The mineralogical and chemical characteristics, based on X-ray diffraction (XRD) and scanning electron microscopy, of a kaolin known as DD3, from eastern Algeria were examined in the present study.
The results showed that kaolin DD3 has an alumina content of 39%. The SiO2/Al2O3 molar ratio of 2.14 is close to that of a pure halloysite. The hematite concentration is relatively large and the flux oxides ratios remain as acceptable impurities. Microscopic observations showed a predominant tubular halloysite phase, flattened hexagonal platelets corresponding to the presence of kaolinite and its polymorphs (nacrite, dickite), and hydrated alumina. The SiO2/Al2O3 molar ratio and tubular DD3 suggest possible uses in technical ceramics and nanotechnology applications.
Analysis by XRD revealed the presence of many phases. Thermal treatment at 450 °C and chemical treatment with HCl confirmed the presence of halloysite. The inclusion in the clay of organic molecules (dimethylsulfoxide (DMSO), DMF, and diluted glycerol) showed that the DMSO led to expansion of the inter-planar distance. The intercalation by DMSO molecules resulted in a shift of the basal peak from 10 to 11.02 ¿ and partial displacement of the peak from 3.35 to 3.65 ¿. These two peaks are characteristic of halloysite. The presence of residual nacrite was also confirmed by the shift of the peak observed at 3.35 ¿.
A full analysis of the XRD patterns using the Match software, based on these results, showed that the DD3 clay consists of >60% halloysite.
En el presente estudio se examinaron las características mineralógicas y químicas, basándonos en análisis mediante difracción de rayos X (DRX) y microscopio electrónico de barrido, de una caolinita denominada DD3, procedente del este de Argelia.
Los resultados reflejaron que la caolinita posee un contenido de alúmina del 39%. La proporción molar SiO2/Al2O3 de 2,14 es cercana a la de la halloysita pura. La concentración de hematites es relativamente grande, y la proporción de óxidos fundentes se muestra como impurezas aceptables. Las observaciones al microscopio reflejaron una fase predominante de halloysita tubular, plaquetas hexagonales aplanadas que se corresponden con la presencia de caolinita y sus polimorfos (nacrita, dickita), y alúmina hidratada. La proporción molar SiO2/Al2O3 y la DD3 tubular sugieren posibles usos para cerámica técnica y aplicaciones de nanotecnología.
El análisis mediante DRX reveló la presencia de diversas fases. El tratamiento térmico a 450 °C y el tratamiento químico con HCl confirmaron la presencia de halloysita. La inclusión en la arcilla de moléculas orgánicas (dimetilsulfona (DMSO), DMF, y glicerol diluido) mostró que la DMSO conducía a la expansión de la distancia interplanar. La intercalación por parte de las moléculas de DMSO originó un cambio del valor máximo basal de 10 a 11,02¿ y un desplazamiento de dicho valor máximo de 3,35 a 3,65¿. Dichos dos valores máximos son característicos de la halloysita. También se confirmó la presencia de nacrita residual mediante el cambio del valor máximo observado a 3,35¿.
Un análisis completo de los patrones de la DRX, utilizando el software Match, basado en dichos resultados, reflejó que la arcilla DD3 se compone de >60% de halloysita.
The most important minerals in kaolin are kaolinite and halloysite. Kaolinite Al2Si2O5 (OH)4 is often used as a base for the synthesis of new organo-mineral nanomaterials designed for applications in industries and for environmental protection ([12]). Halloysite had a similar composition with some excess water molecules present between the layers and often exhibits a tubular morphology. Halloysite loses water easily from the intermediate layer and is frequent in some partial dehydration state. Fully hydrated halloysite Al2Si2O5 (OH)4·2H2O becomes increasingly important because of its use in nanotechnology applications which takes advantage of its tubular habit ([18]).
Typically, clays have a hexagonal structure oriented perpendicularly to the C axis. The inter layers distance varies according to the type of clay.
