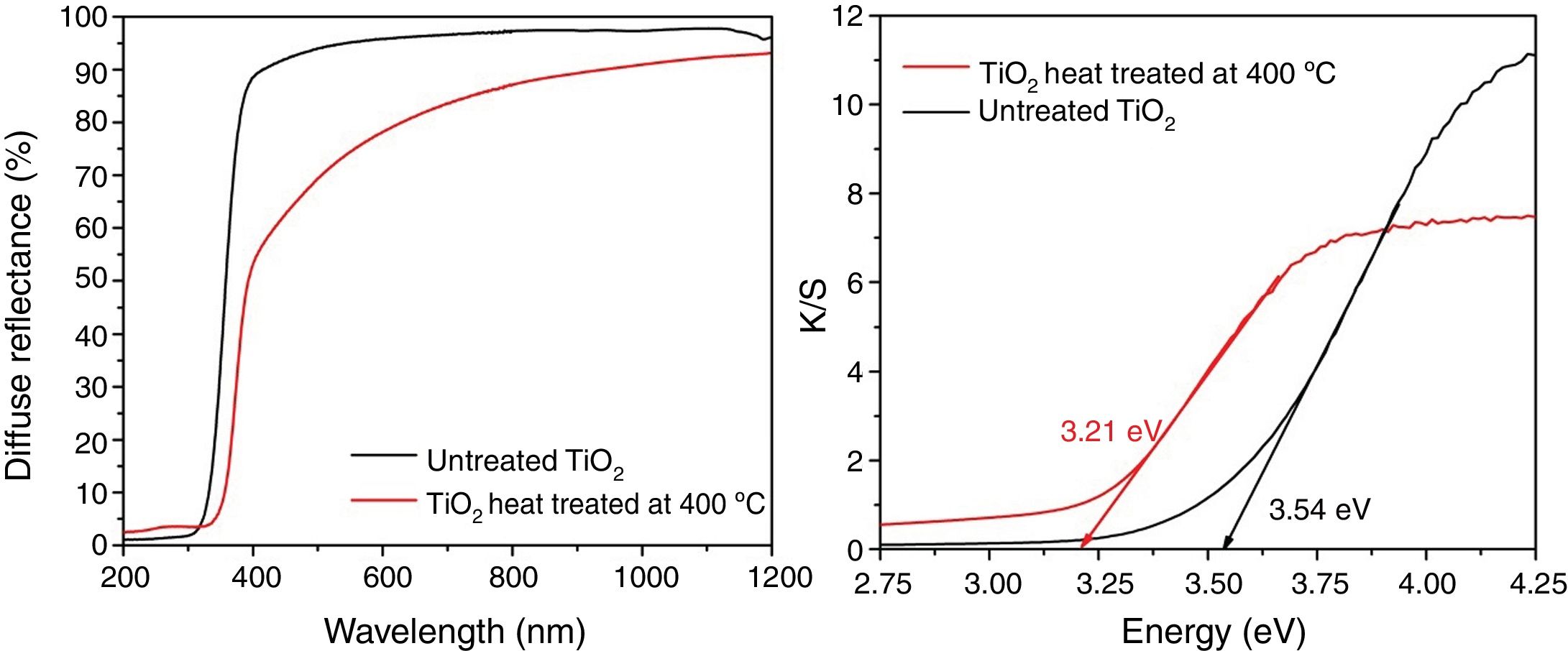
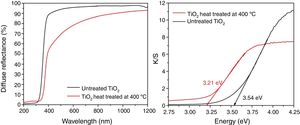
The UV–Vis diffuse reflectance spectra of the TiO2 powders synthesized heat-treated at 400°C, and untreated TiO2 (xerogel) are shown in Fig. 5. An evident semiconducting behavior can be inferred from the optical assays in both samples. To estimate the optical bandgap of the different samples, the Kubelka–Munk method was applied to the reflectance curves. The estimated band gap value is 3.21eV. It worth noting which it is typical of quantum effects in nanocrystallites, and it has been found that the bandgap shifts to a lower wavelength (or higher energy) with decreasing crystallite size [32]. Moreover, the usual value of Eg for the anatase phase synthesized by sol-gel is 3.2eV [21,33]. Amorphous TiO2 usually present Eg values greater than crystalline forms, mainly due to the structural defects [34]. The xerogel dried at 100°C show a bandgap of 3.54eV, which is also a semiconductor but can absorb less light than the anatase phase (crystalline).
