Introducción: El objetivo de este trabajo fue determinar la prevalencia de disfunción diastólica subclínica del ventrículo izquierdo (DDVI) y su asociación con el descontrol metabólico en adolescentes con diabetes tipo 1.
Métodos: Se trató de un estudio en 53 adolescentes con diabetes tipo 1 en dos fases: primero, un estudio transversal descriptivo y, después de realizar un ecocardiograma, un transversal comparativo. Se consideró DDVI cuando tuvieron tres o más datos ecocardiográficos alterados: velocidad de contracción auricular (relación E/A), tiempo de desaceleración (TD), tiempo de relajación volumétrico (TRIVI) y función sistólica mayor de 50%. Además, se les determinaron los niveles de glucosa, de hemoglobina glucosilada y microalbuminuria.
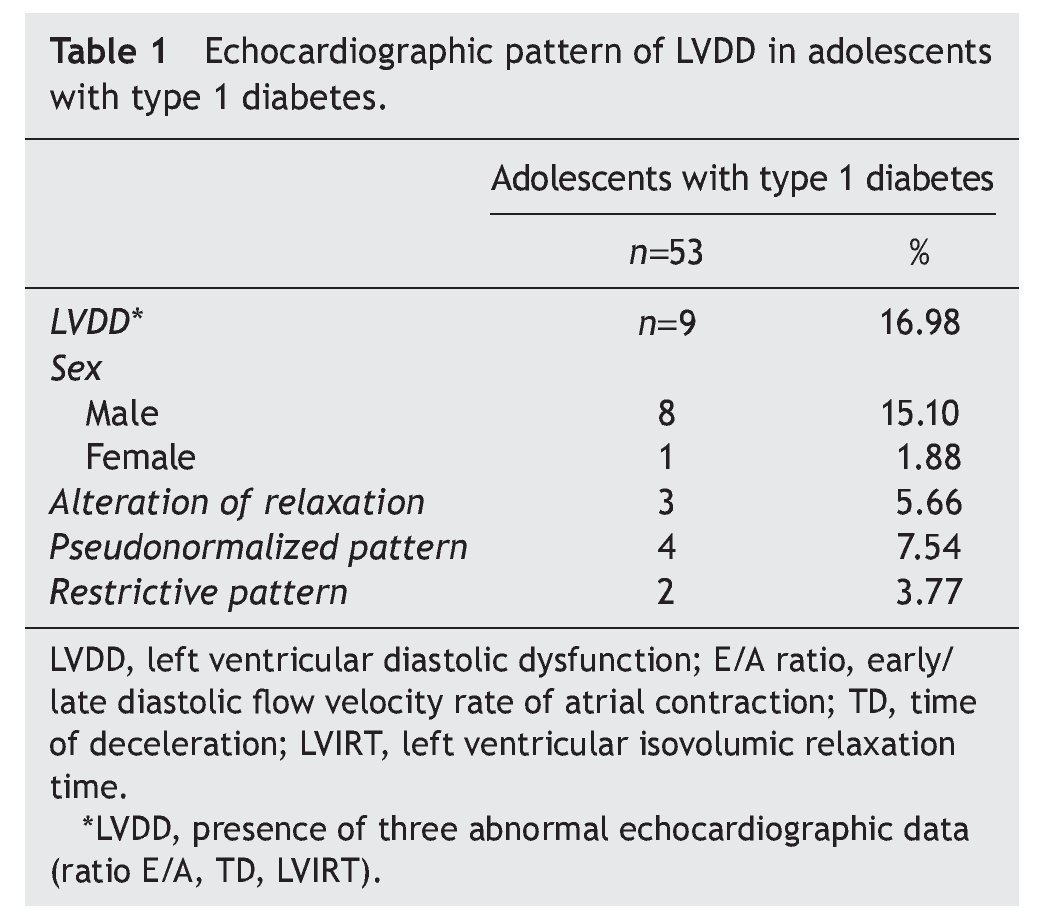
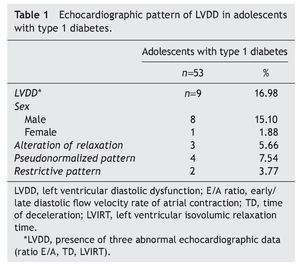
Resultados: El 16.98% de los adolescentes diabéticos mostraron datos ecocardiográficos de DDVI, y el 15.10% correspondió al sexo masculino. El patrón pseudonormalizado se observó en 7.54%, en relación con el 5.66% del patrón de alteración de la relajación y del 3.77% del restrictivo. Estos pacientes, además, mostraron mayor tiempo de la enfermedad, obesidad y un aumento en la glucemia, en la hemoglobina glucosilada y de la microalbuminuria.
