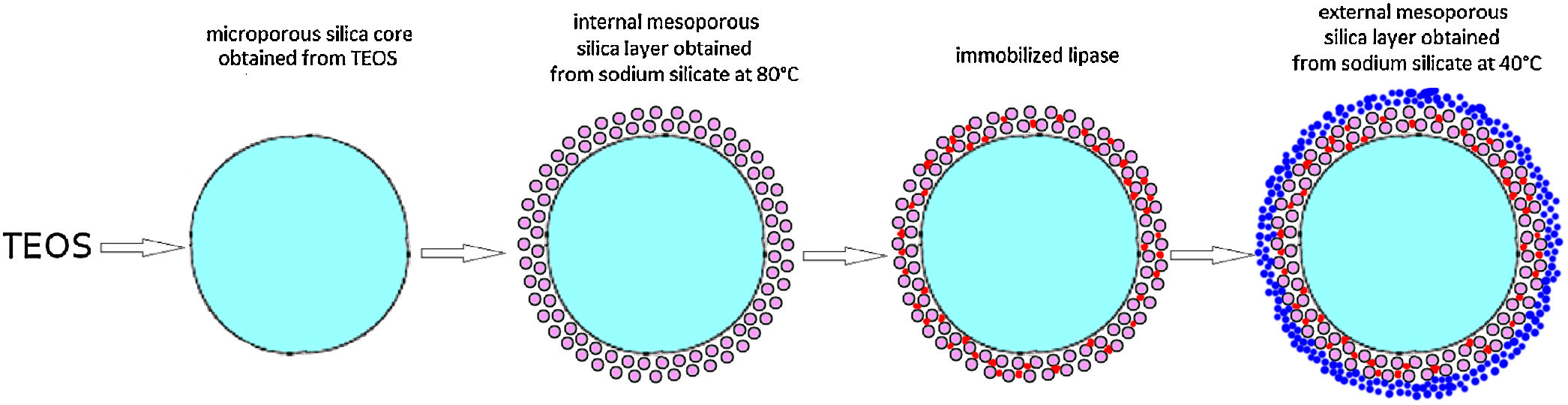
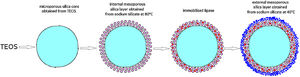
Two methods of enzyme immobilization onto silica core-shell particles were developed. The first method involved the immobilization of Candida rugosa lipase inside a previously synthesized mesoporous silica layer (deposited at 80°C) surrounding a dense silica core. To prevent lipase leakage from the support, an outer mesoporous silica layer was deposited at 40°C around the first silica layer containing the immobilized lipase. The deposition of the second layer was performed at a relatively lower temperature, to prevent thermal inactivation of the immobilized enzyme. The internal silica layer was obtained by assembling primary silica nanoparticles generated from highly basic sodium silicate solution at 80°C on the surface of poly (diallyldimethylammonium chloride) (PDDA) functionalized silica core particles. The average shell thickness and pore size of the internal silica layer was ∼60nm and 24nm, respectively. The effect of process parameters on generation and aggregation of silica nanoparticles prepared from highly basic sodium silicate solution was also investigated. The aggregation of silica particles generated at 40°C and 80°C took place after 840s and 570 of reactions, respectively. The immobilization efficiency of lipase on the mesoporous silica monolayer was 80%. A decline of immobilized lipase activity was approximately 6 times after 10 reaction cycles due to lipase leakage from the monolayered shell. An outer mesoporous silica layer was deposited at 40°C onto the surface of previously PDDA-functionalized monolayered silica core-shell particles containing the immobilized lipase. The average thickness and pore size of outer mesoporous silica layer was ∼60nm and 17nm, respectively. The activity of lipase immobilized inside the bilayered shell was further reduced due to diffusion resistance within the outer silica layer and PDDA layer however, it was retained for the next reaction cycles.
The pore size of mesoporous silica layer obtained at 80°C was insufficient to allow invertase immobilization. Thus, the second method for the immobilization of invertase was developed. It involved the preparation of the mesoporous silica layer simultaneously with invertase immobilization at 40°C. The immobilized invertase showed decreased activity, but it was not hampered by substrate inhibition, as in the case of the free enzyme, due to the location of the enzyme inside the mesoporous silica layer, where the mass transfer resistance for the substrate to the enzyme active site was present.
Han sido desarrollados dos métodos de inmovilización enzimática sobre las partículas de la núcleo-cubierta de sílice. El primer método implicaba la inmovilización de Candida rugosa lipasa dentro de una capa de sílice mesoporosa previamente sintetizada (depositada a 80°C), la cual rodea un núcleo denso de sílice. Para evitar fugas de la lipasa del soporte, se depositó una capa externa mesoporosa de sílice a una temperatura de 40°C sobre la superficie de las partículas de la núcleo-cubierta de sílice que contenían la lipasa inmovilizada. La deposición de la segunda capa se realizó a una temperatura relativamente más baja para evitar la inactivación térmica de la enzima inmovilizada. La capa interna de sílice se obtuvo ensamblando nanopartículas de sílice primaria generadas a partir de una solución de silicato de sodio altamente básica a 80°C en la superficie de las partículas del núcleo de sílice funcionalizadas con poli (cloruro de dialildimetilamonio) (PDDA). El grosor medio de la cubierta y el tamaño de poro de la capa interna de sílice fue de ∼60nm y 24nm, respectivamente. También se investigó el efecto de los parámetros del proceso sobre la generación y agregación de las nanopartículas de sílice preparadas a partir de una solución de silicato de sodio altamente básica. La agregación de las partículas de sílice generadas a 40°C y 80°C tuvo lugar después de 840s y 570s de reacción, respectivamente. La eficiencia de la inmovilización de la lipasa en la monocapa de sílice mesoporosa fue del 80%. La disminución de la actividad de la lipasa inmovilizada fue de aproximadamente 6 veces después de 10 ciclos de reacción debido a la fuga de la lipasa de la cubierta monocapa. Se depositó una capa de sílice mesoporosa externa a 40°C sobre la superficie de las partículas de la núcleo-cubierta de sílice monocapa previamente estratificadas de PDDA que contenían la lipasa inmovilizada. El grosor promedio y el tamaño de los poros de la capa de sílice mesoporosa externa fue de ∼60nm y 17nm, respectivamente. La actividad de la lipasa inmovilizada dentro de la cubierta bicapa se redujo aún más debido a la resistencia a la difusión dentro de la capa de sílice externa y la capa de PDDA y, sin embargo, se retuvo para los siguientes ciclos de reacción.
El tamaño de poro de la capa de sílice mesoporosa obtenida a 80°C fue insuficiente para permitir la inmovilización de la invertasa. Así, El segundo método para la inmovilización de la invertasa fue desarrollado debido a que la invertasa era mayor que el poro de la capa de sílice mesoporosa previamente sintetizada. Lo mismo implicaba la preparación de la capa de sílice mesoporosa simultáneamente con la inmovilización de la invertasa a una temperatura de 40°C. La invertasa inmovilizada mostró una actividad disminuida, pero no fue obstaculizada por la inhibición del sustrato, como en el caso de la enzima libre, debido a la ubicación de la enzima dentro de la capa de sílice mesoporosa, donde existía resistencia a la transferencia de masa del sustrato a la enzima activa.
Enzymes are versatile biocatalysts used in processes for production of a wide variety of fine chemicals [1], medicines [2] and biosensors [3]. Enzyme-catalyzed reactions are environmentally friendly [4], but enzymes have high activity and selectivity under relatively mild reaction conditions. However, under extreme conditions (high temperatures, extreme pH, organic solvents), enzymes are easily inactivated due to denaturation. Moreover, the application of natural enzymes is hampered by difficulties in reuse, product contamination and separation. The approach taken to resolve these difficulties is to immobilize enzymes onto solid supports, in order to enable their reuse and the formation of stable heterogenous biocatalysts. As a result of their excellent properties, such as adjustable size, morphology and porosity, along with their chemical stability, biocompatibility and non-toxicity, mesoporous silica particles have widely been used for enzyme immobilization [5,6]. In recent years, silica core-shell particles (solid core-porous shell or superficially porous particles) have demonstrated a wide range of applications, including separation [7], drug delivery [8], chemical catalysis [9] and enzyme immobilization [10]. Uniform silica core particles are often synthesized by the Stöber process [11] which is based on hydrolysis and condensation of highly reactive tetraethylortosilicate (TEOS) precursor catalyzed by ammonia with water in low molecular weight alcohol. In addition, it has been shown [11–13] that size and distribution of the synthesized silica particles are very sensitive to the TEOS:NH4OH:H2O molar ration and TEOS concentration.
The formation of mesoporous silica layers around silica cores is mainly based on the use of surfactants that are aggregated on the surface of silica cores, followed by condensation of TEOS on the surface of modified cores [8–10,14]. In these cases, calcination or extraction is performed to remove the template from the structure. The different silica coating thicknesses can be prepared by varying the core/TEOS ratio in the initial suspensions as it was found for the precipitation of silica onto the surface of previously obtained alumina cores [15]. The neutralization of sodium silicate solution induces nucleation of silica nanoparticles, which aggregate to form particles with mesoporous structures [13]. Therefore, mesoporous silica shells can be synthesized without templates by a simple procedure involving the electrostatic deposition of silica nanoparticles prepared from highly basic sodium silicate solution on the surface of functionalized cores [16,17]. The use of a cationic polyelectrolyte layer on the surface of core particles improved the electrostatic deposition of precipitated silica and the formation of uniform and continuous silica layers [16,18].
The growing interest in processes with the use of immobilized enzymes leads to the development of new supports suitable for enzyme immobilization. Thus, the design and characterization of new supports as well as the methods for enzyme immobilization have been increasingly valued. In this research, two processing methods for the immobilization of lipase and invertase onto silica core-shell structures were developed. The reason was the different sizes of these two enzymes. Candida rugosa lipase has an apparent molecular weight of 60kDa [19] and a molecular volume of 5×4.2×3.3nm3[20] while invertase from Saccharomyces cerevisiae has a molecular weight of 270kDa [21]. Bilayered silica core-shell structures were developed for lipase immobilization while monolayered silica core-shell particles were developed for invertase immobilization.
