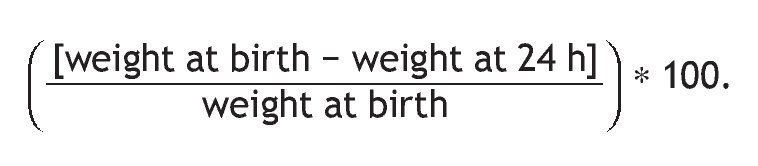Introducción: La indicación del aporte por fórmula al recién nacido puede ser a libre demanda (LD) o por capacidad gástrica calculada (CGC). El objetivo de este trabajo fue determinar si la técnica de alimentación (LD vs. CGC) influye en el volumen ingerido, la tolerancia a la fórmula y el riesgo de hipoglucemia.
Métodos: Se diseñó un ensayo clínico abierto no aleatorizado en neonatos a término, sanos, vigilados durante 24 h. Se determinó el volumen total ingerido (ml/kg), la tolerancia oral (vómitos-regurgitaciones, perímetro abdominal), la repercusión en el peso y datos de hipoglucemia.
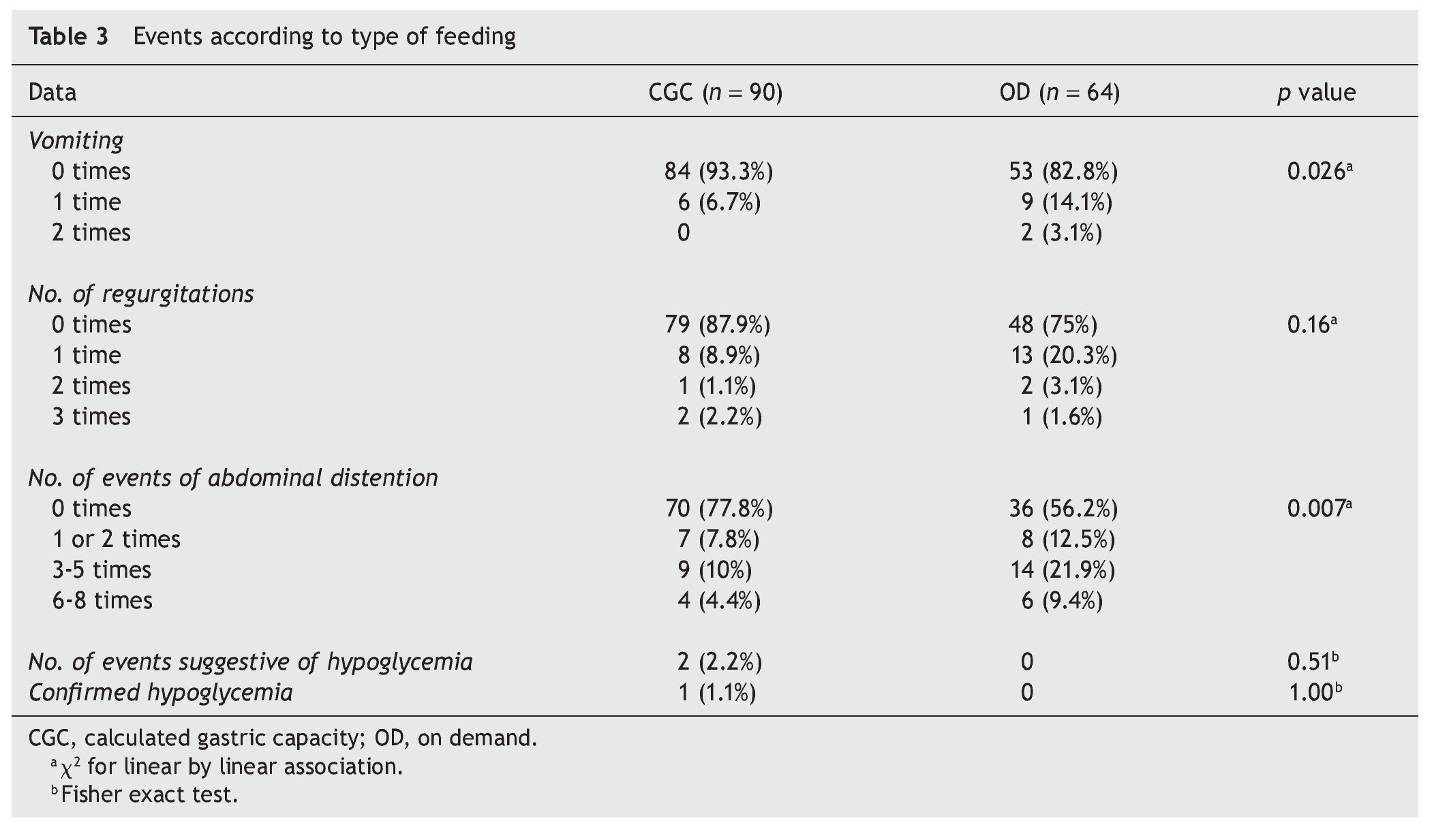
Resultados: Se analizaron en total 154 neonatos (CGC = 90 y LD = 64). Los neonatos en LD consumieron una mayor cantidad de fórmula (8 ml/kg; IC 95% 5-11) con mayor variación entre toma. No hubo diferencias en el porcentaje de pérdida ponderal (2.1% vs. 2%, p = 0.80). Los neonatos en LD mostraron más vómito (17.2% vs. 6.7%, p = 0.02) y mayor distención abdominal (43.8% vs. 22.2%, p = 0.007). Solo un neonato del grupo CGC mostró hipoglucemia (p = 1).
Conclusiones: La alimentación por CGC permite una ingesta constante con menor riesgo de intolerancia, sin aumentar la posibilidad de hipoglucemia o pérdida de peso.
Background: Newborn formula feeding can be given ad libitum (AL) or as calculated gastric capacity (CGC). The objective of the study was to determine if the technique used to offer the newborn formula (AL vs. CGC) modifies volume intake, tolerance and risk for hypoglycemia.
Methods: The study design was an open, nonrandomized clinical trial. Patients were healthy term newborns. All newborns were followed for 24 h. We determined the total volume ingested (ml/kg), oral tolerance (vomiting, regurgitation, abdominal circumference), impact on weight and hypoglycemic events.
Results: One hundred fifty four infants were included (CGC = 90 and AL = 64). The AL group consumed slightly more formula (8 ml/kg, 95% CI 5-11) with greater variation between intakes. There was no difference in the percentage of weight loss (2.1% vs. 2%, P = 0.78). AL group also showed more vomiting (17.2% vs. 6.7%, P = 0.02) and increased abdominal distension (43.8% vs. 22.2%, P = 0.007). Only one newborn in the CGC group had hypoglycemia (P = 1.00).
