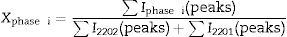In this work, we report on the effect of Y3+ doping on structural, mechanical and electrical properties of Bi-2202 phase. Samples of Bi2Sr1.9Ca0.1−xYxCu2O7+δ with x=0, 0.025, 0.05, 0.075 and 0.10 are elaborated in air by conventional solid state reaction and characterized by X-ray diffraction (XRD), scanning electronic microscopy (SEM) combined with EDS spectroscopy, density, Vickers microhardness and resistivity measurements. A good correlation between the variations of the bulk density and the Vickers microhardness with doping is obtained. The SEM photograph shows that the samples are composed of grains with a flat shape that characterizes the Bi-based cuprates. Quantitative EDS analysis confirms the reduction of Ca content and the increase of Y content when x is increased. The variation of resistivity with temperature shows that only samples with x=0, 0.025 and 0.05 present an onset transition to the superconducting state. The higher onset transition temperature is obtained for x=0.025 and is about 93.62K. The transition is wide and is realized in two steps confirming then the presence of the low Tc Bi-2201 phase in the samples. For x=0.075 and 0.10, a transition to a semiconducting state is seen at low temperatures. Some physical parameters are extracted from these curves and discussed.
En este trabajo se describe el efecto del dopado con Y+3 en la estructura y las propiedades mecánicas y eléctricas de la fase Bi-2202. Las muestras de Bi2Sr1.9Ca0.1-xYxCu2O7+δ, con x=0, 0,025, 0,05, 0,075 y 0,10, se prepararon en aire por reacción en estado sólido y fueron caracterizadas por medio de difracción de rayos X (XRD), microscopia electrónica de barrido (SEM) combinada con espectroscopia EDS, densidad, microdureza Vickers y resistividad eléctrica. Se ha obtenido una buena correlación entre las modificaciones en densidad y microdureza inducidas por el dopado. Las micrografías han mostrado que las muestras están formadas por granos laminares, lo que es típico en los superconductores basados en Bi. El análisis cuantitativo realizado con el EDS ha confirmado la disminución en el contenido de Ca y el aumento de Y, cuando se aumenta el valor de x. Las variaciones de la resistividad eléctrica en función de la temperatura mostraron que solo las muestras con x=0,025 y 0,05 Y presentaban una transición al estado superconductor. La mayor temperatura de transición se ha obtenido en las muestras con x=0,025, alcanzando 93,62K. La transición es ancha y se realiza en 2 pasos, lo que confirma la presencia de la fase de baja Tc, Bi-2201, en las muestras. Para x=0,075 y 0,10 se observa una transición a un estado semiconductor a bajas temperaturas. Se han extraído y discutido algunos parámetros físicos relevantes de esas curvas.
Intensification of synthesis and study of Bi-based superconductors are still of interest for researchers [1–3]. Superconductivity at 9–20K in ternary bismuth cuprates was first reported by Michel et a1. [4] for the BiO–SrO–CuO system. Addition of calcium oxide to this system led Meada et a1. [5] to discover bulk superconductivity in (Bi,Pb)2Sr2Can−1CunO2n+4+δ (n=1–3) and their critical temperatures are approximately 20K, 85K and 110K for the Bi-2201, Bi-2212, and Bi-2223 phases, respectively [4–6]. The only difference between these two consecutive phases is the addition of a double CaCuO block in the perovskite subunit resulting in an increase of the c-axis parameter. In addition, the Pb-doped Bi–Sr–Ca–Cu–O system has higher thermodynamic stability than the Pb-free one. This is because lead substitutes at Bi sites and reduces the incommensurate modulation [7]. Chemical doping in high Tc superconducting cuprates (HTSC) has generated a great interest because it represents an easily controlled, non-destructive and efficient tool for improving the mechanical, structural and superconducting properties of these compounds [8–10]. The doping element nature and its ionic radii, preparation method, sintering temperature, thermal processing time, synthesis atmosphere, precursor compositions and doping or substituting various cations and anions have an important influence on the properties of superconducting materials. In many HTSC families of compounds, the rare-earth elements play an important role in establishing the proper structure. The substitution of Ca2+ (divalent) by trivalent rare-earth elements in HTSC causes a drastic change in the carrier concentration and results in transition from superconductor to an insulator [11–13]. Many reasons have been suggested for the decrease in the carrier concentration such as structural modulations or change in oxygen stoichiometry change in the Cu valence or both [14]. The substitution by different elements of the rare earths in HTSC concerned usually at high doping levels.