First order X-ray Bragg reflection did not totally identify the minerals present in the clay. The compounds complexity caused a multiple reflection of higher orders (n = 2, 3, 4, …) promoting the superposition of the first order peaks with those of higher orders. The presence of chlorite in the kaolinite clay led to the superposition of the peak corresponding to the reflection of the first order of the kaolinite (d = 7 ¿) with that of the second order reflection of chlorite. To remove ambiguity in the identification of these minerals, auxiliary techniques tailored to the specific thermal properties and chemical properties of these clay minerals were used. The common technique used consists of the intercalation of the clay mineral by organic molecules. The latter resulted in the expansion of the interlayer distance of basal layers. This induced a shift of the first order peak reflection to smaller angles. The organic molecules usually used are: ethylene glycol or glycerin, N-methylformamid, hydrazin, potassium acetate and dimethyl sulfoxide (DMSO).
The intercalation of these molecules between layers of nacrite not only caused an increase in the interlayer distance, but also some inter-sheets translation ([1]). Among the organic molecules used, the DMSO reduced the translation of the octahedral site. In this case, the guest molecules occupied the space between the two sites causing a decrease of the coherent domain size consistency both in the direction of the layers and in the perpendicular direction (d002 = 11.19 ¿). This intercalation caused cleavage of the particles. A similar result was obtained by Bookin et al. ([2]) for dickite. Another study is concerned with the intercalation of formamide, potassium acetate and hydrazine in halloysite enriched by kaolinite clay ([4]). The treatment of the first two molecules, followed by washing with water or water and glycerol, led to the total expansion of halloysite. The peak at 7.2 ¿ moved to 10.1 ¿ and remained in this position. With kaolin, these treatments did not lead to such displacement. Nevertheless, intercalation by formamide and potassium acetate molecules underestimated the proportion of halloysite compared to the use of hydrazine. This was because to the presence of the kaolinite disordered and that halloysite maintained its hydrated state after the removal of hydrazine by water washing.
Therefore, these treatments did not allow a quantitative estimation of the proportion of halloysite in kaolin–halloysite mixtures. The quantification of kaolin is possible by using a prolonged intercalation method (over 18 days). In this context, it appeared that the hydrazine was the most appropriate intercalation molecule. It led to an almost total displacement. It was also noted that the crystalline and the grain size had some influence on the intercalation quality ([4]).
Formamide molecules were used to differentiate kaolinite and halloysite ([7]). Immersion of halloysite in a formamide solution for a short time (<1 h) enabled a complete expansion of the clay. Kaolinite was rather susceptible to such treatment only after a long period (4 h). At a temperature greater than 110 °C, this treatment was no more effective on halloysite. The higher temperature treatment caused the transition of halloysite (10 ¿) to definitive metahalloysite (7.2 ¿).
Jackson and Abdelkader ([13]) obtained the total expansion of fine type IV kaolinitic fire clays. They used ground kaolinite with CsCl and the resulting mixture was interposed with hydrazine and heated at 65 °C for 24 h. A second interposed procedure was made with DMSO and the mixture obtained was heated at 90 °C for 24 h. This multiple treatment led to a shift of the XRD peak from 7.2 to 11.2 ¿.
The intercalation of well crystallized halloysite with ethylene glycol confirmed the results of Hillier and Mac Ewan ([5], [17]).
This led to a decrease in the intensity of 7.2 ¿ peak and an increase in the intensity of 3.58 ¿ peak, which resulted in a ratio reduction I (7.2 ¿)/I (3.58 ¿). The intercalation of ethylene glycol promoted a shift in (7.2 ¿) peak to 2.58 ¿ leading to a third order reflection peak close to the peak of halloysite (3.58 ¿).
Other authors have studied the de-intercalation of kaolinite and its effect on the crystalline of the host material ([11]). It was reported that the intercalation of N-methyl formamid, especially DMSO, caused a large structural disorder. Octavian and Rouxhet ([3]), however, concluded that the treatment of kaolinite with DMSO did not alter the original configuration. The heat treatment between 100 and 250 °C, water washing and the methanol prevented the deformation of the crystal lattice. The proposed method for de-intercalation by heating from 100 °C to 250 °C, washing with water and methanol or CS2 ([8]) was probably the reason for the apparent contradiction.
The preceding works mentioned revealed that a thorough analysis of multi-component natural clay required associating several other techniques in addition to SEM, EDX and XRD. Attempting a different type of intercalation molecules may remove some ambiguities. In this work, a complete process analysis of DD3 kaolin, based on morphological observation, chemical analysis and XRD diffraction associated with tailored intercalation treatments, was presented.
ExperimentalRaw materialThe material studied is a kaolinitic gray clay designated DD3 extracted from a mountain of eastern Algeria (Djebel Debagh).