Conclusions: CGC feedings allows constant intake with less risk for intolerance without increasing the possibility of hypoglycemia or weight loss.
1. Introduction
The recommendation with greatest scientific support for nutrition of the healthy newborn is exclusively breast milk, started early and “on demand (OD).”1,2 However, up to 45% of newborns require supplemental feeding, even in the best hospitals that support breastfeeding, due to the mother’s fatigue and the time of birth, among other reasons.3 This is without taking into consideration the absolute contraindications due to maternal illness that make a separation necessary or because of an obstacle due to drug ingestion or radioactive material.1
When supplementary feeding is necessary, it should provide the necessary nutrients for the metabolic requirements of the newborn.4,5 Also, the contribution must conform to the physiological changes during the transition phase. For this reason, different authors recommend a diet with controlled amounts.6,7 Unlike being fed from the mother’s breast, being bottle fed does not seem to have self-regulation of the amount consumed.8 National guidelines stipulate that the volume should have as a baseline an amount of 70-80 ml/kg of weight at birth and continue with an increase of 10 ml daily up to the seventh day of life, always evaluating how the newborn is adapting.9 However, in clinical practice there are two thoughts: one recommends to provide the newborn with supplemental nutrition (maternalized formula or initial infant formula) OD, emulating the behavior of the mother’s breast. The other recommends to provide amounts of feedings based on a calculation of the gastric capacity (CGC) expected for the newborn’s body weight [(weight in grams −3)/10].10
Although both behaviors appear to be adequate, there is concern about their consequences. For OD nutrition, although one would wait until the newborn self-regulates his intake, the volumes ingested may surpass his gastric capacity and, with it, present regurgitations or vomiting11 as well as postprandial abdominal distention.