Conclusiones: La DDVI es una complicación frecuente en los adolescentes con diabetes tipo 1. Aquellos con DDVI presentaron con mayor frecuencia obesidad, mayor tiempo de evolución de la enfermedad y un peor control metabólico. Se propone que en estos pacientes se realice un diagnóstico oportuno y sistemático a través de un ecocardiograma.
Background: To determine the prevalence of subclinical left ventricular diastolic dysfunction (LVDD) and its association with metabolic control in adolescents with type 1diabetes.
Methods: We carried out a study in 53 adolescents with type 1 diabetes in two phases: cross-sectional and after performing two-dimensional M-mode echocardiogram and color Doppler, a cross-sectional comparison. Subjects were divided into two groups: the first without LVDD and the second with LVDD. LVDD was considered when there were three or more alterations according to echocardiographic data (rate of atrial contraction, time of deceleration, time of volumetric relaxation) accompanied by systolic function>50%. We also determined glucose, hemoglobin, glycosylate, and microalbuminuria.
Results: Of the adolescents with diabetes, 16.98% showed echocardiographic data of LVDD; 15.10% were male. Pseudonormalized pattern was observed in 7.54% compared to 5.66% with impaired relaxation pattern and 3.77% with restrictive pattern. Furthermore, there was a longer time of disease evolution, obesity and a significant increase of glycemia, glycosylated hemoglobin and microalbuminuria.
Conclusions: LVDD is a frequent complication in adolescents with type 1 diabetes. Those with LVDD had a higher prevalence of obesity, longer time of disease, and poorer metabolic control. Therefore, we propose that a timely and systematic search with echocardiogram is important in patients with type 1 diabetes.
1. Introduction
Cardiovascular disease (CVD) is one of the principal causes of morbidity and mortality in patients with type 1 and type 2 diabetes. The most frequent complication is coronary artery disease (CAD) and it represents>50% of deaths in patients with type 2 diabetes and almost 25% in all type 1 diabetics.1 It is known that the greater the metabolic disorder, the greater the incidence of cardiovascular complications.2-6 In the Framingham study, it was reported that the presence of diabetes increases 2.5-5 times more the risk of developing cardiac insufficiency.7-9 In addition, it has been noted that patients who have cardiac insufficiency and who develop diabetes have a poor prognosis for function and life.
Currently, myocardial lesions in diabetic patients not associated with coronary atherosclerosis or arterial hypertension are described as diabetic cardiomyopathy (DCM) where there are changes in the microcirculation, metabolic disorders and, in late phase, autonomic neuropathy. This leads to the deposit of collagen fibers in the cardiac interstitium, hypertrophy of the cardiomyocytes and lipid deposits.10-12 The result of all of these changes is reflected in myocardial functioning that begins with a subclinical left ventricle diastolic dysfunction (LVDD) and in late phases presents systolic dysfunction of the same ventricle.13-15
LVDD in diabetic adolescents without prior cardiovascular disease could be considered as an initial marker of DCM. The method most used for its diagnosis is echocardiography because of its greater diffusion, high sensitivity and lack of complications.16-19 Echocardiography evaluates the filling flow of the mitral valve and pulmonary veins and the times and velocity of mitral and tricuspid rings and relaxation. At present, it allows the study of regional diastolic function of the basal and mid-segments of the left ventricle (LV). These echocardiographic methods have enabled diverse disorders of diastolic function to be demonstrated, ranging from a mild deterioration of myocardial relaxation to restrictive changes of ventricular filling.16,19 Therefore, LVDD is considered when there is a relaxation disorder or ventricular distention or both, with an increase of left ventricular filling pressures and pulmonary capillary pressure accompanied by a systolic function >50%.16-19 Systolic function in turn depends on the mechanical function of the LV, of the ventricular-arterial coupling and of the volemia. Shortening of the longitudinal and circumferential fibers cause a greater thickening of the ventricular wall with an increase in the expulsion of the systolic volume (SV); 14% of the shortening of the myocardial fibers produce ∼40% ventricular wall thickening.20 Currently it is known that various indices related with the shortening (longitudinal global strain, torsion and twist) could be altered in the presence of a normal ejection fraction (LVEF).20-23 In Mexico, the frequency of LVDD is not known in adolescents with type 1 diabetes, although in other countries it has been reported that it varies between 18 and 69%.20-23 Therefore, the objective of this present study was to evaluate the prevalence of LVDD by echocardiography and its association with the metabolic derangement in adolescents with type 1 diabetes without cardiovascular complications.