For a better understanding the mechanism of the superconductivity and their unusual physical properties, the influence of small concentrations of doping atoms should be considered. In particular, we have studied the effect of doping by low content of yttrium at Ca and Sr sites on the structural and microstructural properties of Bi(Pb)-2212 superconducting ceramics [15], where the superconductivity properties are better when the doping is realized between the CuO2 planes at Ca site. In comparison with the Bi-2201, Bi-2212 and Bi-2223 compounds that have been extensively studied, only two papers were published on the Bi-2202 phase [4,16]. The influence of the annealing atmosphere on the superconductivity has been mostly studied [17–20], which revealed that the oxygen content and its distribution are the key points to the improvement of superconducting properties in HTSC. The Bi-2202 phase could show a superconducting behaviour only if it is doped by small amounts of Ca at the Sr [16]. Indeed, a sample of stoichiometry Bi2Sr1.9Ca0.1Cu2O7+δ elaborated in air shows a highest onset transition temperature (Tc,on) of about 90K. The oxygenation at high temperatures seems to have no effect on the value of Tc,on.
In this paper, we focused on the effect of doping with low content of yttrium (Y3+) on structural, microstructural, mechanical and electrical transport properties of the Bi2Sr1.9Ca0.1−xYxCu2O7+δ cuprate.
Experimental procedureChemical synthesisThe polycrystalline samples of Bi2Sr1.9Ca0.1−xYxCu2O7+δ cuprate ceramics with x=0, 0.025, 0.05, 0.075 and 0.10, each of about 2g of total weight, were prepared by solid state synthesis method, using high purity chemicals of Bi2O3, SrCO3, CaCO3, CuO and Y2O3 (Aldrich >99.9%). Stoichiometric amounts of the ingredients were accurately weighed using an electronic balance, thoroughly mixed and ground using an agate mortar. The mixture was then subjected to a stage calcination process in air at 800°C/24h. After calcination, the samples were formed into cylindrical pellets under the pressure 30kN at room temperature. The process of sintering comprised three steps. The first step of sintering was conducted at 820°C for 24h. Next, the samples were crumbled, wet milled and again formed into pallets. The second step of sintering was carried out at the temperature of 835°C for 24h, consecutively. The procedure of crushing and milling was repeated before the final sintering at 850°C for 24h.
MeasurementsPhase analysis of the samples was done using X-ray diffraction (XRD) on a Siemens D8-Advance powder diffractometer by use of Cu Kα radiation (λ=1.5418Å) with an angle step of 0.02. The diffraction data was taken at room temperature since there is no change in the lattice constant and the structure of high-Tc superconductors above and below the transition temperature [21]. Phase identification was performed using Janna2006 software, in support with ICDD-PDF 2 database. Microstructural examinations of the samples were done on a scanning electron microscopy (JEOLJSM-6390LV). Elemental analyses of the samples were done using energy dispersive X-ray analysis (EDS) attached to the SEM. The Vickers microhardness (VHN) is determined using a B3212001 Zwick microhardness tester with an applied force of 2N. The electrical transport properties of the samples from 2 to 300K were measured using the four probe method on a Cryodine CTI-Cryogenics closed cycle cryostat.