Physico-chemical treatmentThe identification of the different phases present in the DD3 kaolin from XRD measurements required the use of different treatments.
The first was a thermal dehydration at 450 °C. The second was made up of a dehydration step at room temperature followed by dissolution in hydrochloric acid (4 N) for half an hour at 20 °C. The third treatment was made up of the intercalation of organic molecules in the clay by spraying a water solution of specific additives. The used organic molecules in a water solution (10% water) are Glycerin, dimethylformamide (DMF) ([7]) and Dimethylsulfoxide (DMSO). The DMSO was also intercalated in vapor form ([8], [14]). One gram of kaolin with 20 ml of DMSO was placed in a pyrex™ sealed enclosure. The mixture was stirred until a homogeneous solution was reached and heated at 60 °C for 12 h.
Analysis of solid phases by XRDThe samples were analyzed using a Phillips PW3050/60 diffractometer with a copper anticathode (λ = 1.540598 ¿). The ground powder (particle size <50 μm) was compacted and flattened between two glass slides to increase the preferential grain orientation. The samples were then scanned from 4 to 90° with a step of 0.02° per second. Match software was used to identify the main phases using the COD Inorganic 20130415 database.
Microscopy analysis by SEM-EDSThe observations of the morphology of the various minerals in the kaolin DD3 were made using a Hitachi S4700 SEM. Before observation, the powder of kaolin was dispersed in acetone under ultrasonic vibrations and dried. Chromium metallization was performed by vacuum deposition.
Furthermore, an Energy-Dispersive X-ray Spectrometry (EDS) was used in tandem with the SEM for identification of kaolin DD3 chemical elements.
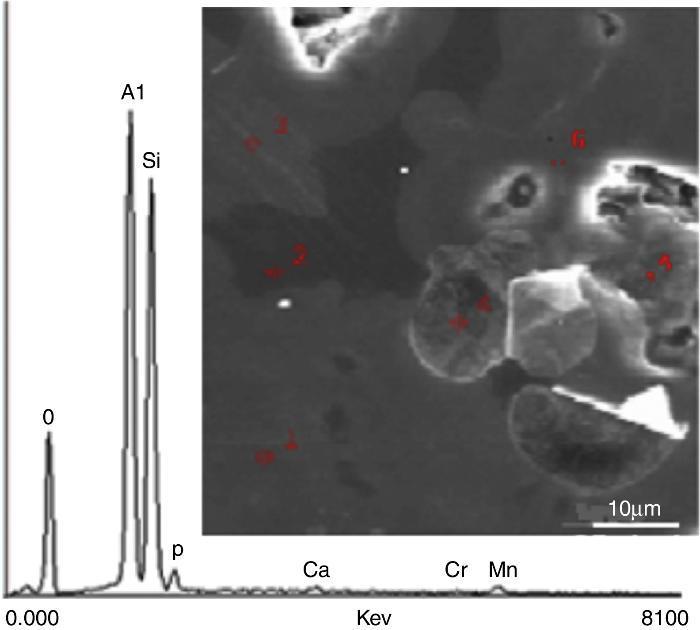
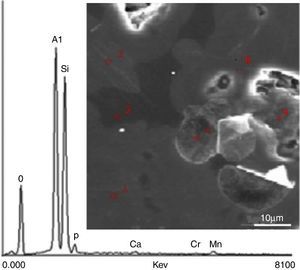
Results and discussionSEM-EDS analysesThe main elements and their corresponding oxides were determined by X-EDS. The localization of six analyzed sites designated by numbers and a typical X-EDS curve is shown in Fig. 1.
Fig. 1. Typical X-EDS results and localization of the analyzed areas on sintered DD3 kaolin.