Deposition of TiO2 in the ceramic substrateAfter a complete characterization of the TiO2 synthesized by sol-gel, TiO2 was supported in the porous ceramic. The supports were weighed before and after the impregnation process and the difference, after the dry process, we consider as the supported amount. The weight of TiO2 impregnated in the porous ceramic, from the suspension containing only water was 50.5±4mg. On the other hand, from the suspension containing alcohol as dispersing media, it was possible to impregnate 70.2±1mg. One perceives that the alcohol suspension proved to be more efficient as it was possible to impregnate a more significant amount of catalyst in the ceramics substrate. This phenomenon indicates that the nanoparticles were more dispersed in alcohol remaining in suspension and having a viscosity more suitable for deposition, giving greater homogeneity to the deposited layer [28]. The alcohol addiction changed the pH of the system from 6.8 to 5.1. It is reported that the addition of alcohol to the suspension containing TiO2 and H2O may affect the potential zeta values of the particles and increase the viscosity of the suspension [35]. In this case, this situation is beneficial as a slightly higher viscosity can improve particle adhesion to the substrate surface. To evaluate the deposition of TiO2 nanoparticles from the suspension containing alcohol as a dispersing agent, the samples were assessed by SEM. Fig. 6 shows the SEM micrograph of the porous ceramic support before TiO2 impregnation and porous ceramic supported TiO2 nanoparticles. The micrographs show the porous surface of the substrate without deposition of TiO2 and subsequent deposition. It is observed a good homogeneity of the photocatalyst on the support without excess agglomeration of the particles.
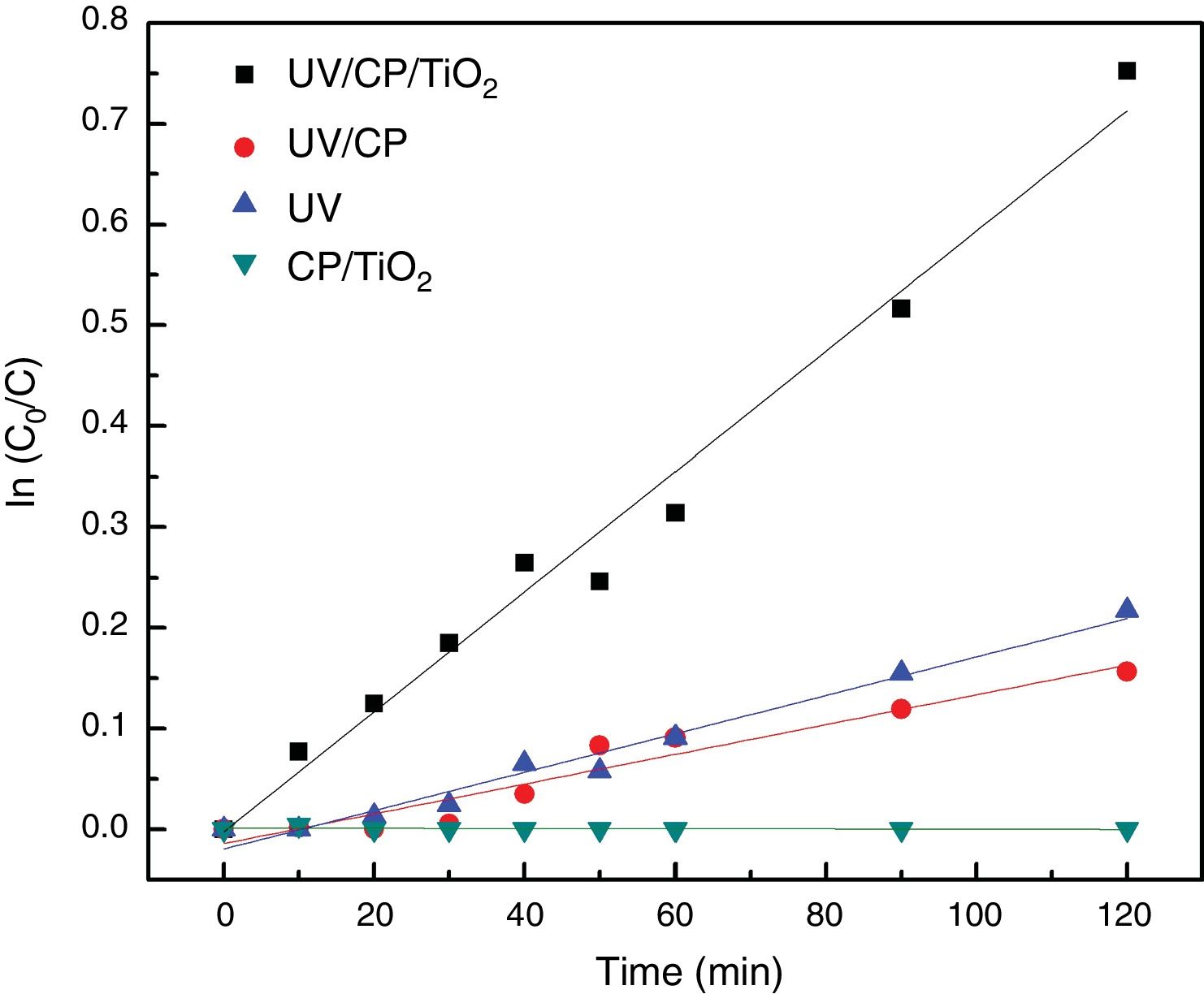
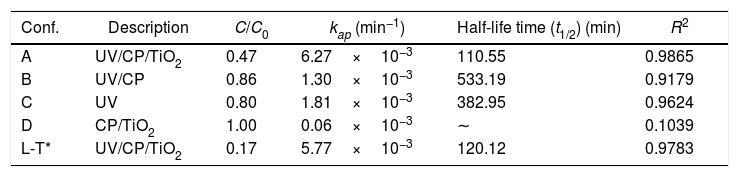
Heterogeneous photocatalysisTo evaluate the photocatalytic properties of the catalyst, photocatalysis tests of RhB dye were performed. Table 2 shows the results for the remaining concentration of Rhodamine B (C/C0), reaction kinetics kap (min−1), half-life time (min) and determination coefficient (R2) obtained in the four configurations tested for degradation of Rhodamine B in 120min. The L-T* configuration refers to the long-term performed with the A configuration by 5h.
Results of heterogeneous photocatalysis applied to Rhodamine B degradation.
| Conf. | Description | C/C0 | kap (min−1) | Half-life time (t1/2) (min) | R2 |
|---|---|---|---|---|---|
| A | UV/CP/TiO2 | 0.47 | 6.27×10−3 | 110.55 | 0.9865 |
| B | UV/CP | 0.86 | 1.30×10−3 | 533.19 | 0.9179 |
| C | UV | 0.80 | 1.81×10−3 | 382.95 | 0.9624 |
| D | CP/TiO2 | 1.00 | 0.06×10−3 | ∼ | 0.1039 |
| L-T* | UV/CP/TiO2 | 0.17 | 5.77×10−3 | 120.12 | 0.9783 |
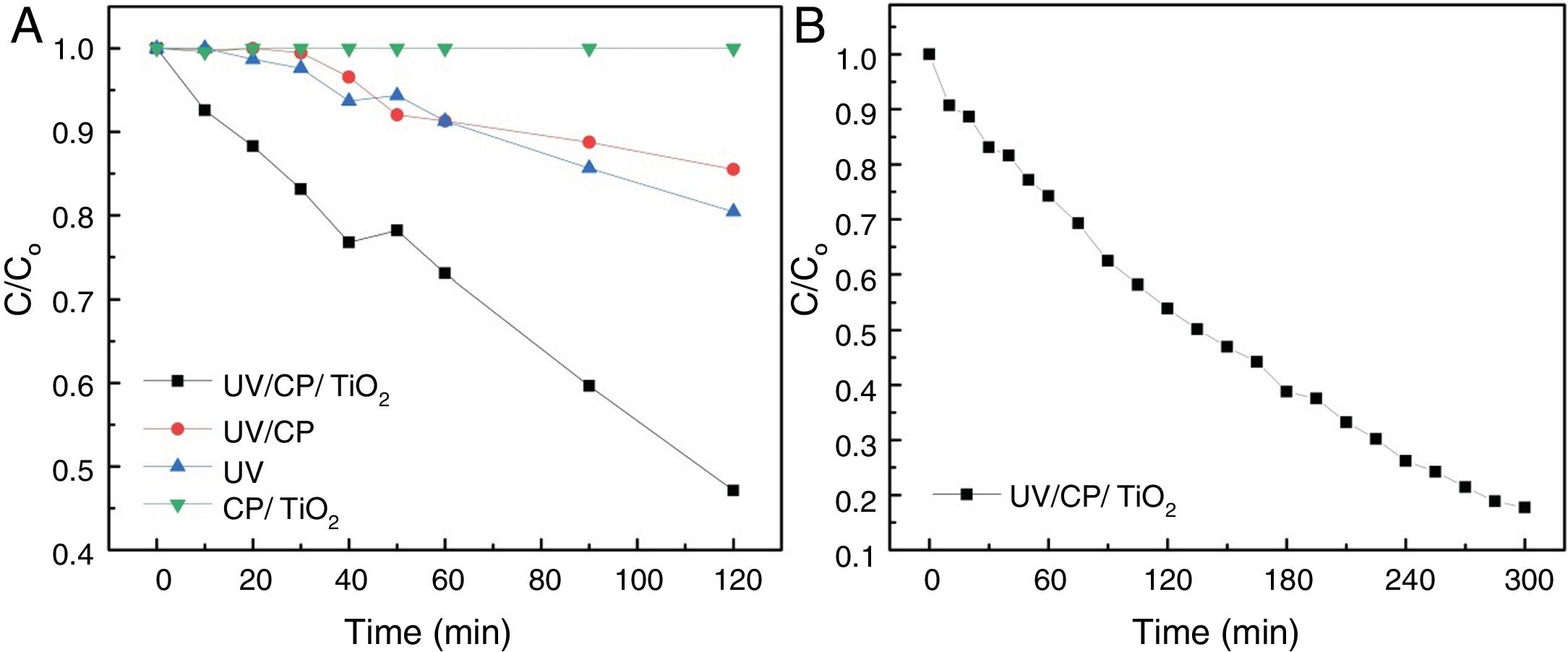
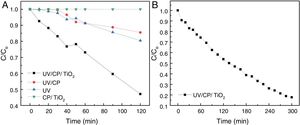