In contrast, an inadequate intake can be associated with the risk of hypoglycemia, a phenomenon already observed in newborns exclusively fed breast milk in the first hours of feeding.12 On the other hand, nutrition restricted in volume may not provide sufficient nutrients and thereby increase early weight loss.13 Currently, the existence of a study that determines if there are differences with clinical repercussions between both recommendations and which is the better option is not known. The hypothesis of this study was that CGC feeding maintains a better relationship with the physiological conditions and adaptation of the digestive apparatus as it allows for greater oral tolerance, carries the necessary nutritional requirements and does not favor events of transitory hypoglycemia or greater weight loss.
2. Methods
A prospective cohort study was carried out in Nuevo Sanatorio Durango in Mexico City for a period of 8 months (from November 2012 - June 2013). Two cohorts were studied: newborns who had maternalized formula indicated at 13% at OD and newborns with indication for CGC feeds. Term newborns included those of 37-41 weeks of gestation by Capurro method, with adequate weight (percentiles 10-90, national graphs),8 of either gender, with an Apgar score of 9 or 10 at 5 min, vaginal birth or cesarean, and without major malformations. Newborns at high risk due to maternal gestational history were excluded.
The decision to begin maternalized formula feeding as well as the technique to offer it (OD or CGC) was made by the treating physician (nonrandom allocation). The medical justification was by separation of the binomial, maternal medication, low milk production, or in common agreement due to fatigue of the mother. In all cases the parents were informed and discussion was held about the use of the milk formula, indication, risks and benefits.
OD was defined when, at the time of the feeding, the newborn was offered the formula volume until he stopped sucking. For indication for CGC, the newborn was only allowed to consume the volume calculated for his weight [(weight in grams −3)/10].
Independently of the indication for the feeding technique, all received the same commercial formula, which was begun after the first 3 h of life. The feedings were provided every 3 h, with exceptions being made for medical reasons, i.e., to give a larger quantity of the calculated formula. The feedings were done by health personnel trained in the technique of bottle feeding (nursery).
None of the newborns was given any other type of fluids. In all cases, when there was no absolute contraindication, the mother was allowed to attempt to feed the infant. Before each feeding, the supraumbilical abdominal perimeter was measured with a metric tape. When it increased >2 cm with respect to a previous perimeter, it was considered to be significant abdominal distention. For each feeding, the volume ingested was recorded, measured as the difference between the volume before and after bottle feeding. During the next 3 h the infant was monitored for the presence of regurgitation (formula coming out of the mouth without the presence of nausea or arching) or vomiting (exit with previous arching). Glucose levels were also monitored to determine hypoglycemia, with support from the “Current Official Standard for the Diagnosis and Treatment of Neonatal Hypoglycemia.”14 Using this as a basis, the capillary glucose was measured. When a capillary glucose concentration <45 mg/dl was determined, it was confirmed by venous puncture and the treatment indicated in the standard was continued.
Monitoring was done only during the first 24 h of life due to the high rate of discharges from this point in time. All newborns were weighed on two occasions, the first within the first hour of birth and the final at 24 h of birth. Weight was determined by placing the newborn naked on a digital scale (Seca Model 231, accuracy 5 g) with an automatic calculation of the average. The 24-h measurement was done just when this time was met and prior to being fed. Weight loss was calculated with the formula:
Staff members who conducted the surveillance were unaware of the project or its objectives.
Adequate consumption was defined as when the newborn ingested volumes of formula as indicated in a calculated range for gastric capacity with a difference of not more than 10% above or below it (e.g., for a calculated volume of 30 ml the range was 27-33 ml). When the consumption was less than this range it was considered to be low or, when greater, as high.
Size of the sample. Sample size was calculated assuming an average weight of 3 kg, with which the group being fed according to gastric capacity would consume an average of 216 ml/day (27 ml per feeding) with an SD ±20 ml. A median difference in consumption was calculated of at least 10 ml. Statistical power of 80% and probability of type I error <5% (n = 63 patients per group) was calculated.
2.1. Statistical analysis
Patients were analyzed according to the indication of feeding (independent variable). The dependent variable was considered the consumption of formula per day. The difference in weight at 24 h, the presence of vomiting, regurgitation, events of abdominal distention and hypoglycemic events were considered as secondary variables.