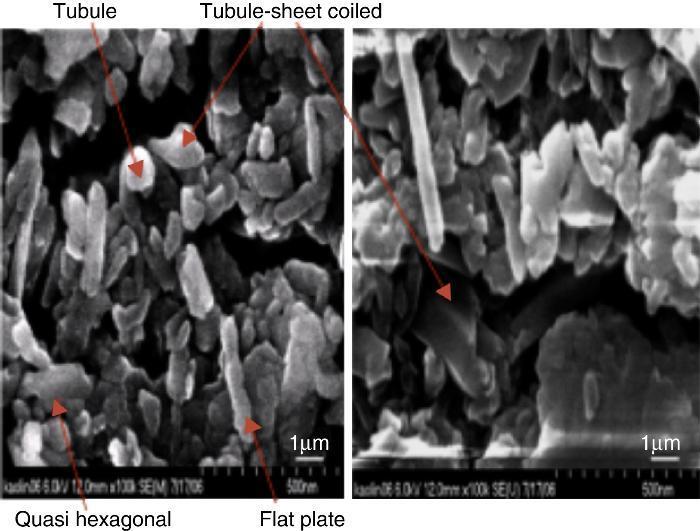
SEM observationsSEM photographs of DD3 kaolin powder (Fig. 2) showed irregular grain sizes and various particle shapes. This imperfect structure was linked to some disturbances in the octahedral layer in which iron may replace aluminum ([10]). Grains exhibited irregular tubular shapes, sometimes elongated (rolled sheets) and in some cases spherical. These shapes are indicative of halloysite. The tubular form was predominant. It allowed the storage of water, conferring plasticity. The tubular rods were entangled (Fig. 2a), giving good tear strength to the material. Additionally, the presence of near hexagonal shapes and flattened platelets probably indicated the presence of nacrite, dickite and hydrated alumina, characterizing the presence of kaolinite, whose basal surfaces were perpendicular to the (001) direction and side faces perpendicular to (010) and (110) directions. The predominant tubular shaped grains revealed that halloysite was the main mineral constituent of the studied clay.
Fig. 2. SEM observation of DD3 kaolin.
The main chemical elements detected and their oxides (Table 1) confirmed the origin of clay. The predominant constituents were silica and alumina. The SiO2/Al2O3 molar ratio was 2.14. This ratio was very close to that of a pure halloysite. The dispersion of the observed values for these two oxides showed that the chosen sites exhibited variable compositions. The proportions of other oxides (Fe2O3, MgO, Na2O, K2O and MnO) remained acceptable such as impurities relative to conventional application. The presence of these oxides in such concentration may be a sign of the existence of chlorite in the clay. Their presence played an important role in the formation and evolution of crystalline phases during the heat treatment of kaolin. It appeared that the concentration of hematite was relatively large.
Table 1. Weight percentage of different chemical element sand calculated oxides in DD3 kaolin.
| Element | wt (%) | SD | Calculated oxides | wt (%) | SD |
| O | 47.65 | 0.69 | – | – | – |
| Si | 23.08 | 1.97 | SiO2 | 49.00 | 4.22 |
| Al | 20.84 | 3.56 | Al2O3 | 39.00 | 6.74 |
| Fe | 3.12 | 0.61 | Fe2O3 | 4.50 | 0.87 |
| Na | 1.78 | 0.91 | MgO | 2.90 | 0.61 |
| Mg | 1.74 | 0.36 | Na2O | 2.41 | 1.22 |
| Ca | 1.15 | 1.03 | CaO | 1.63 | 0.84 |
| Mn | 0.49 | 0.15 | MnO | 0.62 | – |
| K | 0.23 | 0.11 | K2O | 0.34 | 0.14 |
SD, standard deviation; wt, weight average amount.
The analysis of XRD spectra was difficult because DD3 kaolin contained several mineral phases and many impurities. The observed overlapping peaks made complex their full identification ([9]). Additional treatments were used to remove some ambiguity. These were a low-temperature thermal treatment (450 °C), a chlorhydric acid chemical attack (4 N) and a clay interlayer intercalation with organic molecules.