2. Methods
This study was approved by the Ethics and Research Committees of the Cardiology Hospital, CMNSXXI, IMSS (registry R-2011-3604-1) and was carried out according to the Helsinki Declaration. All patients parents provided written informed consent and all adolescents signed a consent letter. A study on 53 adolescents of either gender with type 1 diabetes mellitus was carried out according to the criteria of the American Association of Diabetes.24 The study was done in two phases: descriptive cross-sectional to determine the prevalence of LVDD and comparative cross-sectional to evaluate the association between LVDD and metabolic derangement.
Patients were selected from the Endocrinology Service of the Pediatric Hospital, Centro Medico Nacional Siglo XXI, Instituto Mexicano del Seguro Social. They were divided into two groups after having M-mode, bidimensional color Doppler echocardiography study: the first group, which did not provide echocardiographic data of LVDD. The second group had data of LVDD, but did not have cardiovascular complications or any other added pathology and on the echocardiogram had normal systolic function (inclusion criteria). Diabetic adolescents with congenital heart disease, arterial hypertension, arteriosclerosis, acute or chronic infection, electrolyte imbalance, liver disease, using medications such as antihistamines, antithrombotics or antihypertensives were not considered for the study (exclusion criteria).
All adolescents received a normal diet of 55 kcal/kg/day (15% proteins, 55% carbohydrates, 30% lipids). In addition, they were administered a combination of rapid- and intermediate-acting insulin at a ratio of 1-1.5 U/kg/day. Between 07.00 and 08.00 h and 12 h after the last meal, 3 mL of blood was collected by venipuncture to determine glucose levels, glycosylated hemoglobin, cholesterol, TG, HDL, LDL and microalbuminuria. Bidimensional M-mode transthoracic echocardiogram and color Doppler were also done to evaluate LVDD.
2.1. Biochemical trials
Serum glucose was determined by the glucose oxidase method (normal values 60-100 mg/dL),25 glycosylated hemoglobin by cation-exchange chromatography (normal values 4.8-5.9%),26 and microalbuminuria (normal value <300 mg/24 h) by the method of Doumas et al.27 Cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were quantified with a Kit Flex reagents cartridge (Dade Behring Inc., Newark, DE) that uses a colorimetric enzymatic method.