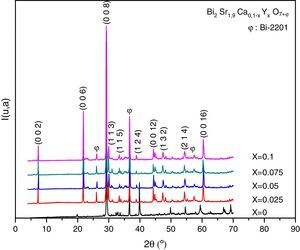
Results and discussionX-ray diffraction analysisThe X-ray diffractographs of the samples after final sintering are given in Fig. 1 where the indexed peaks are shown. In all samples, the major identified phase is Bi-2202. In addition to the lines of the main phase, some small peaks are also marked with φ symbol are observed. By analyzing the patterns using PCPDFWIN software, it has been found that these peaks correspond to that of Bi-2201 secondary phase [JCPDS-ICDD 46-0392 file], as referred in the database. This result was expected in accordance with previous report [16]. The absence of any Y2O3 containing phase proves that the solubility limit of yttrium in Bi-2202 system is above x=0.10. Orthorhombic lattice cell parameters were refined by the use of JANA2006 software [22] in the space group Bbmb[15]. The starting values of cell parameters are (Å) a=3.628Å, b=6.915Å and c=24.61Å [16]. The lines intensities are fitted by a pseudo-Voigt function. A 36 terms of Legendre polynoms is used to describe the background and the asymmetry correction is made by the Berar–Baldinozzi method. The obtained cell parameters as well as the agreement factors (Rp, Rwp) and the goodness of fit (GOF) are presented in Table 1. The obtained values are in good agreement with those reported in the literature [16].
Cell parameters (a, b, c, V), proportion of Bi-2202 phase (f2202), agreement factors (Rp, Rwp) and goodness of fit (GOF) of samples.
| x | a (Å) | b (Å) | c (Å) | V (Å3) | (Rp, Rwp) (%) | GOF | f2202 (%) |
|---|---|---|---|---|---|---|---|
| 0 | 3.6016(8) | 6.895(1) | 24.446(3) | 607.1(2) | (11.57, 17.54) | 2.83 | 75.46 |
| 0.025 | 3.6035(6) | 6.900(1) | 24.405(1) | 606.8(1) | (9.95, 14.54) | 2.81 | 81.08 |
| 0.05 | 3.606(2) | 6.901(4) | 24.358(4) | 606.3(3) | (11.96, 17.91) | 3.41 | 80.34 |
| 0.075 | 3.5664(8) | 6.901(2) | 24.352(6) | 599.3(4) | (11.37, 16.56) | 3.13 | 79.37 |
| 0.1 | 3.5619(9) | 6.905(1) | 24.347(2) | 598.8(1) | (11.84, 17.84) | 3.37 | 78.72 |
The c axis length decreases with the yttrium concentration from 24.446(3) to 24.347(2) Å. The increase in b-lattice parameter can be due to the extra electrons introduced by the doping ions. The extra electrons reduce the Cu valence and thus lead to an increase in CuO bond length [23]. Nevertheless, the a-parameter goes through a maximum for x=0.05 and then decreases with increasing x. This can be ascribed to the change in the planar CuO bond distance due to hole compensation in the system. For the cell volume V, it decreases gradually with x of yttrium. This decrease in V values can arise from the smaller ionic radius of the yttrium (1.04Å) compared to that of calcium (1.14Å) [24], and the substitution of higher valence cation Y3+ with an ion smaller than that of Ca2+. There can be however another explanation for the decrease in V. The rare earth ion at calcium site introduces excess oxygen between the Bi–O double layers [25]. The decrease of V in Bi2Sr1.9Ca0.1−xYxO7+δ indicates that the Y ions are readily incorporated into the structure.
The relative volume fractions of the Bi-2202 and Bi-2201 phases in all the samples were estimated from the intensities of the reflections using the following well-known formula [26]:
Phase i=2202 or 2201, where I2202 and I2201 are the XRD intensities of Bi-2202 and Bi-2201 phases, respectively. These calculations are believed to give reliable phase compositions of the samples. Sample with x=0.025 has higher volume fraction of the Bi-2202 phase (81.08%) than other samples. The samples with x=0 and 0.1 contained a smaller proportion of Bi-2202.