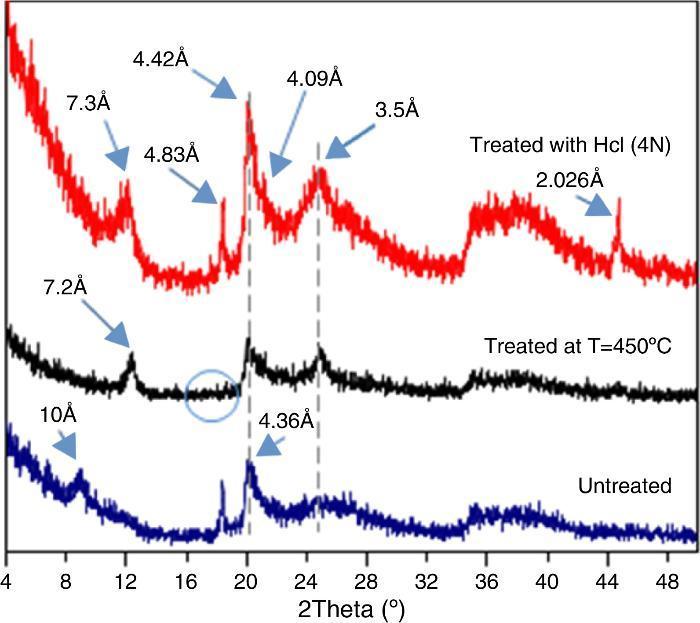
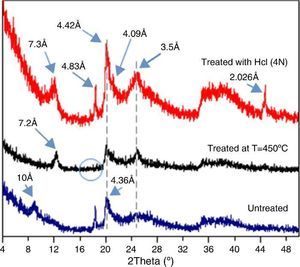
The obtained XRD spectra of DD3 (ϕ < 50 microns) for the thermally or chemically treated samples in comparison with an un-treated case is shown in Fig. 3. At low diffraction angles, there was a fairly large continuous background because of Lorentz polarization ([15]) and the presence of amorphous phases. Two broad peaks (the first one between 19° and 33° and the second one between 34° and 43°) were also observed. Furthermore, the low intensity and the more or less pronounced asymmetry of the peaks at the smallest angles were likely as a result of the greater disorder of the structure of this kaolin.
Fig. 3. Effect of heat treatment at 450 °C and HCl on the XRD spectra of DD3 kaolin.
The peak at 10 ¿ could be attributed to both hydrated halloysite-type (10 ¿) than illite, muscovite, or phlogopite phases. The thermal treatment caused the displacement of the 10 ¿ peak to 7.2 ¿ with improved peak symmetry. Halloysite was the only dioctahedral 1:1 layer silicate that dehydrates at such low temperature ([7]). This caused a shift of its XRD peak confirming that the 10 ¿ peak corresponds to hydrated halloysite (001) (DRX data: halloysite PDF file 29-1489, to metahalloysite PDF file 29-1487). In an obvious way, the gibbsite dehydroxylation at such temperature give amorphous alumina ([6]), led to the disappearance of the peak at 4.83 ¿. The remaining peaks kept their original position. The same effect was obtained by dehydration with hydrochloric acid HCl for half an hour. In this case, the halloysite peak shifted a little to the new position 7.30 ¿. The shrinkage because of the heat treatment at 450 °C was slightly higher than that obtained chemically with HCl. In this last case, an increase in the peak intensity was observed probably because of impurities dissolution and the rearrangement of the disordered phases. The (001) peak of hallosyite (4.36 ¿) also shifted to 4.09 ¿ after HCl treatment. A new peak appeared at 2.026 ¿ and was attributed to iron oxide (Fe3O4) (JCPDS file 65-3107) resulting in various minerals acidic attacks such as chlorite.