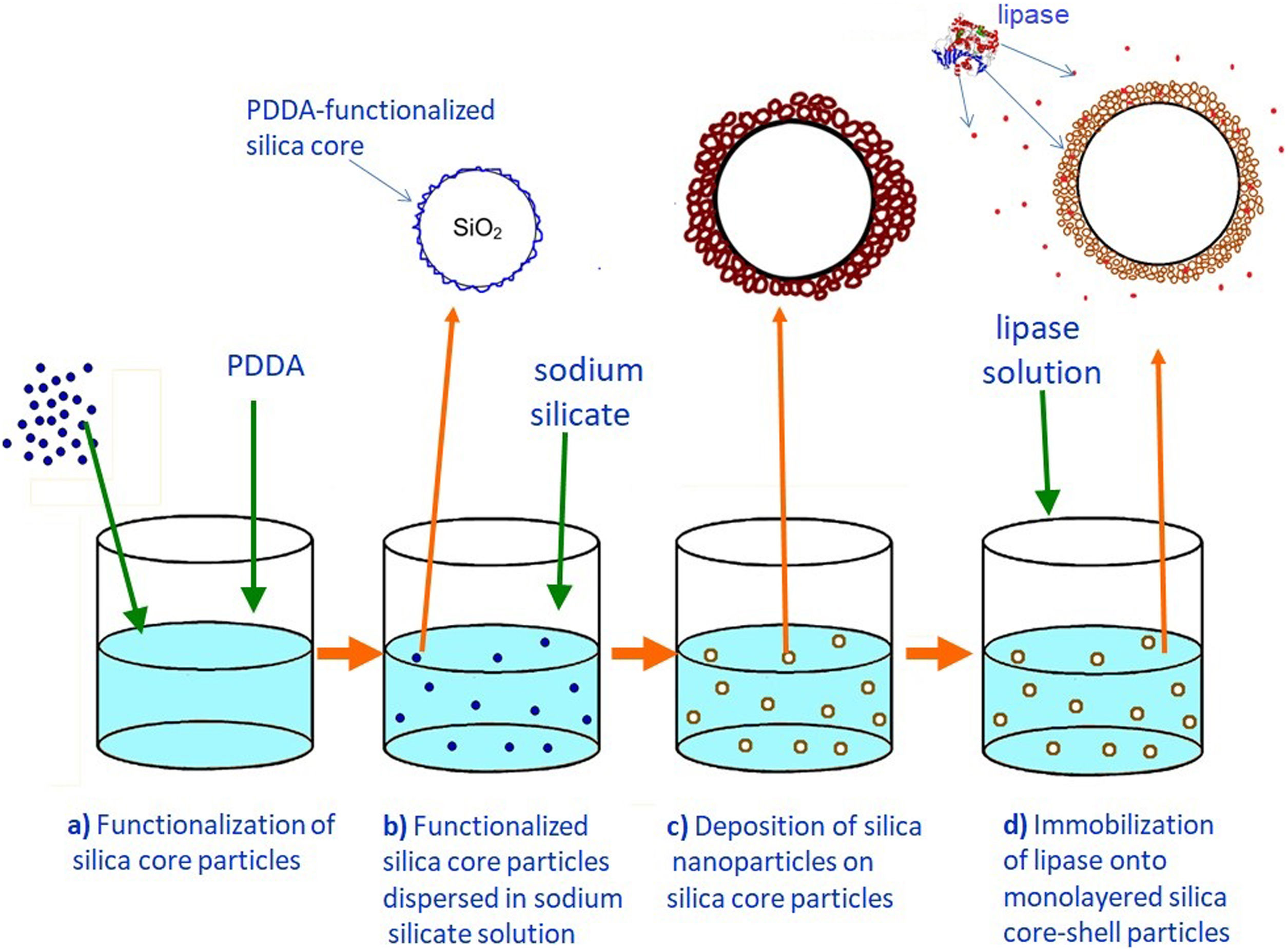
ExperimentalBilayered silica core-shell particles containing immobilized lipase were prepared using a three-step process. In the first step, silica core particles were synthesized by the hydrolysis and condensation of tetraethylortosilicate (TEOS) by the Stöber process [11] and functionalized with poly(diallyldimethylammonium chloride) (PDDA). In the second step, a mesoporous silica layer was prepared by the deposition of silica nanoparticles generated by the neutralization of highly basic sodium silicate solution on the surface of the previously synthesized positively charged silica core particles. The as-synthesized silica core-shell particles were used as a host for lipase immobilization. In the third step, an outer mesoporous silica layer was deposited on the surface of the silica core-shell particles containing the immobilized enzymes to prevent enzyme leakage from the support.
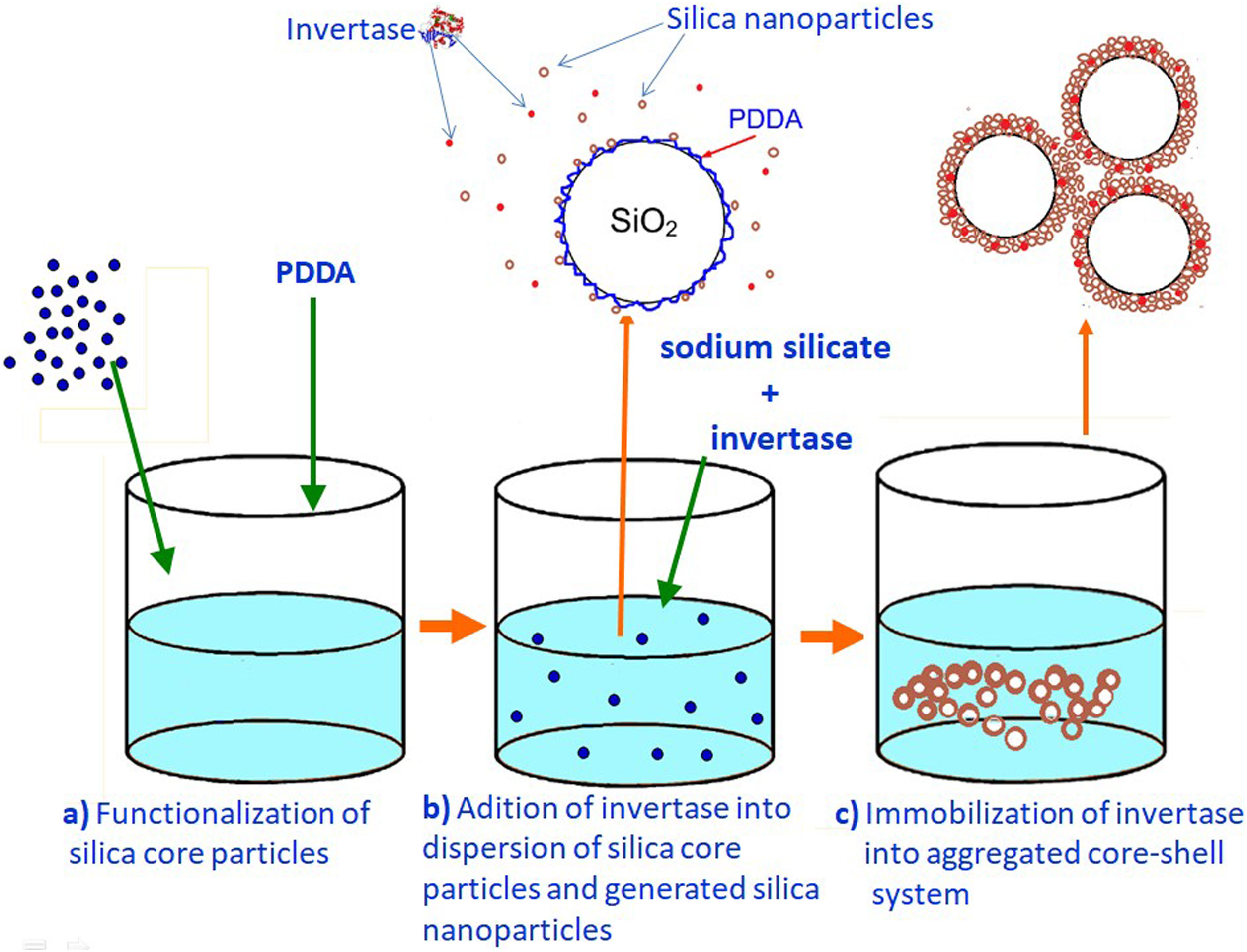
Monolayered silica core-shell particles containing immobilized invertase were prepared by a two-step process. In the first step, silica core particles were prepared from TEOS and functionalized with PDDA. In the second step, a mesoporous silica layer containing immobilized invertase was prepared by dispersing functionalized silica core particles in an aqueous solution containing invertase and sodium silicate. The mesoporous silica layer with the entrapped enzyme was deposited on the surface of positively charged silica core particles.
Synthesis of silica core particlesSilica core particles (sample C) were synthesized by the hydrolysis and condensation of tetraethylortosilicate (TEOS), dissolved in anhydrous ethanol, with distilled water under basic condition (25% NH3, Merck). The sample was prepared by the Stöber process using a molar ratio of TEOS:H2O:NH4OH=1:40:2 and a TEOS concentration of 0.25mol/l [16]. After feeding, the product suspension was continuously stirred at room temperature for 1h. The white precipitated powder was centrifuged and washed with distilled water until the pH of effluent was approximately 7, and finally dried at 120°C for 1 day.
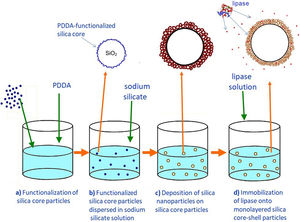
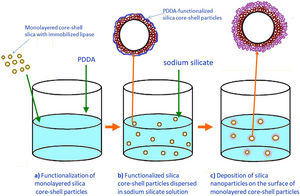
Preparation of the first mesoporous silica layer with immobilized lipaseIn order to enable the electrostatic assembly of primary silica nanoparticles on silica core particles (sample C), their surfaces were functionalized with poly(diallyldimethylammonium chloride) (PDDA) (Fig. 1a). PDDA-functionalized silica particles (sample CP) were prepared by suspending silica core particles (1.5g) in 70ml PDDA solution (at a concentration of 12mg/ml), which also contained 0.05M NaCl [16]. The obtained suspension was stirred for 20min at 70°C, and the functionalized particles (sample CP) were centrifuged, washed with distilled water, and finally dried at 100°C.
