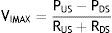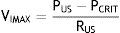The onset of nasal breathing sets a genetically determined impulse to aerate the face cavities or paranasal sinuses, which in turn initiate their growth creating a useful trafficable space for air during the development of the midface. Considering the evidence that the upper airway obstruction has a primary role in the pathogenesis of respiratory sleep disorders, any condition that causes a permanent difficulty to nasal airflow during breathing will cause hypo-development of the required amplitude in this passage, reducing the growth stimulation of the sinus cavities and altering the development of the whole midface.
El inicio de la respiración nasal marca un impulso genéticamente determinado para airear las cavidades de la cara o senos paranasales, que a su vez inician su crecimiento y forman el espacio útil transitable desde el punto de vista respiratorio durante el desarrollo del tercio medio facial. Considerando la evidencia de que la obstrucción de la vía aérea superior tiene un rol primordial en la patogénesis de los trastornos respiratorios del sueño, cualquier patología que cause dificultad permanente al flujo aéreo nasal durante la respiración llevará a un hipodesarrollo de la amplitud requerida en esta vía, disminuyendo la estimulación del crecimiento de las cavidades sinusales y alterando el desarrollo del tercio medio facial en su conjunto.
Human breathing is a basic function of life. The human being is born conditioned to breathe through the nose and to feed through the mouth, and the process of breathing requires the free passage of air through the nasal and naso-oro-pharyngeal spaces. An adequate respiratory function associated with a correct masticatory, swallowing and labial and lingual muscular action will stimulate facial growth and development in its whole given that bone growth responds to the adequate functioning of the muscles and facial soft tissues, as it is described in Moss’ theory.1 Growth of the midface and the conformation of the dental arcade initiate from the first respiration and finalize at the end of the second childhood,2 linked to the adequate ventilation of the paranasal sinuses in relation to the volume of air that can pass through the nose.3 The midface is integrated by the bones that form the roof of the mouth, the floor and the lateral walls of the orbit, a large part of the nasal cavity, which accommodates the nasal septum, the inferior, middle and superior turbinates, as well as the multiple cavities of the maxillary and ethmoidal sinuses, which serve as a support and give shape to the soft tissues from which the external configuration of the face will depend. Therefore, these tissues have a great physiological and esthetic relevance. Any disease that involves these structures can contribute to an alteration of their growth and development.