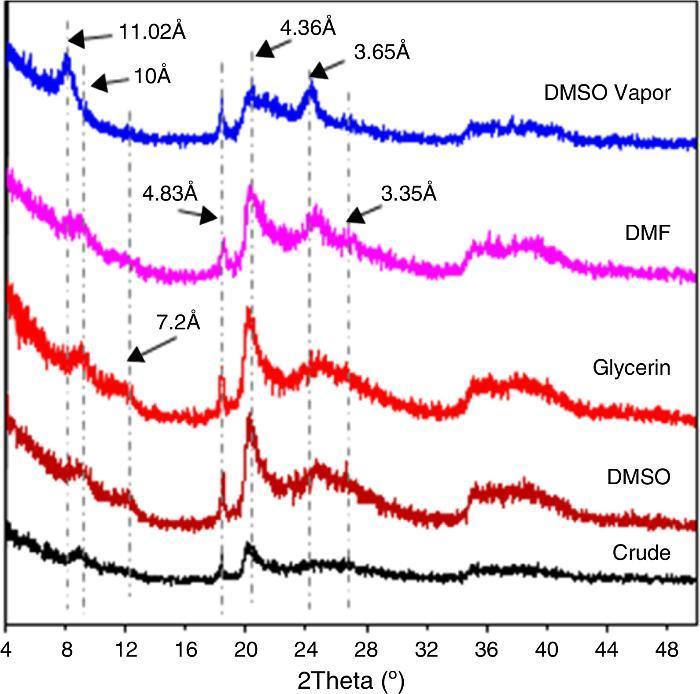
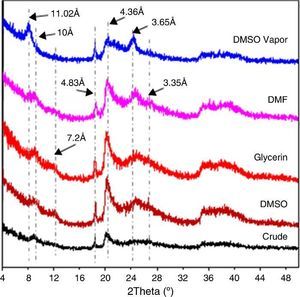
XRD diagrams after the insertion of organic molecules are shown in Fig. 4. Expansion is reduced after DMSO was inserted in solution form. This could be explained by the presence of a more resistant to intercalation such as kaolinite and/or its polymorphs ([16]). Indeed, the 7.17 ¿ peak corresponding to kaolinite underwent a slight shift to 7.2 ¿. No such effect was observed in the XRD diagram after a treatment with glycerol. The intercalation of molecules of DMSO and DMF in vapor form resulted in a complete displacement of the base peak from 10 ¿ to 11.02 ¿. A minor shift of another peak from 3.35 ¿ to 3.65 ¿ was also observed. The expansion was more pronounced in the case of DMSO whose molecules were more or less bulky. The two shifted peaks belonged to (001) and (003) halloysite. This is consistent with the other results that showed that intercalation of formamides was effective even during an exposure of less than one hour ([7]). Peaks corresponding to kaolinite, however, did not undergo any change. The present treatment was probably insufficient as Costanzo et al. reported ([14]). The kaolinite underwent total expansion when it was subjected to an intercalation treatment with a steam DMSO at 60 °C only after a period of ten days. The 3.25 ¿ peak shift to 3.65 ¿ may be due to the possible presence of nacrite and certainly quantity of kaolinite, dickite and nacrite together.
Fig. 4. Effect of intercalation of organic molecules in the DD3 kaolin on XRD spectra.
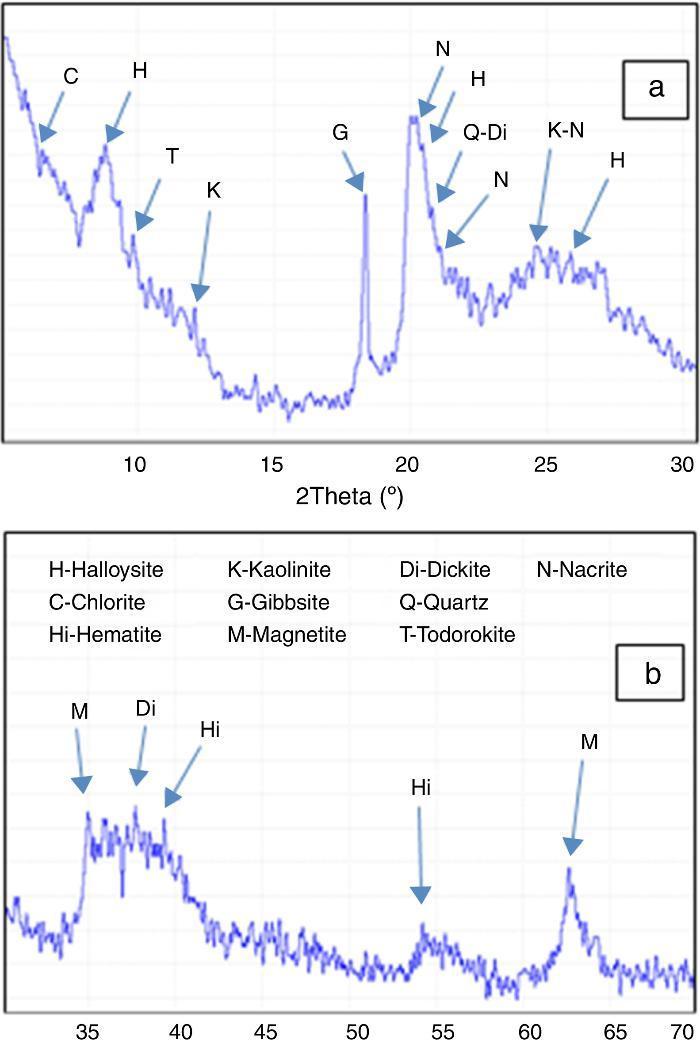
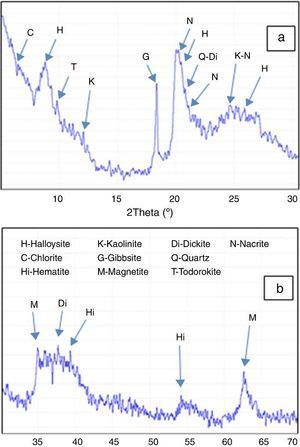
Match software was used to analyze the obtained DD3 clay XRD diagrams. Polynomial low-pass smoothing interval convolution of eleven points was done to reduce the effect of background noise. For the obtained diagram (Fig. 5), the main peaks were indexed by the most likely minerals (indexed by letter). In this figure, peaks close to 10 ¿, 4.36 ¿ and 3.35 ¿ were attributed to quartz and the 4.83 ¿ peak was attributed to gibbsite. Several weak reflection peaks, however, did not seem to correspond to these two phases.