For qualitative variables a simple and relative difference in percentage was obtained. The comparison between the curves was done with x2 test. Continuous variables were summarized as mean and standard deviation when they showed normal behavior and were contrasted with the parametric Student t test for independent data. Non-normally distributed variables were summarized with median and interquartile range. For analysis, Mann-Whitney U test for between-group feedings ingested per day, distribution graphs in bars and whiskers were done (median and 1 and 3 quartile ends). Given its low frequency for the events searched, Fisher exact test was used. For categorical variables of frequency, χ2 test for linear trends was used.
To control for potential confounding factors (birth weight, birth route and gender) in relation to the indication of offering the formula, multiple linear regression analysis was used. All analyses were performed with the SPSS statistical package v.20. Statistical significance with a probability of error a <5% (p <0.05) was considered.
The study was approved by the Research and Ethics Committee of the hospital. Because medical indications were approved by the parents, verbal consent was requested regarding the measurements and parents were informed about the confidentiality of the data collected.
3. Results
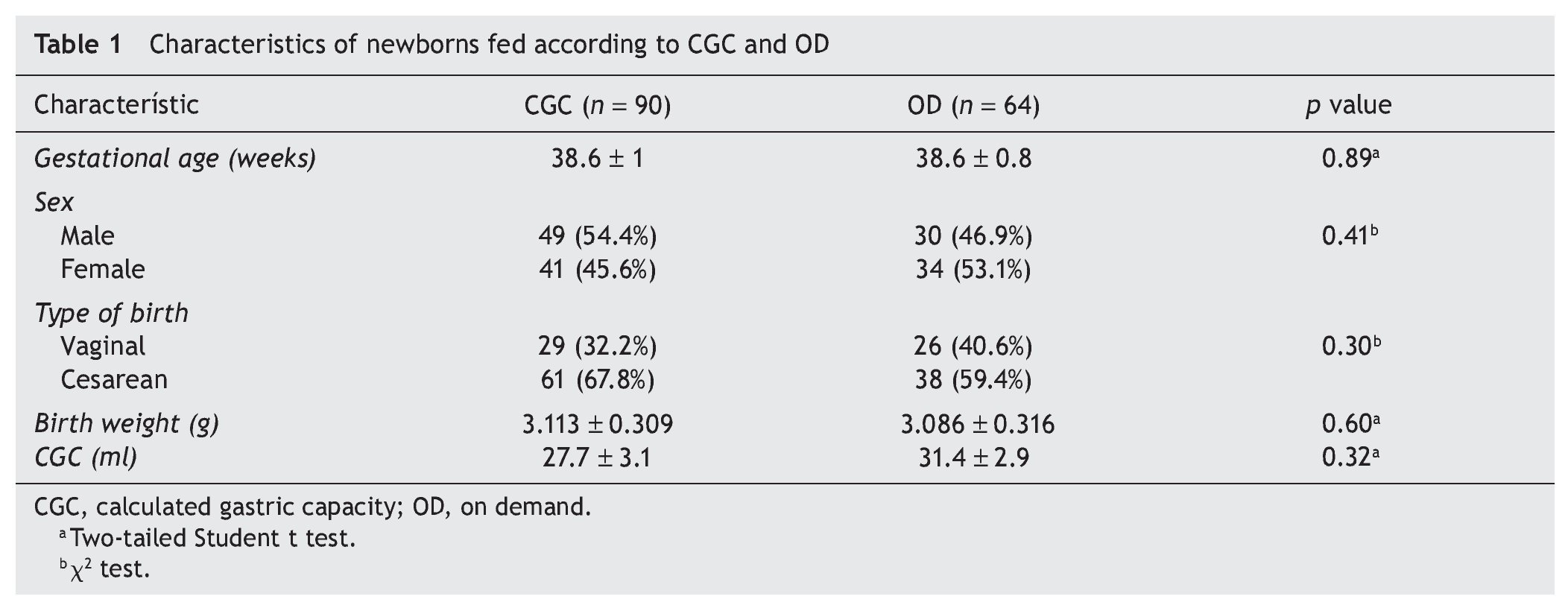
A total of 154 term newborns were included; 61.1% (90) were indicated to be fed by CGC and 38.9% (64) by OD. Newborns were monitored only for the first 24 h, given that the patients born by vaginal birth were discharged on average at 24 h of life. Only those born via cesarean section were discharged after 48 h without any associated complications. Table 1 compares the characteristics of the newborns according to their assigned group. Both groups were similar in gestational age, proportion by gender, birth route (those born via cesarean section predominated), birth weight and CGC.