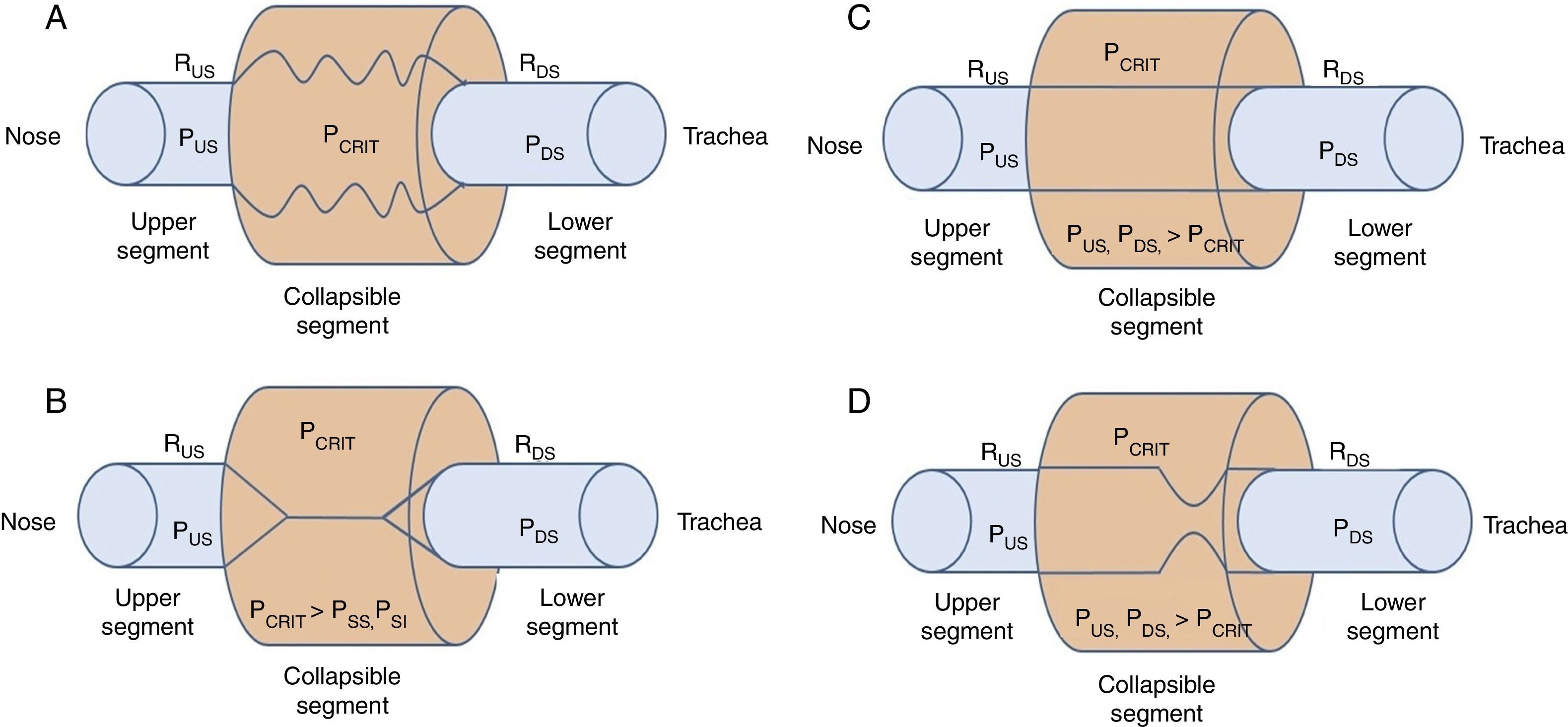
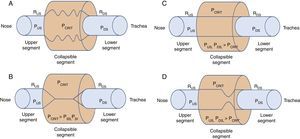
2Biomechanics of the upper airwayPrevious research has outlined the dynamic alterations of permeability as a function of the intraluminal pressure throughout the flexible segments of the cardiovascular, gastrointestinal and genitourinary biological conduits.4–8 In the case of the upper airways, the permeable and flexible segment, which corresponds to the pharynx, is connected through two rigid segments. The upstream segment corresponds to the nose and the downstream segment to the trachea (Fig. 1A).
Starling's upper airway resistance model. A. A flexible segment is connected to an upstream rigid segment (nasal fossa) and a downstream rigid segment (trachea). These rigid segments are characterized by the intraluminal pressures in the upstream and downstream segments (PUS and PDS), respectively, and airflow resistance in the upstream and downstream segments (RUS and RDS), respectively. B. When the PCRIT>PUS, PDS, the airway is closed. C. When both, PUS and PDS are maintained above the PCRIT, the airway is permeable. D. When the PUS is greater than the PCRIT, but the PDS is lower than the PCRIT, the airway presents a limited flow during inspiration and it can rapidly change between an open and closed state9,10.
The air conduits of the upper and lower segments of the flexible site have fixed diameters and resistances: upstream segment resistance (RUS) and downstream segment resistance (RDS); and variable pressures—upstream segment pressure (PUS) and downstream segment pressure (PDS). It is important to mention several characteristics of this model, known as Starling's model of resistance, highlighting the following concepts:
- A)
The pressure outside the rigid conduits and the flexible conduit is positive; inside, the pressure is negative, which allows the air current to flow freely through the conduits
- B)
The components of the system generate resistance to the passing of air; by increasing resistance, more pressure is required for the air to flow to the interior of the system
- C)
The rigid segments of the conduit do not have a risk to collapse, only the flexible portion. Based on this, a new concept is generated: the critical pressure (PCRIT), which represents the risk of total or partial collapse of the flexible portion, and results in a greater or lesser obstruction
- D)
When the upper portion of the segment is obstructed, the pressure inside the conduit is modified, which increases the usual pressure from -18 to -10 cmH2O to +4 cmH2O, which closes or collapses the airway in the flexible segment during sleep.10 When the PCRIT is greater than the PUS and PDS, which connect with the flexible segment, the intramural pressure is positive, the airways close and airflow ceases (Fig. 1B).