The first mesoporous silica layer (with somewhat larger pores) was prepared by the deposition of silica nanoparticles generated by the neutralization of highly basic sodium silicate solution on the surface of the previously synthesized positively charged PDDA-functionalized silica core particles [16]. The PDDA-functionalized SiO2core particles were dispersed in highly basic sodium silicate solution (Water Glass, Birač Alumina Factory, Zvornik) having a Na2O/SiO2 molar ratio of 0.4 and a SiO2 concentration of 1.25mol/l (Fig. 1b). Sulfuric acid (1mol/l) was slowly added to a well-stirred sodium silicate solution (containing dispersed PDDA-functionalized SiO2core particles) at 80°C to decrease the pH value and enable particulate silica layer formation. The optimum reaction temperature and time were selected for the preparation of an appropriate mesoporous structure for enzyme immobilization (Fig. 1c). When the reaction finished, the obtained particles (sample CPS) were separated from the liquid phase by centrifugation, washed with distilled water, and finally dried at 120°C for one day.
The immobilization of lipase from C. rugosa (Sigma–Aldrich) onto the mesoporous silica layer was performed according to the following procedure: 1.3g of as-synthesized silica core-shell particles and 100ml of lipase solution (0.065mg/ml) in phosphate buffer (0.05M and pH=7.0) were added to a flask and stirred magnetically for 1h at ambient temperature (Fig. 1d). The reaction mixture was centrifuged and the supernatant was used for the determination of the amount of lipase remaining in the solution. The concentration of lipase solution was measured by the Bradford method [22], with BSA (bovine serum albumin) as a standard at 595nm. A similar procedure was used for the immobilization of invertase from baker's yeast (S. cerevisiae) (Sigma–Aldrich) onto the mesoporous silica layer, but it was unsuccessful.
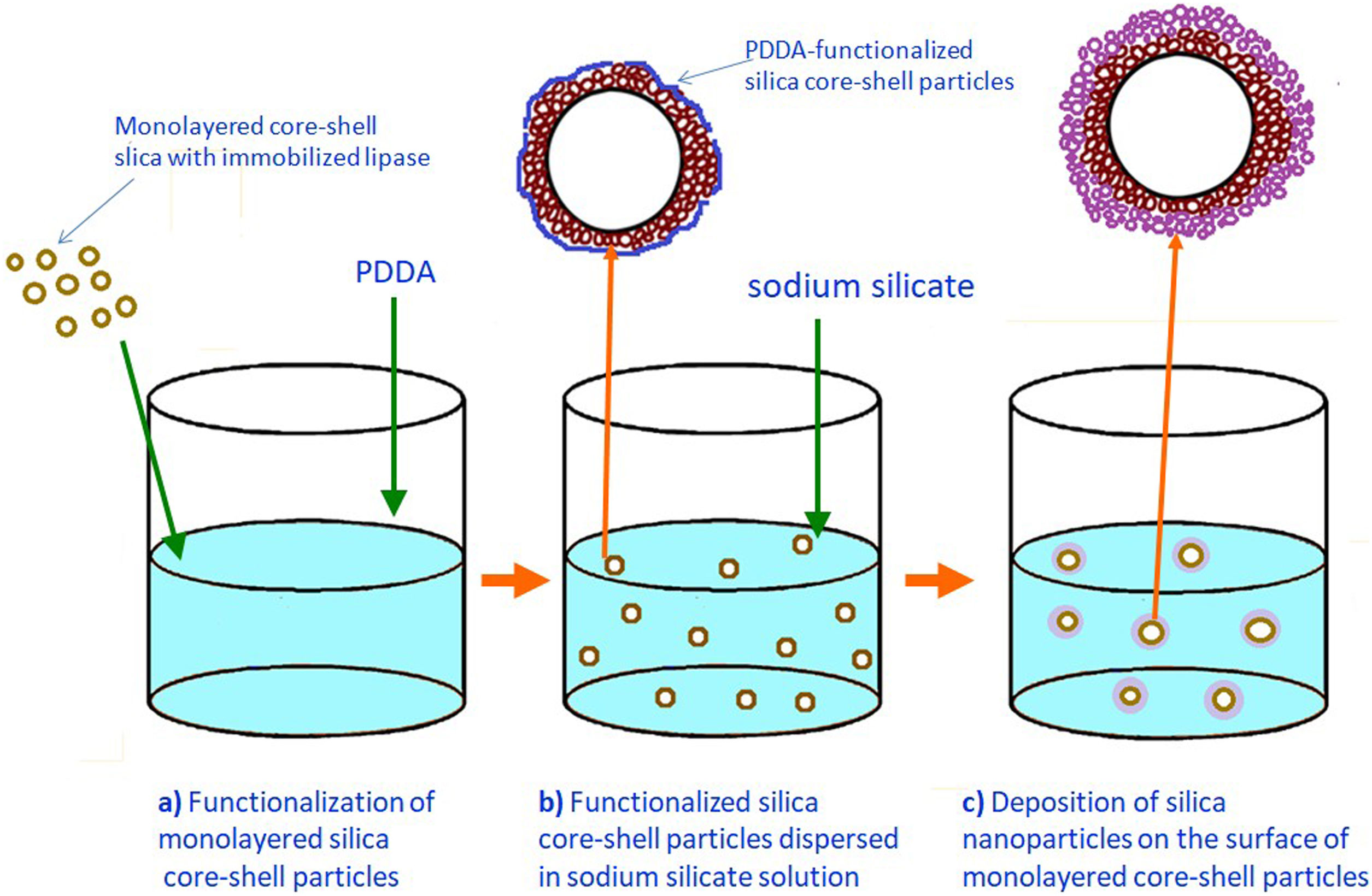
Formation of an outer silica layer around lipase-immobilized silica core-shell particlesAn outer mesoporous silica layer (with somewhat smaller pores) was deposited on the surface of silica core-shell particles containing the immobilized lipase to prevent enzyme leakage from the support. To achieve the electrostatic assembly of silica nanoparticles on the surface of the previously obtained enzyme-immobilized silica core-shell particles, the latter were functionalized with PDDA (Fig. 2a). The PDDA-functionalized lipase-immobilized silica particles were prepared by dispersing 1.3g of enzyme-immobilized silica particles in 50ml PDDA solution (at a concentration of 12mg/ml), which also contained 0.05M NaCl. The suspension was stirred for 20min at room temperature. The modified particles (sample CPSP) were centrifuged and successively washed with distilled water.
The PDDA-functionalized silica core-shell particles containing the immobilized lipase were used as templates for assembling an external silica layer. These particles were dispersed in highly basic sodium silicate solution having a Na2O/SiO2 molar ratio of 0.4 and a SiO2 concentration of 1.25mol/l (Fig. 2b). Sulfuric acid (1mol/l) was slowly added to this well-stirred dispersion at 40°C to decrease the pH value and enable the generation of silica nanoparticles and their deposition on the surface of the PDDA-functionalized lipase-immobilized silica particles (Fig. 2c). The optimum reaction temperature and time were selected for the preparation of an appropriate mesoporous structure to prevent enzyme (lipase) leakage from the support. When the reaction finished, the obtained particles (sample CP-SP-S) were separated from the liquid phase by centrifugation, washed with distilled water, and finally dried at room temperature.
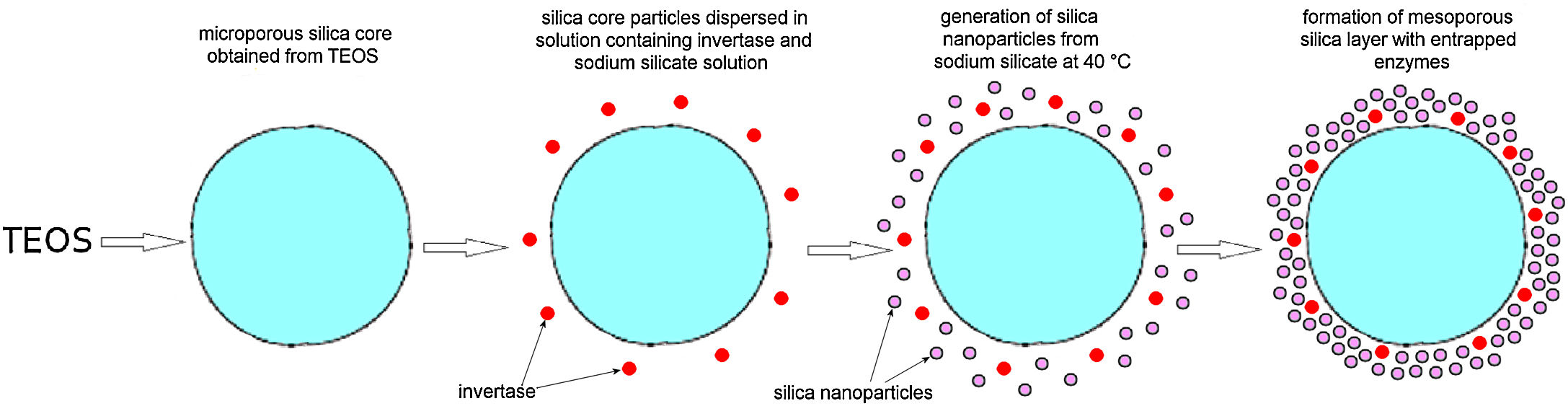
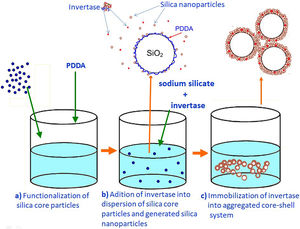
Preparation of a mesoporous silica layer simultaneously with invertase immobilizationThe PDDA-functionalized SiO2 core particles were dispersed in highly basic sodium silicate solution having a Na2O/SiO2molar ratio of 0.4 and a SiO2 concentration of 1.25mol/l. Sulfuric acid (1mol/l) was slowly added to the well-stirred dispersion at 40°C, to decrease the pH value and enable the generation of silica nanoparticles. A small aliquot of invertase (from baker's yeast S. cerevisiae, Sigma–Aldrich) solution (2ml at a concentration of 0.178mg/ml) was added to the reaction vessel before the gel point was reached (Fig. 3b). The reaction lasted for 2min and was stopped when the gel point was reached (Fig. 3c). The white precipitated powder was centrifuged, washed with distilled water, and finally dried at room temperature for 2 days. Viscosity variation of silica sols with neutralization time and the moment of reaching the gel-point were obtained by neutralization of highly basic sodium silicate solution (having a Na2O/SiO2molar ratio of 0.28 and a SiO2 concentration of 0.91mol/l) at 70°C.