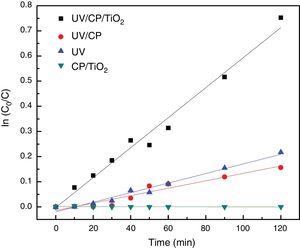
The best results were obtained with heterogeneous photocatalysis (A), with the degradation of 53% in 120min of the test, a half-life time of 110min. The purpose of the catalyst without UV radiation (D) did not change the solution after 120min, showing that there is no adsorption on the supporting material. The contribution of the direct adsorption of Rhodamine B to TiO2 (CP/TiO2) is negligible. This behavior also suggests that the surface of the support is very well coated, since there was no reduction in the color of the solution, as in curve B where the substrate, without TiO2, was exposed to UV light where a decrease of 15% was observed, similar as the photolysis (UV) behavior. Photolysis (C – when only UV radiation is used) presented results about 20% of degradation, proving the efficiency of the use of heterogeneous photocatalysis, associating the use of UV light with TiO2. The configuration without the use of a catalyst (UV/CP) had a reduction of 14%, due to the use of UV light, since there is no adsorption of the material. Due to the linearity of the end of the test with the configuration of heterogeneous photocatalysis (A=UV/CP/TIO2), this was repeated, seeking to evaluate its degradation capacity for a longer time, until there was no variation of the concentration, which was reached with 300min of the test (L-T* of Table 2). The results are shown in Fig. 7.