2.2. Echocardiography study
M-mode bidimensional Doppler study was carried out with an echocardiography device (Philips model iE33) with a transducer of 3.5 MHz. All studies were done and analyzed by the same echocardiology technician. Measurement of the telediastolic and telesystolic diameters of the LV and measurement of the left ventricular ejection fraction (LVEF) were obtained according to the criteria of the American Society of Echocardiography.28 Ventricular mass was determined with the Devereaux formula. Ventricular hyper trophy was defined as the mass index of the LV >110 g/m2 in males and >100 g/m2 in females. Recording of the left ventricular filling flow was obtained through the apical window by the methods described by the Echocardiography Association of the Interamerican Cardiology Society.26 The following measurements were obtained: early diastolic flow velocity (peak E), late diastolic peak flow velocity (peak A), its index (E/A), time of wave E deceleration (TD) and left ventricular isovolumic relaxation time (LVIRT). Diastolic dysfunction was determined in relation with the presence of three patterns of ventricular filling: type 1 (changes in the relaxation with the presence of an E/A ratio ≤1, TD ≥250 msec and TRIVI ≥150 msec); type 2 (pseudonormalized pattern with an E/A ratio of 1:2, TD from 150-250 msec and LVIRT <150 msec). This pattern was confirmed with the presence of a color propagation velocity (Vp) <50 msec or a difference between the time of the duration of the Ar and the duration of the mitral A wave (Ar-A). With increases in left ventricular end diastolic pressure (LVEDP), the Ar velocity increases >35 cm/sec and the duration of the Ar are increased. The restrictive type 3 pattern, with an E/A ratio of >2, TD <150 msec and shortened LVIRT with a velocity of the reverse A wave >35 cm/sec and duration Am