Flow can be reestablished by elevating the PUS above the PCRIT. If the PUS and the PDS are greater than the PCRIT, the intramural pressure is negative, the airways open and allow an adequate airflow (Fig. 1C). In these conditions, flow through the upper airway is proportional to the pressure gradient through the entire airway, and it can be described by the tension-current relation of Ohm's law:
where VIMAX represent the maximal inspiratory volume.In contrast, when the PUS is greater than the PCRIT and the PDS is lower than the PCRIT, the airways operate in a flow-limited condition (Fig. 1D). Since the inspiratory cycle varies rapidly between closed and opened status, the pressure in the flexible segment remains almost constant in relation to the PCRIT. If the pressure in the flexible segment is constant, airflow also remains constant. Under these circumstances, airflow becomes independent of the PDS and reaches a level of VIMAX. Given that the PCRIT replaces the PDS, this favors an effective inspiratory flow return. Therefore, the level of VIMAX is determined by the gradient of the PUS and PCRIT divided by the resistance through the upstream segment in accordance with the following equation:
In this model, a decrease of the PDS does not generate an occlusion of the upper airway and cannot be a factor for the development of sleep obstructive apneas.
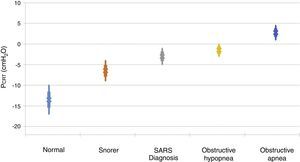
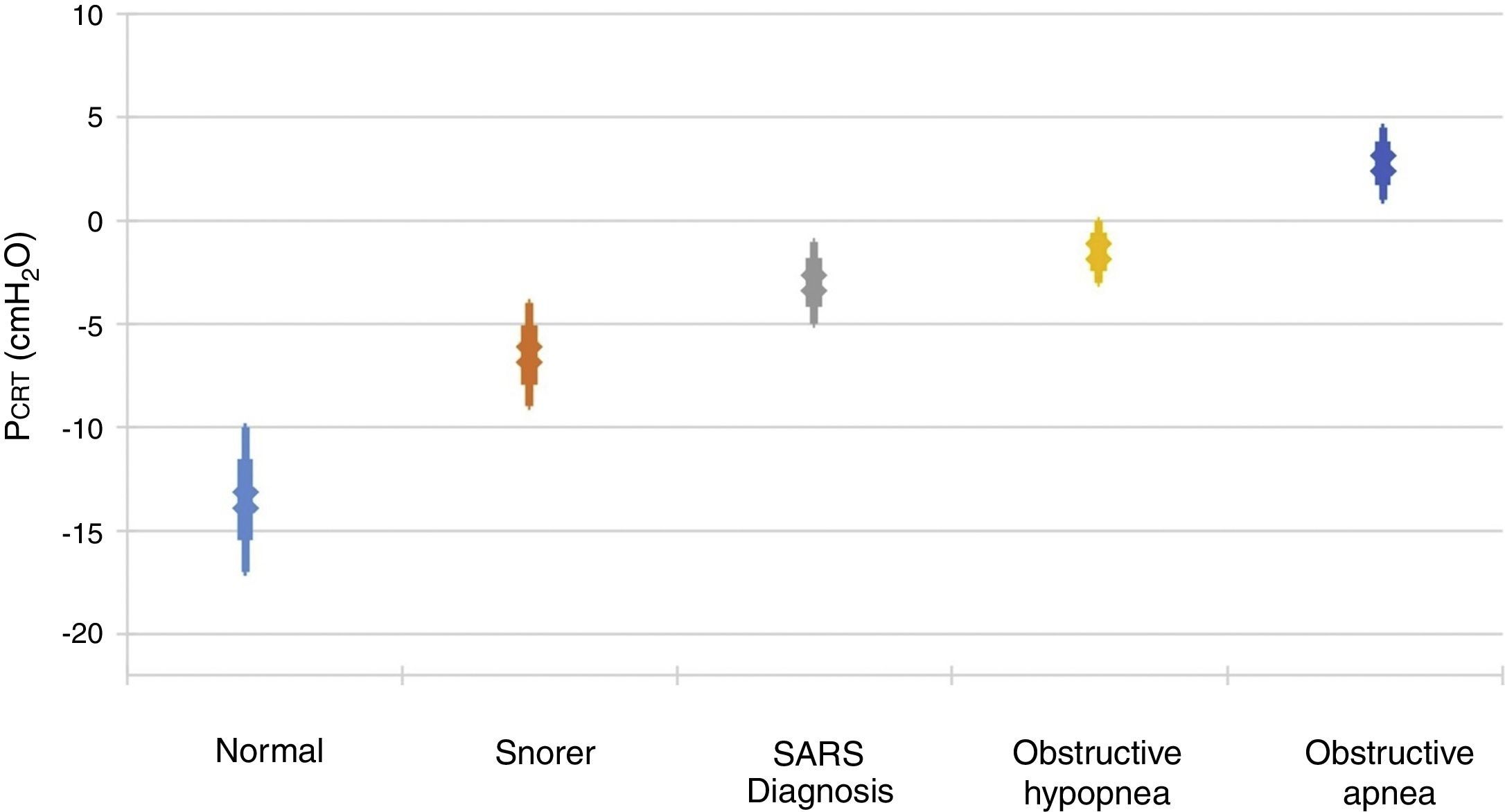
It is important to point out that inspiratory airflow limitation has two implications in the respiratory system. First, upper airway resistance increases notably when flow is limited in comparison with a normal state. In the absence of upper airway obstruction, the combined resistances of the upstream and downstream segment fluctuate between 1 and 2 cmH2O/l/s, which represent approximately half the total resistance of the system during normal breathing. In contrast, during periods of limited inspiratory flow, airflow resistance of the upstream segment can increase up to 20 and 40 cmH2O/l/s. Second, an additional burden to the respiratory system during periods