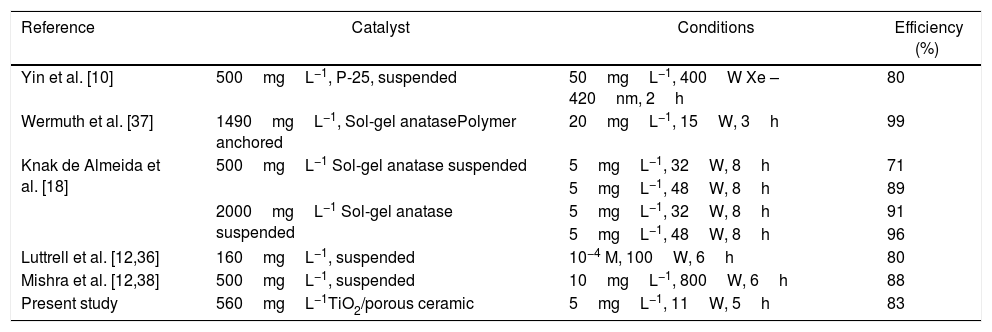
The degradation efficiency of Rhodamine B by TiO2 supported on porous ceramics by heterogeneous photocatalysis was also compared to the literature (Table 3). This demonstrated that the synthesized TiO2 supported at porous ceramic is capable of achieving high removal efficiency of Rhodamine B under a low lamp power, just 11W, thus saving energy. It is important to note the application of only 560mgL−1 of catalyst. The same table demonstrates some results of similar experiments using suspended catalysts [10,36]. It is possible to note similar degradation results, yet in immobilization design, it is possible to recover the catalyst easily.
Comparison of Rhodamine B degradation in the literature.
| Reference | Catalyst | Conditions | Efficiency (%) |
|---|---|---|---|
| Yin et al. [10] | 500mgL−1, P-25, suspended | 50mgL−1, 400W Xe – 420nm, 2h | 80 |
| Wermuth et al. [37] | 1490mgL−1, Sol-gel anatasePolymer anchored | 20mgL−1, 15W, 3h | 99 |
| Knak de Almeida et al. [18] | 500mgL−1 Sol-gel anatase suspended | 5mgL−1, 32W, 8h | 71 |
| 5mgL−1, 48W, 8h | 89 | ||
| 2000mgL−1 Sol-gel anatase suspended | 5mgL−1, 32W, 8h | 91 | |
| 5mgL−1, 48W, 8h | 96 | ||
| Luttrell et al. [12,36] | 160mgL−1, suspended | 10−4 M, 100W, 6h | 80 |
| Mishra et al. [12,38] | 500mgL−1, suspended | 10mgL−1, 800W, 6h | 88 |
| Present study | 560mgL−1TiO2/porous ceramic | 5mgL−1, 11W, 5h | 83 |
Configuration A, which represents the heterogeneous photocatalysis, presented a constant kinetic kap of 6.27×10−3 or 0.00627min−1 (Fig. 9). This result is in good agreement with the literature. However, the main difference is that the Rhodamine B dye, the focus of this study, is considered to be more challenging to degrade due to its molecular structure with more complex carbon chains compared to methylene blue. Wermuth et al., 2013, reached a kap of 28.6×10−3min−1, using different parameters, such as a 15W lamp and a higher concentration of TiO2 and Rhodamine B, factors that may have influenced kinetics.