Fig. 5. Full analysis of DD3 kaolin XRD diagrams (a)°2θ from 3° to 30°, (b)°2θ from 30° to 70°.
Relative amounts of each phase were determined and presented (Table 2). They revealed that the main phase was halloysite with an average amount of 62 wt%.
Table 2. Full identification of minerals present in the DD3 kaolin.
| Mineral phases (DD3) | Weight amount (%) |
| Alumino silicates | |
| Halloysite (H) | 63–66 |
| Kaolinite (K) | 2–3 |
| Nacrite (N) | 7–9 |
| Dickite (Di) | 1–2 |
| Chlorite (C) | 6–8 |
| Silica | |
| Quartz (Q) | 5–7 |
| Alumina compound | |
| Gibbsite (G) | 7–8 |
| Other | |
| Hematite (Hi) | 2–3 |
| Magnetite (M) | 1–2 |
| Todorokite (T) | 2–3 |
The chemical and mineralogical analysis of DD3 kaolin was achieved by using different and complementary intercalation characterization techniques.
EDX chemical analysis indicated the presence of the high amount of alumina (Al2O3) with a (SiO2/Al2O3) molar ratio of 2.14 similar to that of a pure halloysite. In addition, the presence of the high amount of Fe (III), Mg, Na, Mn and Ca may indicate the presence of chlorite and todorokite.
SEM observation showed imperfect tubular grains attributed to halloysite as the predominant structure. The imperfection was because of the disturbances in the octahedral layer or iron replacing aluminum. In addition, the presence of quasi-flattened hexagonal platelets was attributed to kaolinite and its polymorphs (dickite and nacrite) and to hydrated alumina.
A full analysis of XRD diagrams required the use of several additional intercalation treatments to identify several disputable peaks. The heat treatment at 450 °C caused a shift of a first peak from 10.06 ¿ to 7.32 ¿ that is the characteristic of the presence of hydrated halloysite. The same effect was obtained using a treatment with hydrochloric acid HCl (4 N) for half an hour. In this case, the peak barely shifted to the new position 7.30 ¿. Moreover, this acidic treatment promoted the appearance of a peak at 2.026 ¿ which was a result of iron oxide extracted from chlorite by this chemical treatment.
The results obtained by the insertion of DMSO and glycerol by immersion during 1 h in 10% diluted water solution showed an expansion of the interplanar distance of kaolin with DMSO and no change with glycerol. In addition, the intercalation with DMSO in vapor form and DMF resulted in the complete shift of the 10 ¿ basal peak to 11.02 ¿ and partial displacement of peak 3.35 ¿ to peak 3.65 ¿. These two peaks corresponded to (001) and (003) halloysite. The residual peak at 3.35 ¿ observed after vapor treatment was probably because of a polymorph of kaolinite (nacrite).
These results, based on the complementary methods used, showed that the DD3 clay was mainly composed of halloysite. A near 60% halloysite ratio was determined based on Mach software.
Acknowledgments
The authors would like to thank Prof A. LERICHE, Laboratoire des matériaux céramiques et procédés associés and Prof A. Bataille, Laboratoire de structures et propriétés de l’état solide UMR CNRS 8008 France for their contribution to XRD, SEM and EDS analysis.
Received 1 July 2015
Accepted 1 December 2015
Corresponding author. bourahlim@yahoo.fr