Characterization of silica particlesParticle size and zeta potential were measured by dynamic light scattering (Zetasizer Nano ZS, Malvern Instruments). The size and morphology of the particles were examined using a scanning electron microscope (JEOL JSM 6460 LV) operating at 20kV. Prior to SEM imaging, the samples were sputtered with gold. TEM characterization was performed using a JEM 1400-Plus JEOL device operating at 120kV.
The specific surface area (according to the BET method) [23], pore size distribution (according to the BJH method) [24] and pore volume of as-synthesized silica particles were measured by low-temperature nitrogen adsorption using a Quantachrom Autosorb-3B instrument. The samples were outgassed at 150°C for 12h under a vacuum of 10−4 Pa prior to the adsorption measurements.
The viscosity vs. shear-rate relations for silica sols obtained by neutralization of highly basic sodium silicate solution were measured by HAAKE RheoStress 600.
Activity assay of free and immobilized enzymesThe immobilization efficiency (%) of the enzyme was calculated using the equation:
where Ci and Cf are the initial and final concentration of the enzyme, respectively.The activities of the free and immobilized lipase were measured by monitoring the catalytic hydrolysis of 4-nitrophenyl acetate (pNPA) into 4-nitrophenol [25]. Due to poor solubility in water, pNPA was first dissolved in acetonitrile and then dropwise added to the phosphate buffer (0.05M, pH=7). The effect of temperature on the activity of native lipase was performed by adding 400μl of free enzyme solution (at a concentration of 0.03mg/ml) to 10ml of lipase substrate (the concentration of 4-nitrophenyl acetate was 0.55mM) at 30, 38, 46, 54 and 62°C, respectively. Enzymatic activity was calculated based on three assays at each temperature. Activity assays were carried out by adding 400μl of free enzyme solution (at a concentration of 0.03mg/ml) or 150mg of the enzyme-loaded material (the amount of bound enzyme on support CPS and CP-SP-S was 6 and 4mg per gram of support, respectively) to 10ml of lipase substrate (the concentration of 4-nitrophenyl acetate was 1.1mM) at 25°C. Due to diffusion limitations, the reaction time of the immobilized lipase was prolonged (the reaction time of the free and immobilized lipase was 2 and 15min, respectively). Afterwards, the amount of 4-nitrophenol was determined spectrophotometrically at 410nm. Both negative and positive controls were studied. The positive control was the reaction mixture to which the free or immobilized enzyme was added at the beginning of the reaction time. The negative control contained no enzyme.
The activities of the free and immobilized invertase were determined in measurements of reducing sugars produced by the enzymatic hydrolysis of sucrose into glucose and fructose. These activity assays were performed by adding 50μl of the free enzyme in acetate buffer (at a concentration of 0.22mg/ml and pH=4.5) or 0.5g of the enzyme-loaded material to 10ml of sucrose (having three concentrations 0.05, 0.1 and 0.2M) in the same buffer. After exactly 30min of incubation at 25°C, the sample was placed in a test tube containing 3ml of dinitrosalicylic acid reagent (DNS) [26]. The mixture was placed in boiled water for 5min to develop a red-brown color. Subsequently, 1ml of a 40% potassium sodium tartrate (Rochelle salt) solution was added to stabilize the color. After cooling to room temperature in a cold water bath, the mixture was diluted with 10ml of water and finally the absorbance was recorded by a spectrophotometer at 575nm. The calibration curve was based on previous measurements of invert standard solutions (an equimolar mixture of glucose and fructose) of known concentrations.
Results and discussionImmobilization of lipase in the mesoporous silica core-shell structureTo determine an optimum reaction temperature and optimum time for the deposition of both mesoporous silica layers, the following items should be analyzed: (i) the size of C. rugosa lipase, (ii) the effect of temperature on the activity of native lipase and (iii) neutralization conditions of the sodium silicate solution.
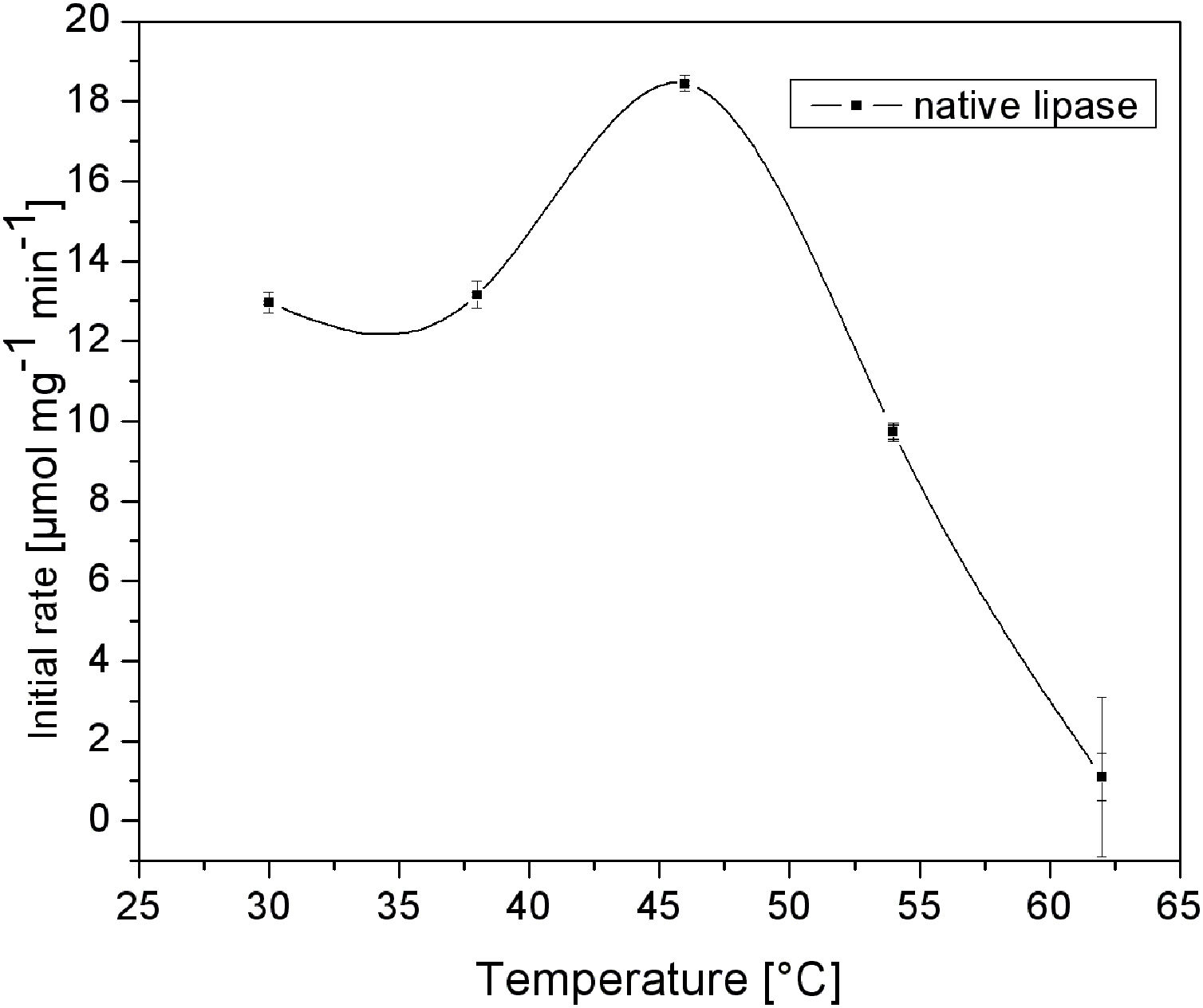
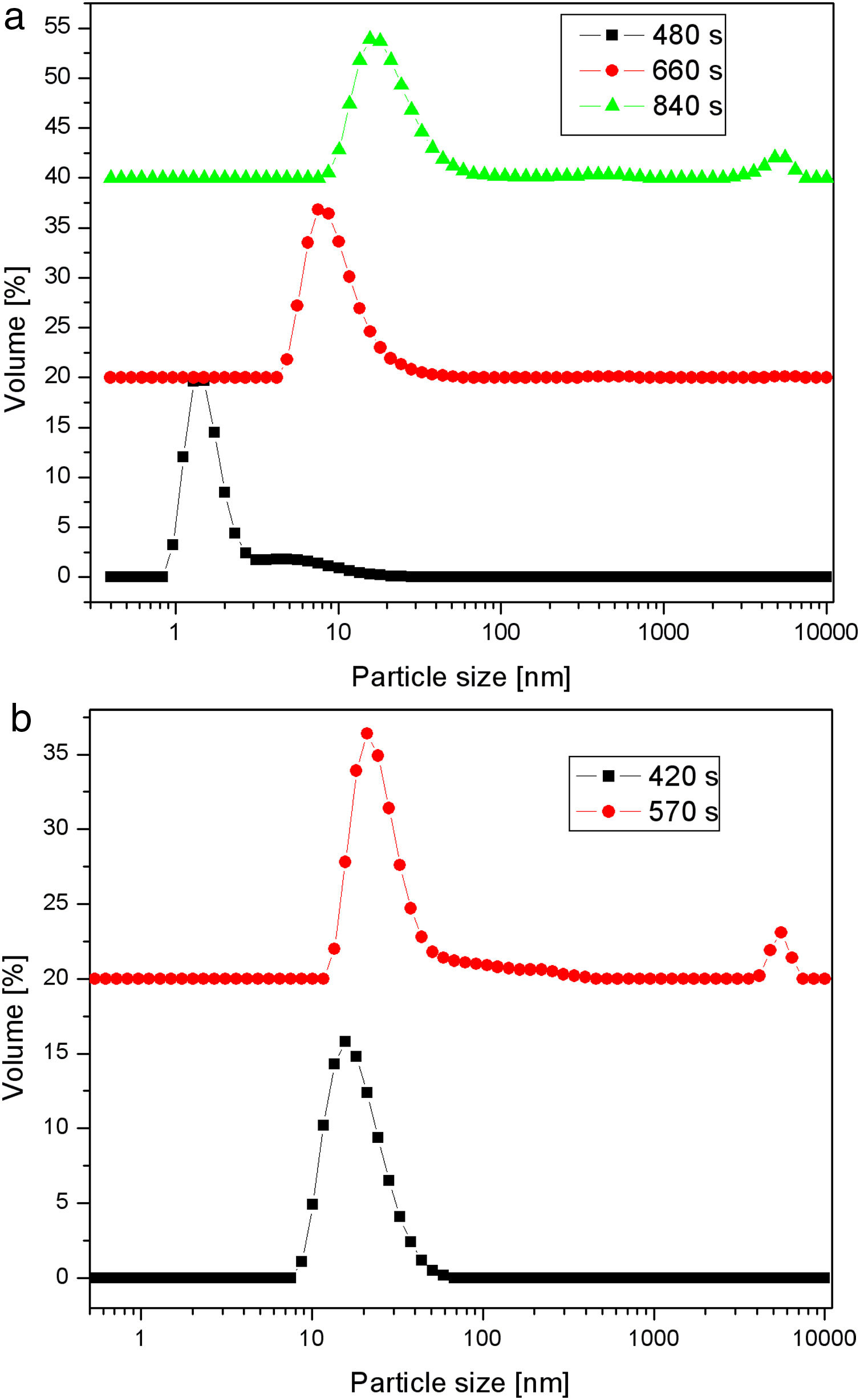
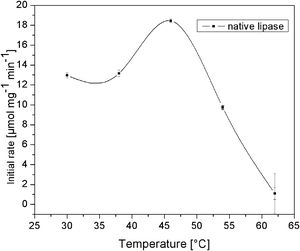
C. rugosa lipase has a molecular volume of 5×4.2×3.3nm3[19]. Therefore, the first silica layer, in which this enzyme is to be immobilized, should have relatively large mesopores. The effect of temperature on the activity of native lipase is shown in Fig. 4. It is obvious that native lipase is completely inactivated above 60°C. Hence, the deposition of the second layer should be performed at a relatively lower temperature, to prevent thermal inactivation of the immobilized enzyme. According to these requirements and our previous results [16,17], two different temperatures (40 and 80°C) and a reaction time of up to 840s were investigated.