2.3. Statistical analysis
Descriptive statistics were used for the demographic variables: percentages, means and standard deviations. For the echocardiographic parameters, descriptive statistics were also carried out. Differences between the average values were analyzed by Student t test and Levene coefficient of variation; p<0.05 was accepted as statistically significant.
3. Results
The most interesting aspect of the present study was to determine the prevalence of LVDD using echocardiogram in adolescents with type 1 diabetes. Of the 53 patients included in the present study, 16.98% had echocardiographic evidence compatible with LVDD, 15.10% were males in relation with 1.88% females. The pseudonormalized filling pattern predominated in 7.54% of the cases compared with the alteration relaxation pattern (5.66%) and with the restrictive pattern (3.7%) (Table 1).
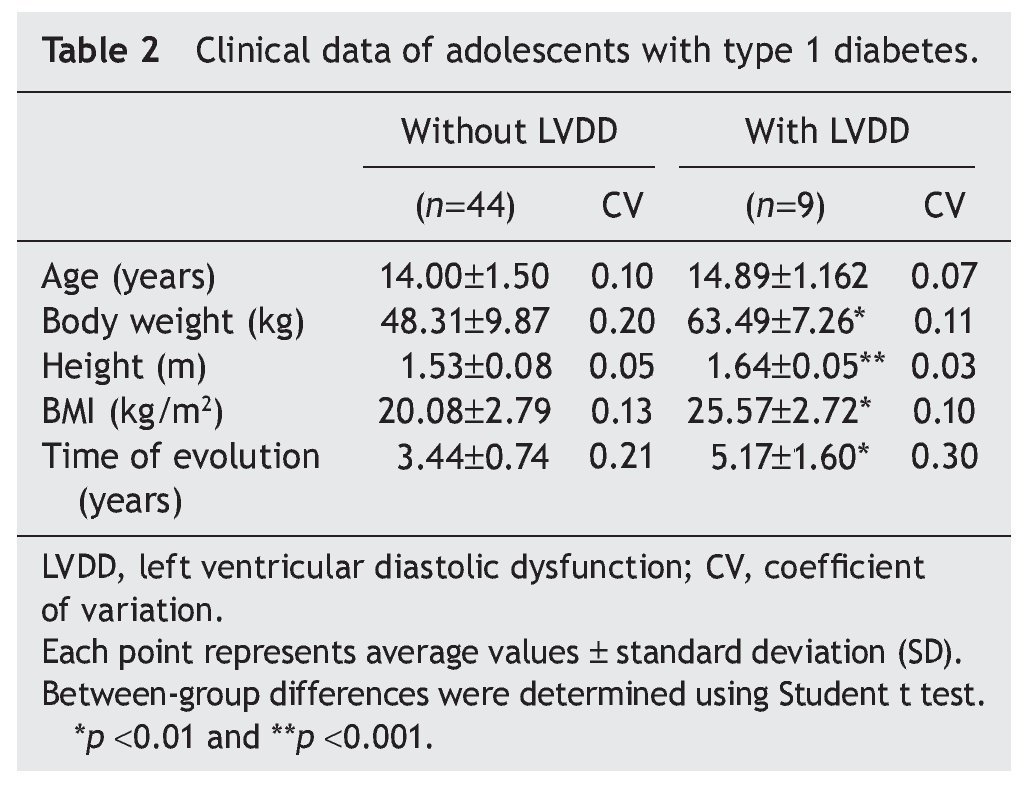
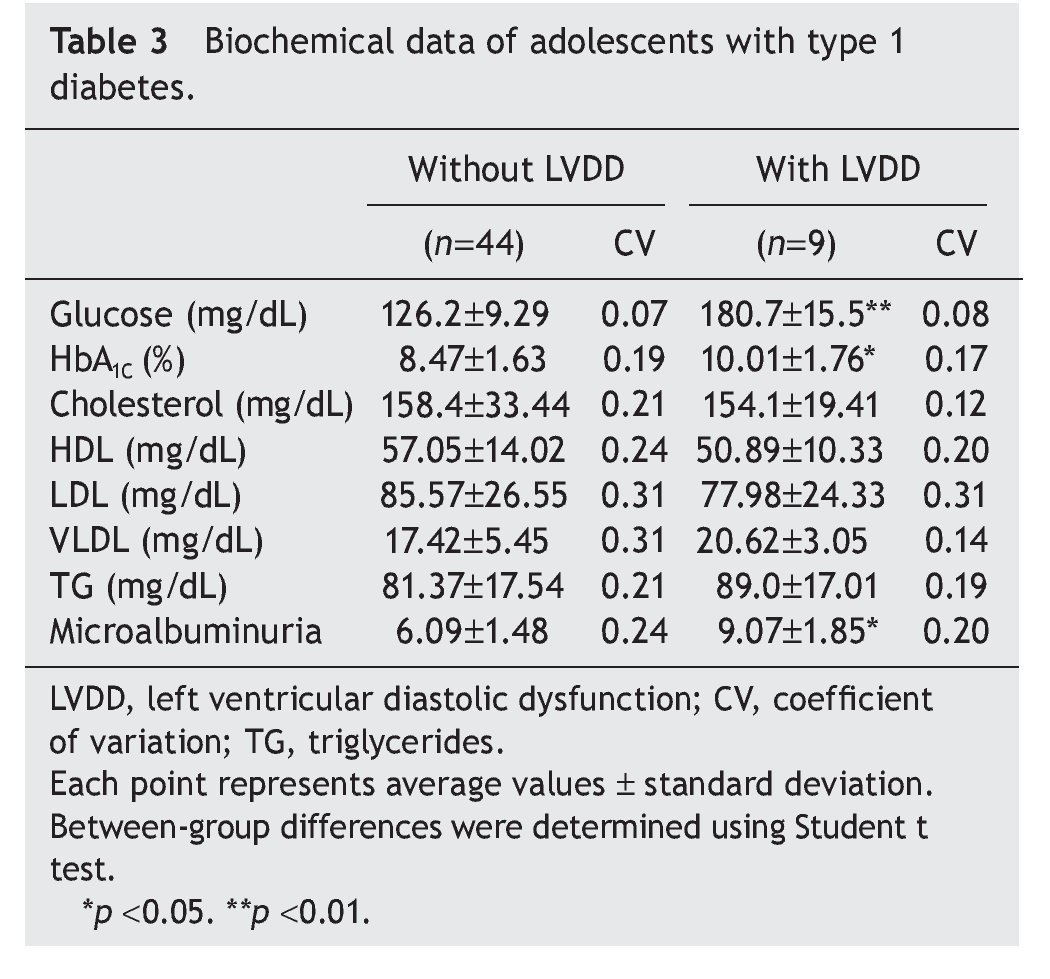
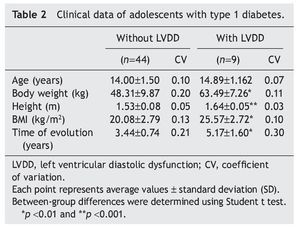
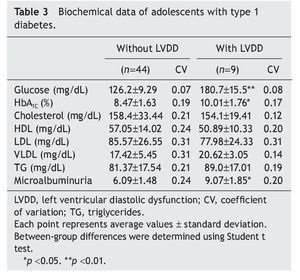
Interestingly, patients with type 1 diabetes who had echocardiographic data compatible with LVDD had a longer time of disease evolution (5.17± 1.60 years vs. 3.44± 0.74 years). They also had higher body weight, height and BMI compared with diabetic adolescents who did not have echocardiographic evidence of LVDD (Table 2). On the other hand, adolescents with type 1 diabetes who had LVDD demonstrated a greater metabolic derangement with significant elevation in glycemic levels (126.2± 9.29 vs. 180.7 ± 15.5), glycosylated hemoglobin (8.47± 1.63 vs. 10.01± 1.76) and microalbuminuria (6.09± 1.48 vs. 9.07 ± 1.85) in relation with the diabetic adolescents who did not have LVDD (p<0.05) (Table 3).