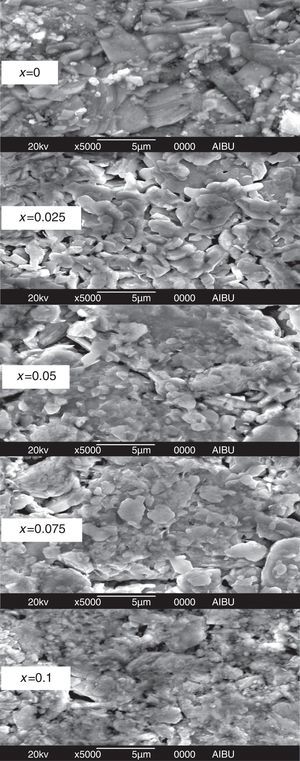
SEM and EDS resultsIn order to explore more detailed information on the effect of Y doping on the morphology characteristics in Bi2Sr1.9Ca0.1−xYxCu2O7+δ ceramics, scanning electron microscopy was employed for imaging in selected areas as well as for microanalysis. The SEM micrographs of the fractured samples after the last stage sintering process are shown in Fig. 2. At first glance, it is found that the grain morphology is not much affected by the doping with Y. All the samples contain long and flaky grains that characterize the BiO–SrO–CaO–CuO system [27–29]. The granular morphology of all samples is very similar, these micrographs reveal that the microstructure is relatively dense and also shows a minor difference in the porosity of the samples. This morphology is expected to be helpful for the enhancement of electrical properties. But with the increase in Y content, the analysis shows that the doped samples exhibit a reduced grain size than that of the undoped one, similarly to the result seen in Eu3+[30] and La3+[27] doped phase at Sr and Ca sites, respectively. The doping element may then act as a growth inhibitor that limits the grains size [28]. The results of the energy dispersive X-ray spectra (EDS) of the pure and Y3+ added samples are summarized in Table 2. Presence of yttrium is detected in all doped samples with a corresponding reduction in Ca content which confirms that the Y3+ atoms are successfully introduced into the samples.
Quantitative EDX results of cation stoichiometry of Y substituted Bi2Sr1.9Ca0.1−xYxCu2O7+δ (x=0, 0.25, 0.05, 0.075 and 0.1).
| Atoms | EDS analyzed composition (wt.%) | ||||
|---|---|---|---|---|---|
| x=0 | x=0.025 | x=0.05 | x=0.075 | x=0.1 | |
| Bi | 36.182 | 43.605 | 39.001 | 39.772 | 40.995 |
| Sr | 32.230 | 30.660 | 33.058 | 30.030 | 33.596 |
| Ca | 0.911 | 0.600 | 0.570 | 0.416 | – |
| Y | – | 0.755 | 0.964 | 1.532 | 1.817 |
| Cu | 15.473 | 9.372 | 9.494 | 13.229 | 8.234 |
| O | 15.205 | 15.009 | 16.913 | 15.021 | 15.357 |
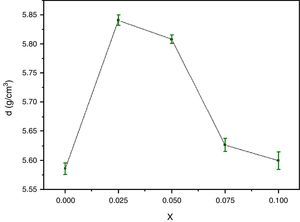
For successful applications of HTSC ceramics in technology, the study of their micromechanical properties is great interesting. The bulk density and microhardness (VHN) are of particular interest because of the possibility of formation of gross fractures or microcracks [31,32] during processing. Fig. 3 shows the density variations of the pellets after elaboration as a function of Y content. The density of the samples is improved by Y doping for x=0.025 and then decreases. The highest values correspond to approximately 85.64% of the theoretical density (6.82g/cm3) for x=0.025. This trend was ascribed to the substitution of yttrium up to 0.025 in Bi-2202 phase can substantially suppress microcracking and improve the ductility of samples. It is also found that Y substitution improves the connection between superconducting grains of the Y-doped Bi-2212 system, and consequently the mechanical and superconducting properties are enhanced [33]. However, the opposite behaviour is reported for Cd substituted at Ca sites in the Bi-2223 system [34].