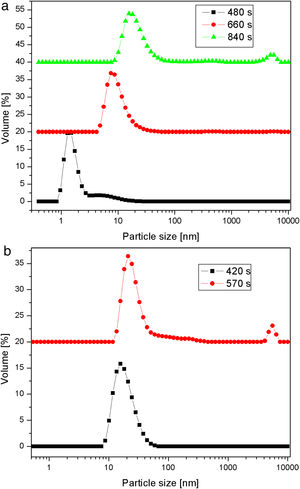
Fig. 5 shows particle size distributions of samples obtained by the neutralization of sodium silicate solution (having a SiO2 concentration of 1.25mol/l and a Na2O/SiO2 molar ratio of 0.4) at 40 and 80°C. The samples were taken from the vessel during the reaction at different time intervals. Sulfuric acid was slowly added to the well-stirred sodium silicate solution, to keep the rate of neutralization constant at both temperatures. The increase in the average particle size with time was less pronounced at the lower temperature. A bimodal particle size distribution, with a high-intensity peak at ∼2nm and a smaller one at ∼9nm, was observed after reaction at 40°C for 480s (Fig. 5a). The prolongation of the reaction led to an increase in primary particle size. However, after 840s, the aggregation of silica particles resulted in the formation of very large particles of about 5μm. Conversely, the increase in the average particle size with time was more pronounced at the higher temperature (Fig. 5b). It was also noted that the aggregation of the primary silica particles obtained at 80°C took place after a shorter period of time (a small peak at ∼5μm was observed as early as after 570s) than in the case of the particles synthesized at 40°C.
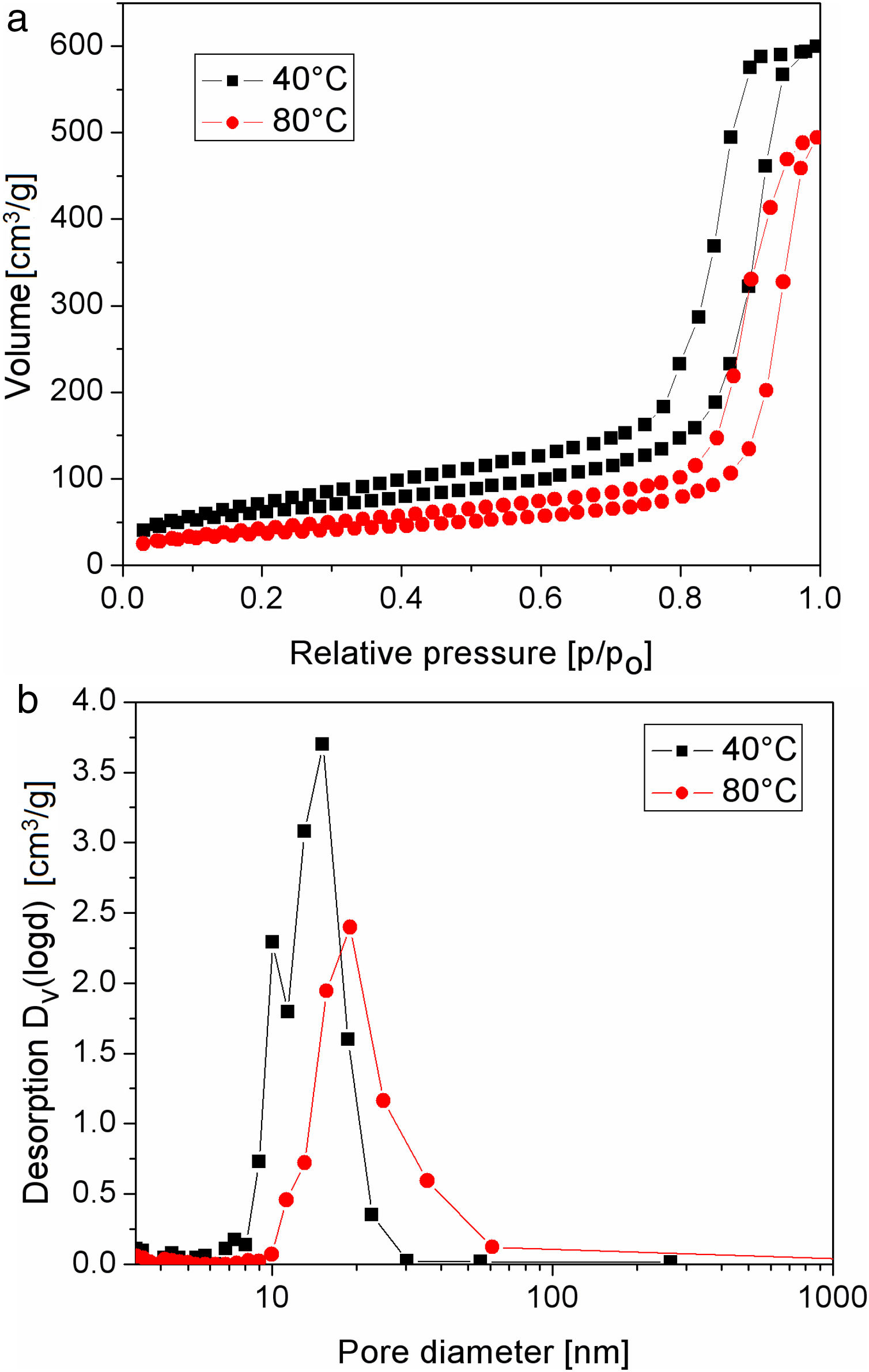
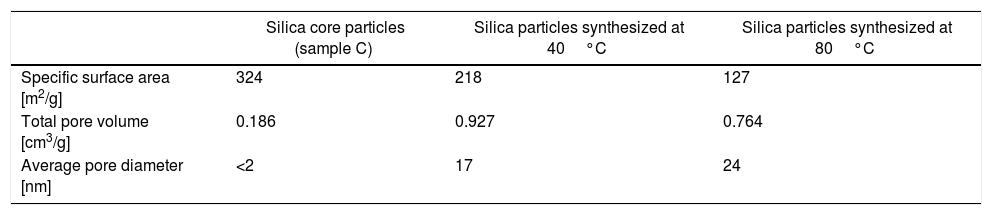
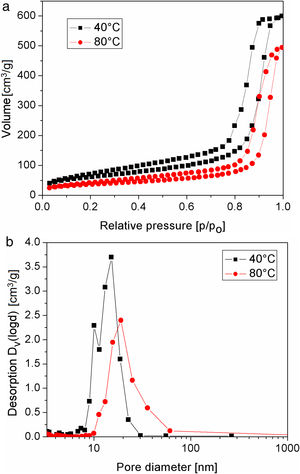
The nitrogen adsorption–desorption isotherms of silica particles obtained from sodium silicate solution at 40 and 80°C are shown in Fig. 6. Both belong to Type IV, indicating that these particles have mesoporous structures. The average pore size distributions of silica particles obtained from sodium silicate solution at 40 and 80°C are given in Fig. 6b, clearly showing that the average pore size of the obtained silica particles is ∼17 and 24nm, respectively (Table 1). On the other side, the average pore size of silica core particles was below 2nm, indicating the microporous structure (Table 1). Additional information, such as total pore volume and specific surface area, are also shown in Table 1. High surface area and very fine porosity of silica core particles correspond to a small size of primary particles obtained by hydrolysis and condensation of TEOS and their fractal structure [13]. As it was shown, an increase of temperature for the reaction of sodium silicate with sulfuric acid resulted in increasing of average pore size and decreasing of the specific surface area and the total pore volume. The reason for the observed differences lies in the fact that at low temperature reactions are slow and, thus, the primary particle size and concentration were small. Thus, the primary interparticle pores were smaller at lower temperature and specific surface area was higher. The total pore volume of silica particles generated at 80°C was lower than that obtained at 40°C. The higher temperature may have resulted in increasing concentration of primary silica particles generated by neutralization of sodium silicate solution. Thus, aggregation of primary silica particles from concentrated silica sols resulted in a dense packing of primary particles and, consequently, the average pore volume of obtained mesoporous volume was decreased.