3.1. Consumption of formula
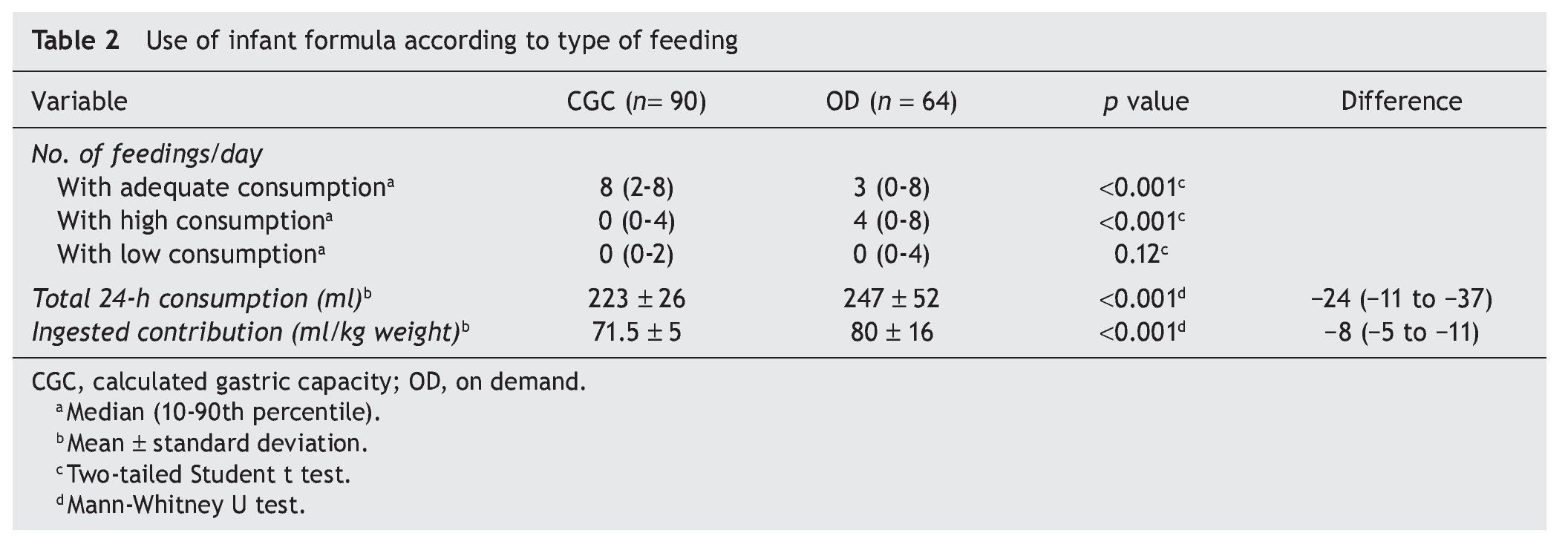
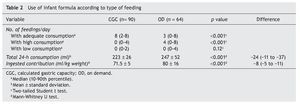
Table 2 shows achievement of the indication assigned to each group. As noted, for the CGC group, the majority consumed the calculated volume in eight feedings. Less than 10% had more than four feedings with a volume >10% of what was indicated or more than two feedings with <10% of the volume indicated.9
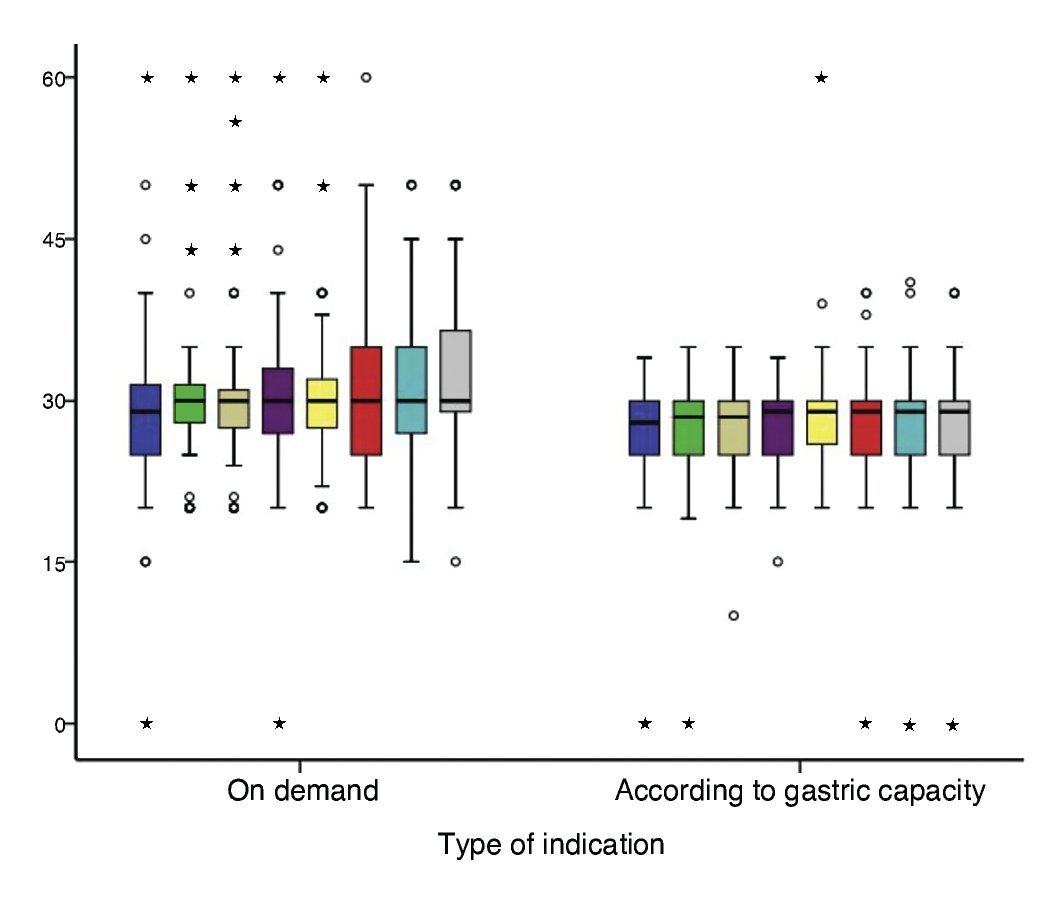
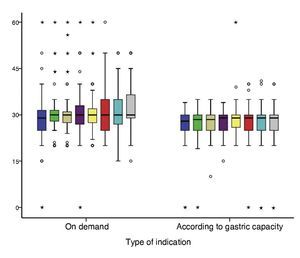
For newborns assigned to OD feeding, three of their eight feedings were with an approximated volume for their gastric capacity. In another four feedings, volumes >10% of their gastric capacity were reported. This indicated that the CGC group had a more regular or constant consumption with respect to the OD group in which the variation of milliliters of formula consumed was greater (Fig. 1).
Figure 1 Behavior of consumption by feeding according to calculated gastric capacity and type of indication.
At the end of the first day of life, the average formula consumption was different according to the indication. The CGC group ingested 24 ml less than the OD group, a difference that resulted in being statistically significant. This represented an average intake <8 ml/kg of weight (Table 2). This difference was maintained when the variables were adjusted for confounders (β-26.4, 95% CI −37 to −15; p = 0.0001).
3.2. Effects of feeding with formula OD vs. CGC
With regard to the consequences of the type of indication with the differential of weight at birth and at 24 h of life, it was found that the CGC group had an average loss of 2% ± 0.2 (from baseline weight). The same figure was observed in the OD group: loss of 2% ± 0.3 g (p adjusted to covariates = 0.71).
The risks and benefits of each type of feedings in the first 24 h were analyzed for oral tolerance, quantifying the number of vomiting, regurgitation and abdominal distention events.