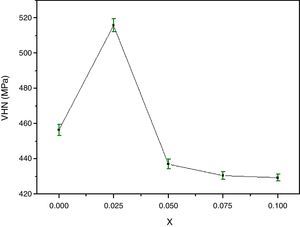
The variation of the bulk density with Y content correlates well with that of the Vickers microhardness (VHN) as it can be seen. In contrast, with enrichment of Y, the microhardness was markedly reduced (Fig. 4). This result could be associated to the presence of secondary phases such as Bi-2201, Ca2CuO3 and CuO and defects at boundaries between regions, grains as well as colonies [35]. However, we showed a similar behaviour in Bi(Pb)-2212 phase with yttrium doping prepared by solid state reaction [15]. The behavior of density and microhardness may be associated to the improvement of the coupling characteristics and mechanical connection between the superconducting grains. Furthermore, the higher VHN values represent the hardness of monocrystalline grains, while the lower microhardness values usually resulted in crack patterns and crack propagation along grain boundaries. We can concluded that the measured VHN and bulk density depend more strongly on the low contents of Y and their maximum value obtained at optimum content of 0.025.
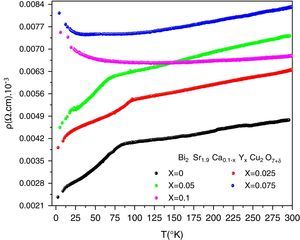
DC electrical resistivity measurementsThe electrical resistivity was measured using the standard four probe technique. Its variation as a function of temperature and doping is shown in Fig. 5. As it can be seen, the samples with x=0, 0.025 and 0.05 show metal like behaviour in the normal state electrical resistivity (dρ/dT>0) and have a wide transition with secondary superconducting phase transition around T=10K. The sample for x=0.075 displays a metallic characteristics and changes to an insulator behaviour (dρ/dT<0) below 70K. This indicates that the metal to insulator transition takes place when 0.05<x≤0.075. The sample with x=0.10 shows a semiconducting behaviour above 87K. Our result agrees well with previous observation [16]. On the other hand, Michel et al. [4] and Campa et al. [16] found that the Bi-2202 phase can show superconducting behaviour when the samples are treated under different atmospheres by quenching the samples.
The Onset critical transition temperature (Tc,on), slope (dρ/dT) of resistivity and residual resistivity (ρ0) of samples are given in Table 3. The Tc,on is taken equal to the temperature of deviation from metallic behaviour in the samples with x=0, 0.025 and 0.05. When the superconductor is cooled below its Tc,on, the electrical resistivity drops abruptly to zero, the external magnetic field is ejected. The Tc,off generally gives information about weak links of the superconductor grains and grain boundaries, while Tc,on can reveal the superconducting phases occurred in the grain structure. We note that Tc,on increases at first, reaching maximum value of 93.62K for x=0.025, and then decrease to 68.97K for x=0.05. Induction of mobile carriers [36], overall oxygen content and the disorder in the CuO2 planes [37,38] are possible mechanisms responsible for a variation of Tc,on. The yttrium doping with valence 3+ may increase the incorporation of oxygen in the structure, will reduce the Cu valence and consequently a degradation the Tc,on. The normal state resistivity of the granular superconducting sample is affiliated to the defect interfaces such as grain boundaries, stacking faults, voids, planar and micro defects of the materials. The metallic normal state resistivity curves can be expressed by a linear equation ρ=ρ0+a.T. The first term ρ0 is called residual resistivity. It is obtained by extrapolating the normal state resistivity to T=0K and is related to chemical impurity scattering, lattice defects and hole concentration [39]. It is clearly shown in Table 3 that the ρ0 increases gradually with the nominal yttrium content. As is shown in Fig. 5, the undoped sample is then more homogeneous among the others. In the second term of equation, the slope a=dρ/dT of resistivity may be considered as a parameter that depends on the intrinsic electronic interactions [39]. The variation of this slope with Y content correlates well with that of the highest Tc,on. The maximum of dρ/dT is also seen for x=0.025.
ConclusionIn the present work, the effect of yttrium doping on the phase formation, microstructure, mechanical and electrical properties of Bi-2202 ceramics was presented. It can be concluded that a good correlation between the variations of the volume fraction of the Bi-2202 phase, Vickers microhardness, bulk density, onset critical transition temperature and slope of resistivity are revealed. These parameters are optimized for a concentration of Y equal to 0.025. Moreover, the electrical behaviour of samples depends more effectively on the yttrium concentration.
The authors express their thanks to Dr. Andrés Sotelo Mieg from the University of Zaragoza, Spain, for helping to translate the abstract in Spanish.