Specific surface area, total pore volume and average pore diameter of as-synthesized silica particles.
| Silica core particles (sample C) | Silica particles synthesized at 40°C | Silica particles synthesized at 80°C | |
|---|---|---|---|
| Specific surface area [m2/g] | 324 | 218 | 127 |
| Total pore volume [cm3/g] | 0.186 | 0.927 | 0.764 |
| Average pore diameter [nm] | <2 | 17 | 24 |
As shown by the results, the first silica layer with larger mesopores (Fig. 7) was deposited at 80°C after a reaction time of 9min, while the second silica layer with smaller mesopores (Fig. 7) was prepared at 40°C after a reaction time of 12min.
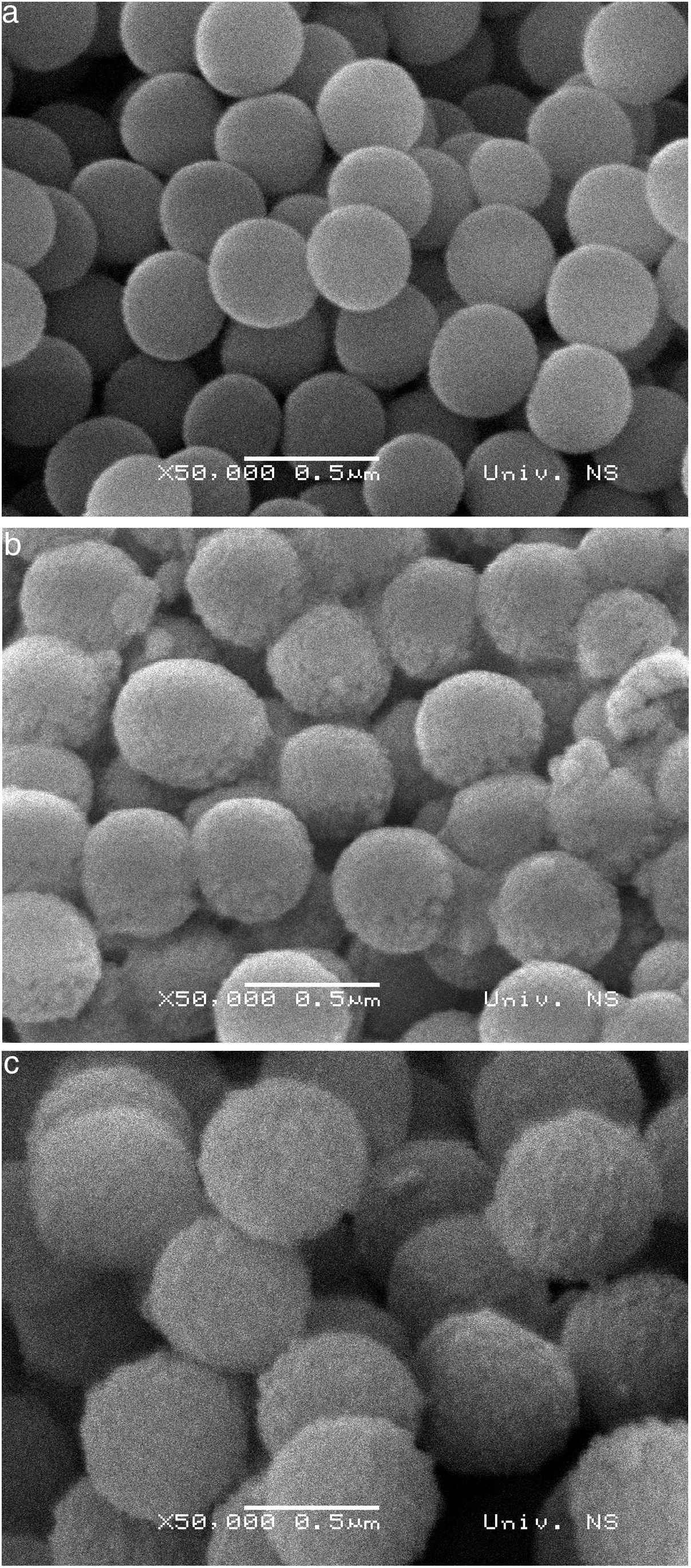
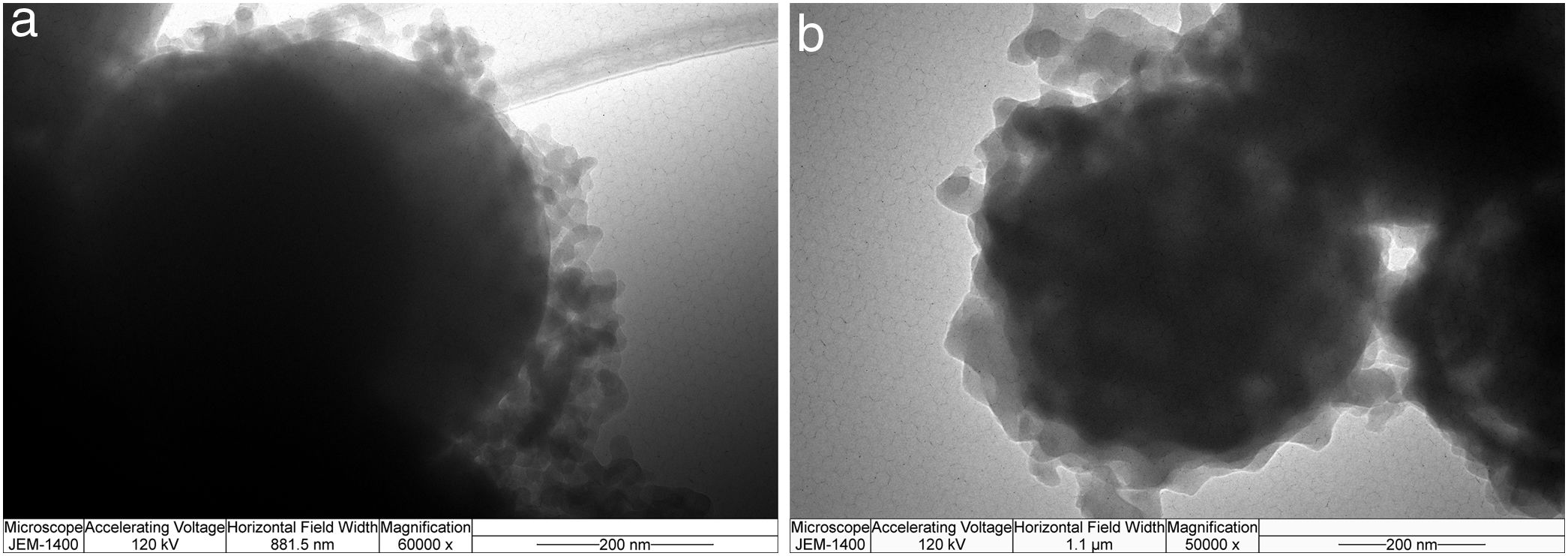
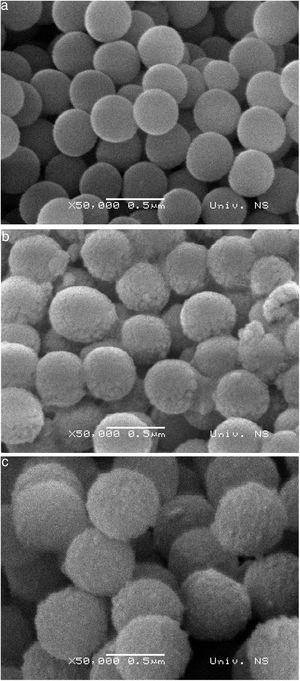
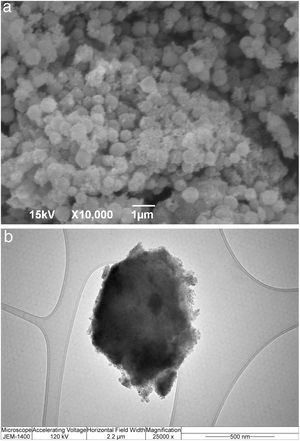
SEM micrographs of silica core particles and monolayered and bilayered silica core-shell particles are shown in Fig. 8. The silica core particles obtained from TEOS are spherical and monodispersed, with a smooth surface and the average particle size of about 400nm (Fig. 8a). A rough surface of silica core-shell particles is clearly visible in the SEM micrographs (Figs. 8b and c), indicating that the silica nanoparticles generated from sodium silicate solution were deposited on the surface of silica core particles. TEM micrographs of monolayered and bilayered silica core-shell particles are shown in Fig. 9. The obtained shells are continuous but slightly inhomogenous. TEM micrograph revealed the average primary particle size of ∼15nm and porosity of mesoporous silica shell (Fig. 9a). The thickness of monolayered silica shell is around 60nm, while the thickness of bilayered silica shell is ∼120nm (Fig. 9b).
The immobilization efficiency of lipase on the mesoporous silica layer (sample CpS) was 80%. When a similar procedure was used for the immobilization of invertase onto the mesoporous silica layer, the efficiency was 0%. C. rugosa lipase has an apparent molecular weight of 60kDa [19] and a molecular volume of 5×4.2×3.3nm3[20] while invertase from S. cerevisiae has a molecular weight of 270kDa [21]. The average pore size of the mesoporous silica layer was 24nm which allowed lipase immobilization within mesopores. However, the pore size was insufficient to allow invertase immobilization, indicating that another procedure should be used for the immobilization of invertase onto mesoporous silica.
The zeta potential of non-functionalized silica core particles (sample C) was −43.7mV while the zeta potential of PDDA-functionalized silica core particles (sample CP) was +54.9mV. The zeta potential of particles obtained by the deposition of silica nanoparticles generated at 80°C from the sodium silicate solution on the surface of positively charged silica core particles was −51.6mV (sample CPS). This could indicate that the surface of PDDA-functionalized silica core particles was covered with a continuous silica layer. Lipase from C. rugosa was immobilized inside the mesoporous silica shell of the previously obtained silica core-shell particles (sample CPS), and then the support particles were functionalized with PDDA. The zeta potential of PDDA-functionalized silica core-shell particles (sample CP-SP) was +39.1mV. Finally, to prevent enzyme leakage from the support, the silica nanoparticles generated at 40°C from the highly basic sodium silicate solution were deposited on the surface of PDDA-functionalized silica core-shell particles. The zeta potential of bilayered silica core-shell particles (sample CP-SP-S) was –33.4mV, which might indicate that a continuous external silica layer was formed around monolayered silica core-shell enzyme support particles.