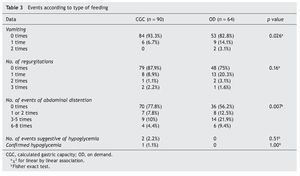
Vomiting was more frequent in the OD group, both in number of times as in the patients with vomiting, with a statistically significant difference (p adjusted to covariates = 0.026) (Table 3).
The same occurred with regurgitations, in number of episodes and time after feedings, a difference that was statistically significant (p adjusted to covariates = 0.14). The greater difference was found in the presence of abdominal distention after feeding, which occurred with greater frequency in the OD group (p adjusted to covariates = 0.012). In this group, 43.8% (n = 28) had abdominal distention at least during one feeding in contrast to the CGC group in which it presented itself in only 22.2% (n = 20).
With respect to the episodes with symptoms suggestive of hypoglycemia, it was observed in two newborns of the CGC group. This condition was confirmed in only one of the patients. In this newborn, the episode occurred after a formula feeding well below the indicated volume (10 ml or −30% of the indicated) because of rejection. For the other infant, the previous consumption was adequate and the serum glucose measured did not confirm hypoglycemia.
There were no events of convulsive crisis or enteral complications in any of the newborns. All were discharged between 24 and 48 h after birth.
3.3. Relationship of volume consumed with symptomatology
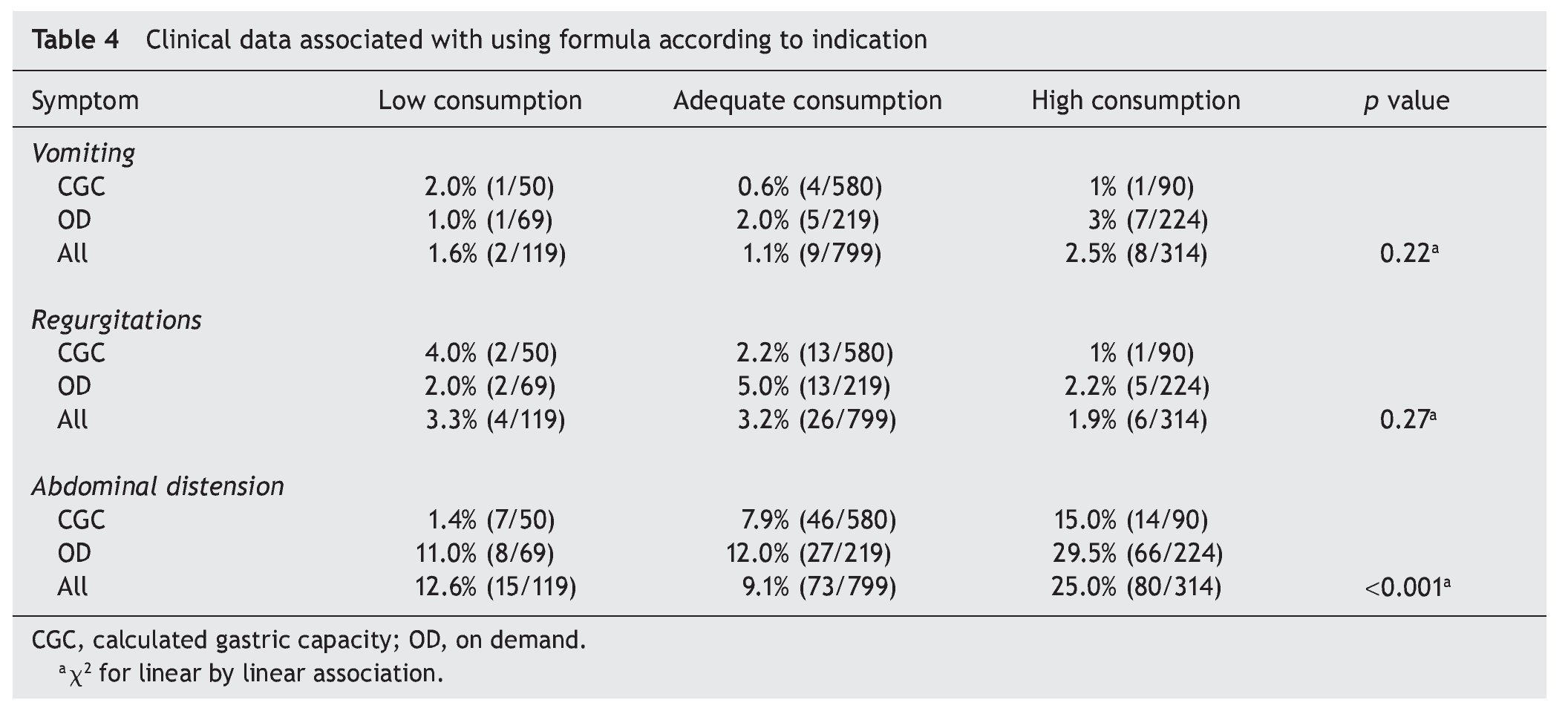
To determine if the symptoms presented by the newborns were related with the quantity of formula consumed in the previous feeding, the rate of events was estimated for each 100 consumptions according to the type of ingestion: low (<10% CGC), adequate or high (>10% CGC). It was observed that neither the presence of vomiting nor regurgitations was related with the volume consumed (Table 4). The rate of events was very similar whether the indication was for CGC or OD.