4. Discussion
Diabetes mellitus induces two types of complications in the heart, coronary atherosclerosis32 and DCM.33 LVDD is believed to be an early phase of DCM10,34 seen in diabetic patients without any cardiovascular symptomatology. The frequency varies between 18 and 69%. This wide difference is possibly due to the different ways of selecting the patients, to the examination techniques and the different definitions of LVDD.20,21 It was noted in this study that 16.98% of adolescents with type 1 diabetes had echocardiographic data compatible with LVDD. On the other hand, different studies carried out in diabetic patients agree that the greater prevalence of LVDD is increased with poor metabolic control of the disease and with patient obesity.35-37 It was also observed in this study that diabetic adolescents had these risk factors: obesity and poor metabolic control.
Diagnosis of LVDD is based on echocardiographic findings because this method fundamentally evaluates the transmitral flow with a Doppler and the measurements of ventricular filling velocity, time of deceleration, isovolumic relaxation time and evaluation of the flow patterns. As the diastolic myocardial function worsens, early diastolic filling (E wave) is reduced and the pattern shows a delay in myocardial relaxation. However, when the pressure of the left atrium increases, the E wave returns to normality with a mitral flow pattern indistinguishable from the normal, for which it is called pseudonormalized pattern.16-19 As LVDD progresses, a pattern of restrictive filling develops, which reflects the elevated pressure of the left atrium and tends to be accompanied by symptoms of cardiac insufficiency. Despite the usefulness of these measures, there is a limitation that lies on the dependence of these techniques during the preload. This implies that the same patient could show a change in the mitral flow pattern as the left atrial pressure increases or decreases. Therefore, evaluation of the flow pattern of the pulmonary veins could limit these variations and allow for the differentiation between normal and pseudonormal patterns.28-30 In the group of diabetic patients who had echocardiographic evidence compatible with LVDD, the type 2 pattern (or pseudonormalized pattern) predominated in 7.5% of the total patients. Pattern 1, alteration of relaxation, and pattern 3 were seen in 5.66 and 3.77%, respectively. These findings are interesting because they are frequently associated with a greater time of evolution of the diabetic picture and with a metabolic derangement of the disease,36-38 aspects that were present in adolescents with type 1 diabetes and LVDD studied in this work.
There is also a general consensus that chronic hyper-glycemia is one of the risk factors often involved in the pathophysiology of LVDD. During the diabetic state, nonenzymatic glycosylation of proteins occurs in the myocardiocytes, increased oxidative stress, activation of protein C kinase and deposit of collagen fibers in the myocardial interstitium.10-12,39 Therefore, the relationship obtained by glycemic control with echocardiographic indices of LVDD supports the hypothesis that hyperglycemia induces DCM.10,34,38 In this context, clinical and biochemical results observed in diabetic patients with LVDD confirmed the fact that poor metabolic control, coupled with obesity and the hyperalbuminuria, are determinant parameters of LVDD.
In conclusion, LVDD is a frequent complication (16.98%) in adolescents with T1D, those with LVDD who presented obesity with greater frequency, greater time of disease progression and who demonstrated poor metabolic control. Therefore, we propose to carry out a timely and systematic search for this complication through an echocardiographic study in all adolescent patients with T1DM.
Conflict of interest
The authors declare no conflict of interest of any nature.
Acknowledgments
This study was carried out with funding provided by the Instituto Mexicano del Seguro Social (grant #FIS/IMSS/PROT/ G11-2/102).
Received 22 October 2013;
accepted 11 April 2014
* Corresponding author.
E-mail:willisga@prodigy.net.mx, gmanjarrezg@gmail.com (G. Manjarrez-Gutiérrez).