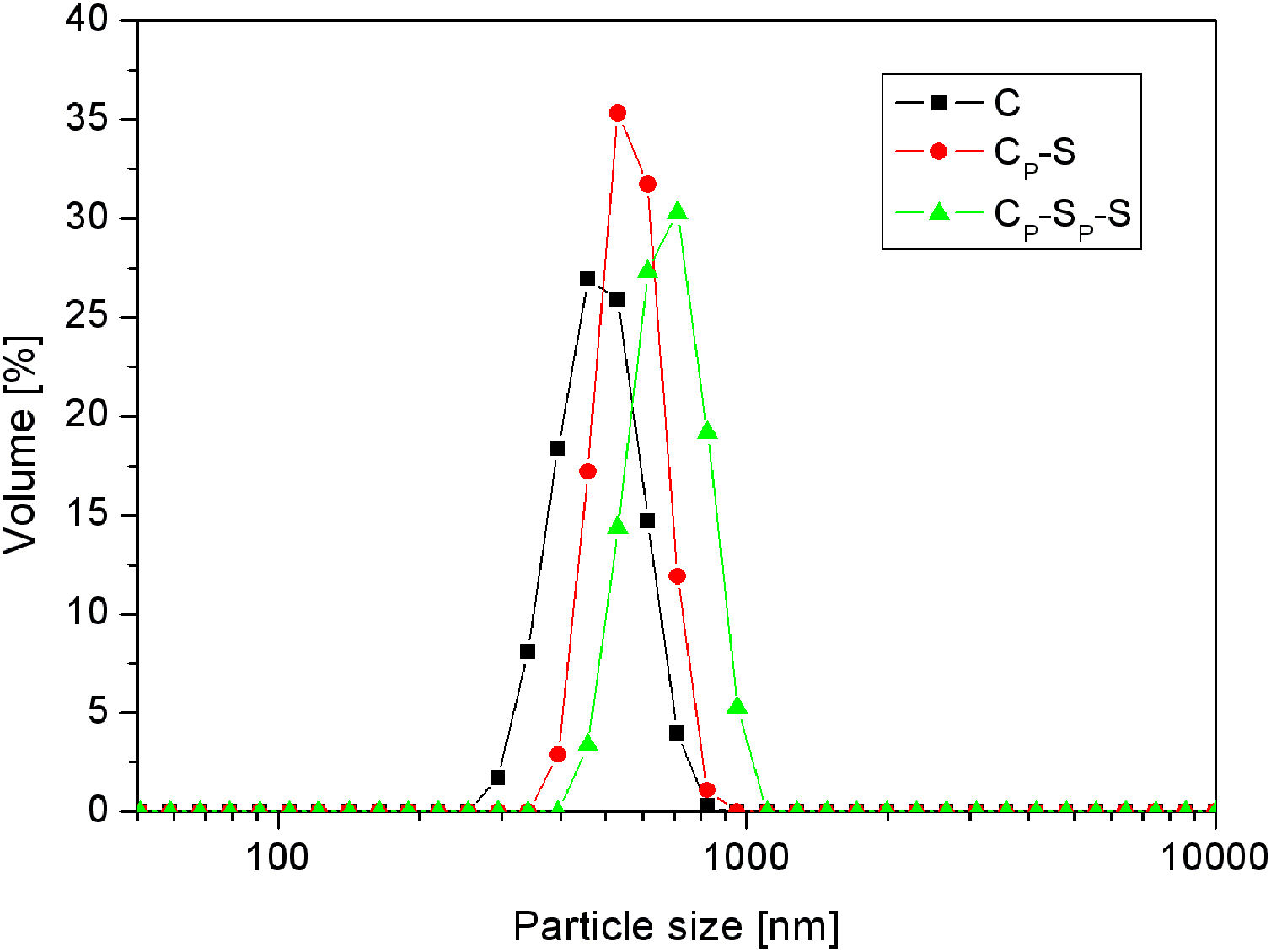
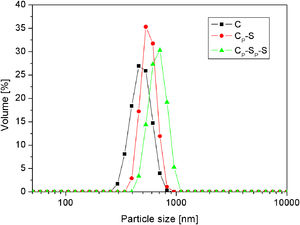
The average particle size distributions of silica core particles and monolayered and bilayered core-shell particles (samples C, CP-S and CP-SP-S) are shown in Fig. 10. The average particle size distributions of these particles are approximately 450, 550 and 650nm, respectively, which indicates increasing of the particle size with successive deposition of two silica monolayers. The average thickness of the monolayered shell is ∼50nm, while the thickness of the bilayered shell is ∼100nm. The similar information was obtained by TEM analysis (Fig. 9).
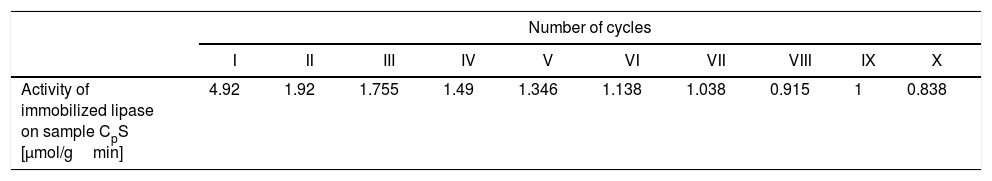
The results of the hydrolysis of 4-nitrophenyl acetate (pNPA) into 4-nitrophenol released per gram of the monolayered silica core-shell material (sample CPS) containing the immobilized lipase are shown in Table 2. As shown in Table 2, the monolayered silica core-shell particles containing the immobilized lipase showed a loss of activity after 10 reaction cycles by approximately six times. The highest activity was after the first cycle, and it was 4.92μmol of 4-nitrophenol per gram of monolayered core-shell silica per minute. In other words, the activity of the immobilized lipase was 0.29μmol of 4-nitrophenol released per milligram of the immobilized lipase per minute. The decrease in the activity of the material (sample CPS) containing the immobilized lipase was probably due to the desorption of lipase from the monolayered silica shell. Conversely, the activity of the free enzyme obtained under similar conditions (the initial substrate concentration of 1.1mM and 25°C) was 29μmol of 4-nitrophenol per milligram of lipase per minute. The activity of the immobilized lipase was lower than that of the free enzyme, probably due to diffusion limitations and the blockage of the enzyme's active site.
The activity of lipase immobilized on monolayered silica core-shell particles (sample CPS) expressed as the amount of 4-nitrophenol released per gram of the support per minute.
| Number of cycles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | |
| Activity of immobilized lipase on sample CpS [μmol/gmin] | 4.92 | 1.92 | 1.755 | 1.49 | 1.346 | 1.138 | 1.038 | 0.915 | 1 | 0.838 |
To prevent enzyme desorption from the mesoporous silica layer, an external mesoporous silica layer was formed by the deposition of silica nanoparticles generated by the neutralization of highly basic sodium silicate solution on the previously obtained silica core-shell particles (sample CPS) containing the immobilized lipase.
The activity of bilayered silica core-shell particles (sample CP-SP-S) containing the immobilized lipase was approximately 0.166μmol/gmin (0.166μmol of 4-nitrophenol per gram of the silica material per minute). In other words, the activity of the immobilized lipase was 0.04μmol of 4-nitrophenol per milligram of lipase per minute. The decreased activity of the lipase immobilized on the bilayered silica shell (sample CP-SP-S) relative to the lipase immobilized on the monolayered silica shell was probably due to mass transfer resistance in the external silica layer. The internal mass transfer resistance occurred in interstitial pores of external mesoporous silica layer as well as in thin PDDA layer surrounding internal mesoporous silica layer.
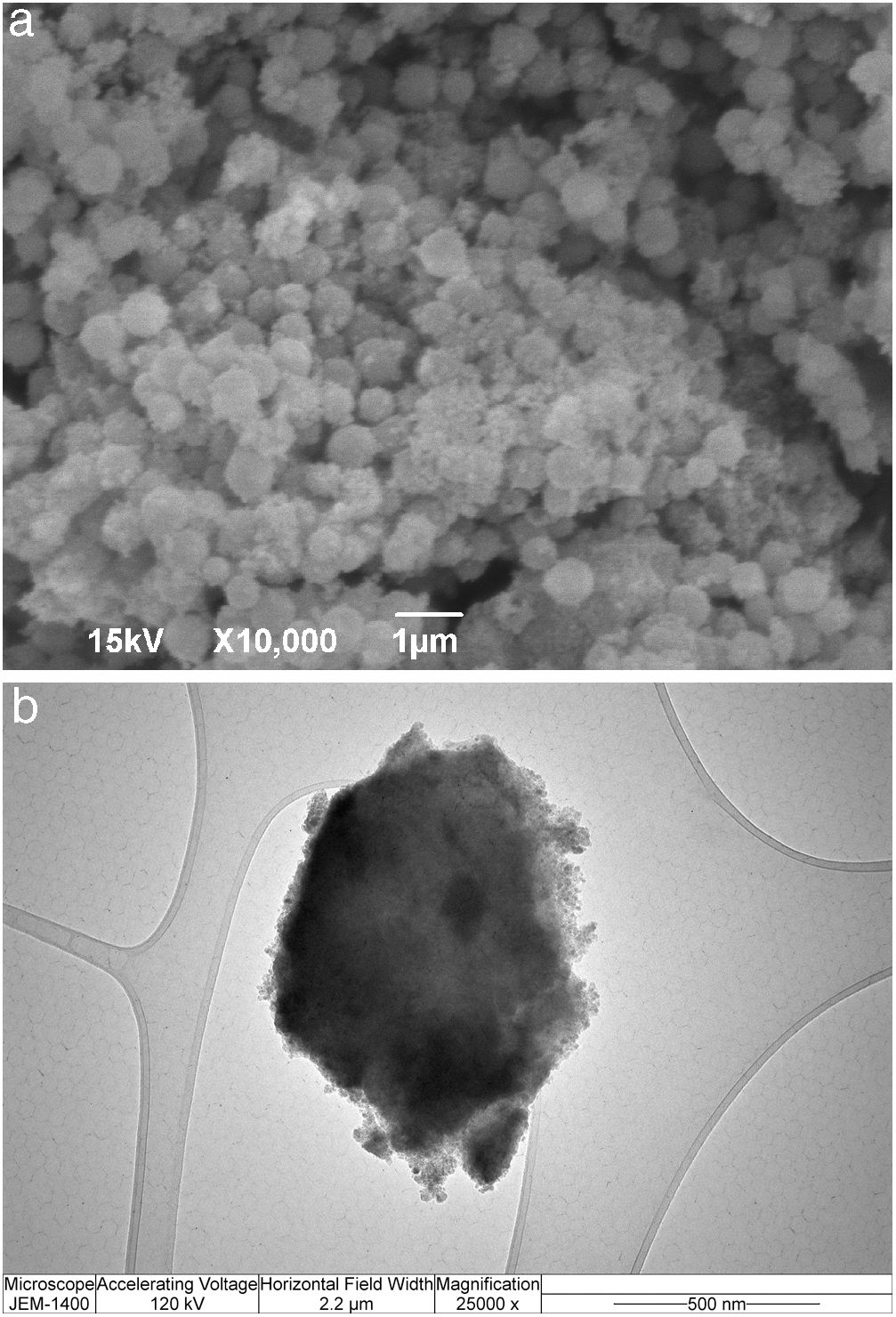
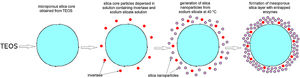
Immobilization of invertase in the mesoporous silica core-shell structureTo allow invertase immobilization inside the mesoporous silica layer, invertase was immobilized inside the simultaneously deposited silica layer (Fig. 11). SEM and TEM micrographs of the silica core-shell particles containing the immobilized invertase are presented in Fig. 12. As shown, a non-uniform shell was obtained since the reaction was prolonged until the gel point was reached to ensure the entrapment of each enzyme dissolved in the reaction vessel inside the mesoporous silica layer.