Abdominal distention was related to high consumption, i.e., the probability of presenting this complication in the newborns who consumed a volume of formula >10% of their CGC was 25% (38.5). One additional observation was that this distention most probably resulted from the OD ingestion.
4. Discussion
Although the decision to feed an infant formula is increasingly difficult given the evidence of the benefits of breastfeeding, this option places the physician in the dilemma of determining the best method for its indication. This study has shown that an indication based on the calculation of the gastric capacity of the newborn would apparently be the best option. Previous studies have shown that infant formulas delay gastric emptying and, with this, it is possible to overfeed the infant.15 Data observed in the present study support better control and tolerance when the volume ingested by the newborn is restricted. A proof of this was the greater regularity in the volumes during feedings. On-demand feeding, although it would appear that it self-regulates by how hungry the child is, may well cause overfeeding during some feedings and rejection during others. In fact, more events of vomiting were frequently observed after low feedings preceded by large feedings. This sign, although not the most important from the clinical point of view, is the most common reason for alarm and concern in parents.
Restriction of gastric capacity did not adversely affect weight loss as it was equal in both groups. The percentages were consistent with that reported in international studies although they reported an expected loss of 4% for the first 72 h of life.15
An inherent risk of low intake is the appearance of hypoglycemic events, which could have later repercussions.14 Previous studies of breast feeding exclusively have reported cases with symptomatic hypoglycemia.12 In this study, although there were three infants with suspected hypoglycemia, it was only confirmed in one of them and was associated with one intake less than estimated for gastric capacity. Thus, it is important to ensure, to the extent possible, these minimum intakes.
It is important to comment that there were violations to the indication in the CGC group during the study. This was tolerated due to the ethical aspects of the study where the criterion of the physician or nurse responsible for surveillance was respected. This allowed us to establish the effect of the over- or underfeeding in the data of tolerance. In this manner, the presence of a greater number of vomiting events during feedings that exceeded the CGC was confirmed.
A strength of our study was that there was an adequate sample to ensure the statistical significance of the main complications evaluated. To ensure the reliability of our observations, staff members who recorded the events and measurements in infants were unaware of the objective of the study.
The main limitation of our conclusions is the non-randomized character of the groups. In an attempt to maintain homogeneity in the characteristics of the newborns, strict inclusion criteria were established to ensure the inclusion of healthy term newborns. Baseline analysis demonstrated that the groups were very similar. Another limitation was that a group bottle fed with maternal milk was not evaluated as has been proposed in different studies including techniques in glass. This behavior could modify the results by changing the time of gastric emptying and possible tolerance by other mechanisms.15 Finally, the comparison was only carried out during a 24-h period so additional studies are necessary to establish if there are differences in more prolonged times.
Formula feeding indicated by the gastric capacity calculated by the weight of the newborn seems to maintain an adequate intake without adverse effects such as oral intolerance, risk of hypoglycemia and weight loss.
Conflict of interest
The authors declare no conflict of interest of any nature.
Received 30 January 2014;
accepted 14 May 2014
* Corresponding author.
E-mail: ferferreiraj23@hotmail.com (T.F. Ferreira-Jaime).