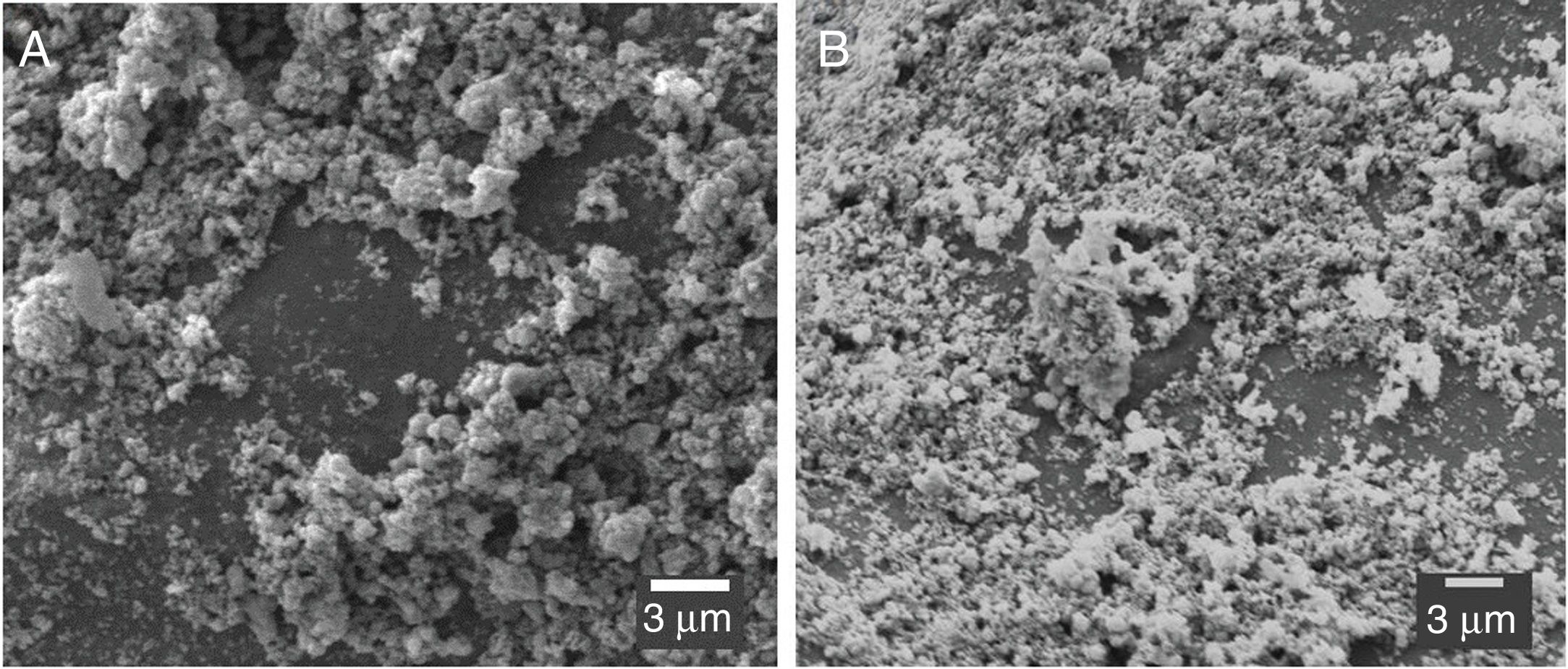
Microstructural images (SEM) of the clay substrates coated before and after use in the photocatalysis process are shown in Fig. 8. The images show that most of the catalyst remained adhered to the ceramics after the degradation of the Rhodamine B effluent. This phenomenon demonstrates that the use of the supported TiO2 is very efficient since the photoactive material can be easily recovered [24]. Also, only a minor quantity of the catalyst is lost in the reactor, along with the effluent. Another possibility of testing is using the continuous flux regime. In this way, it is possible to treat a more significant amount of effluent in this method than in the batch flow with the same catalyst.
It was possible to synthesize titanium dioxide (TiO2) nanostructured by the sol-gel method. The DRX and Raman characterizations showed the oblation of anatase phase and crystallite size of 15nm. Also, the SEM images illustrated that was performed a deposition a uniform layer of 70mg of the catalyst in the ceramic material, using the dip-coating method, and that after the usage, a major part of the catalyst stays at ceramics, it makes possible to recover it or even executes a continuous flow reactor. Higher deposition efficiency was confirmed using alcohol in the solution media compared to only water. The application of the catalyst in the heterogeneous photocatalysis showed excellent results, with the removal of up to 83% of the coloration Rhodamine B, mainly due to the structure of the synthesized catalyst and the anchorage process. It brings an efficient technique for the treatment of this problematic dye and a method of reuse of the residues employed in the manufacture of the ceramic support.
The authors would like to the National Council for Scientific and Technological Development (CNPq) (Process n° 427402/2016-6) and the Coordination for the Improvement of Higher Education Personnel (CAPES) for their financial support.