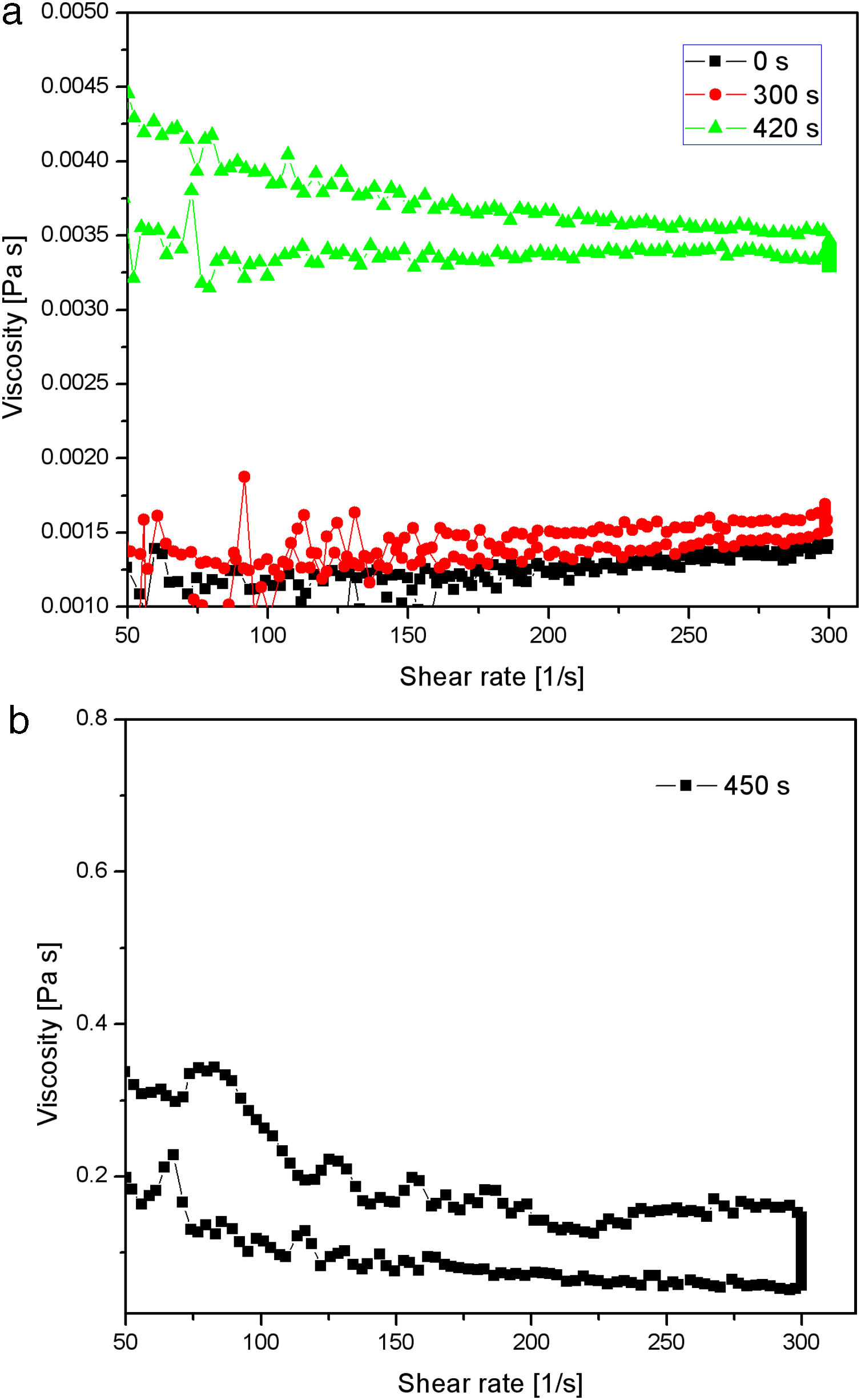
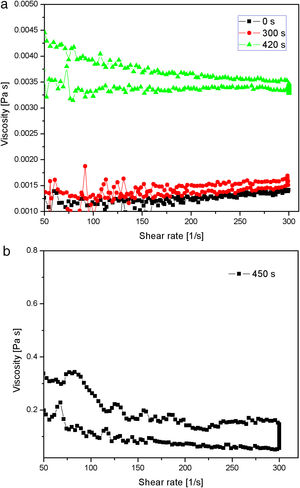
The results about the gel point and network formation are summarized in Fig. 13. The results showed the viscosity change with change in shear rate for the starting sodium silicate solution (having a Na2O/SiO2molar ratio of 0.28 and a SiO2 concentration of 0.91mol/l) and samples obtained by neutralization of sodium silicate solution at 70°C and taken from vessel during the reaction at three different time intervals (300, 420 and 450s), respectively. Sulfuric acid was slowly added into the well-stirred sodium silicate solution at 70°C, so that the speed of neutralization was constant over the whole reaction time. The average viscosity of the starting sodium silicate solution was ∼0.0012Pas (Fig. 13a). The viscosity was marginally changed after 300s of reaction (Fig. 13a). Further prolongation of reaction time (420s) resulted in a slight increase of viscosity (the average viscosity was about 0.0035Pas) (Fig. 13a). The viscosity of sodium silicate solution increased sharply after 450s (the average viscosity was about 0.2Pas), indicating that the gel point was reached (Fig. 13b).
The results of sucrose hydrolysis catalyzed by invertase, either in a free form or immobilized on silica core-shell particles are presented in Table 3. The activities of the free and immobilized invertase were measured at three sucrose concentrations (0.05, 0.1 and 0.2mol/l). The activity of invertase was shown as the amount of inverted sugar (micromoles) released per microgram of invertase after one hour of incubation. The activity of the immobilized invertase was shown as the amount of inverted sugar (micromoles) released per microgram of the immobilized invertase after one hour of incubation.
As shown in Table 3, the activity of the free invertase gradually decreased with increasing sucrose concentration. This indicates that the free enzyme was subject to substrate inhibition at the sucrose concentrations used. The activity of the immobilized invertase gradually increased until the sucrose concentration reached 0.1mol/l. Then, the activity rate rose rapidly when the sucrose concentration reached 0.2mol/l. This indicates that mass transfer resistance for the substrate to the enzyme active site was present inside the mesoporous silica layer and affected the effectiveness of the immobilized invertase. The mass transfer rate was lower at a low sucrose concentration and increased with increasing sucrose concentration.
ConclusionA continuous monolayered mesoporous silica shell with average thickness of ∼60nm was formed by deposition of silica nanoparticles generated at 80°C from highly basic sodium silicate solution on the surface of PDDA-functionalized silica core particles (having an average size of ∼400nm). The average pore size of the monolayered silica shell was ∼24nm, which allowed lipase immobilization inside mesoporous silica layer. The immobilization efficiency of lipase on the mesoporous silica layer was 80%. The activity of the immobilized lipase was lower than that of the free enzyme due to diffusion limitations and the blockage of the active site. Moreover, the activity of the heterogeneous biocatalyst decreased six times through the ten reaction cycles due to lipase leakage from the monolayered shell. To prevent lipase leakage from the support, an outer mesoporous silica layer was deposited at a lower temperature (40°C) on the surface of PDDA-functionalized silica core-shell particles containing the immobilized lipase. The deposition of the second layer was performed at a relatively lower temperature to prevent thermal inactivation of the immobilized enzyme. The average shell thickness and pore size of the outer silica layer was ∼60nm and 17nm, respectively. The reaction time necessary for deposition of outer and internal silica layers was 12 and 9min, respectively, indicating that an increase of reaction temperature increased the tendency for deposition of silica nanoparticles on the PDDA-functionalized silica surface. The activity of the lipase immobilized inside the bilayered shell was further reduced due to diffusion resistance within the outer silica layer and PDDA layer placed between two silica layers however, it was retained for the next reaction cycles.
When a similar procedure was used for the immobilization of invertase onto the monolayered mesoporous silica layer, the efficiency was 0%. The reason was the larger invertase size compared to actual pore size of mesoporous silica monolayer. Therefore, another procedure was developed for the immobilization of invertase in the mesoporous silica layer. It involved the dissolution of invertase in a dispersion of PDDA-functioalized silica core particles and silica nanoparticles generated at 40°C from sodium silicate solution. To allow completely enzyme immobilization, invertase was entrapped by in situ gelling silica system. The immobilized invertase showed lower activity than the free enzyme, but its activity was not hampered by the inhibition of the substrate at its higher concentrations due to the location of the enzyme inside the mesoporous silica layer, where the mass transfer resistance for the substrate to the enzyme active site was present.
Further research will focus on increasing the activity of the enzymes immobilized inside the mesoporous silica shell.
This work was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia under Project OI 172057 and Project III45021