of flow limitation given the fact that patients continue to exert a greater inspiratory effort without increasing airflow. Essentially, a great part of the pressure generated by the inspiratory muscles is wasted by not increasing respiration due to the dynamic collapse of the upper airway. Therefore, the increase in the upper airway resistance and the dynamic collapse of the upper airway lead to an overexertion that will not overcome airflow obstruction. Several studies have demonstrated a directly proportional relationship between pharyngeal collapsibility and the severity of the respiratory obstruction11,12 (Fig. 2). As the PCRIT elevates, an increasing level of upper airway obstruction during sleep is clinically observed. Minimal elevations of the PCRIT have been associated with snoring, meanwhile, moderate elevations (levels of -5 and 0 cmH2O) have been associated with hypopnea and the upper airway resistance syndrome (UARS). With more pronounced increases of the PCRIT, it becomes positive, leading to obstructive sleep apnea. Therefore, the quantitative differences of the PCRIT have been associated with gradual changes in the severity of the airway obstruction during sleep.
Relationship between the critical pressure (PCRIT) and clinical disorders characterized by upper airway obstruction during sleep. As the PCRIT increases, the severity of the upper airway obstruction increases progressively, which suggests a directly proportional relationship between the collapsibility of the upper airway and worsening of the obstruction in asymptomatic snorers, patients with upper airway resistance syndrome (UARS), obstructive hypopnea and obstructive apnea10,12.
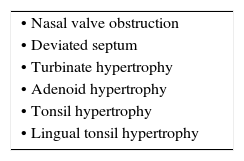
Given the functional importance of the nose in the process of breathing, the presence of a permeable nasal valve, a mid-line positioned nasal septum (both bone and cartilaginous), inferior and middle turbinates of adequate volumes, and a lymphoid tissue that is not hypertrophic, could facilitate said function without altering the development of the midface.
Different researchers have identified a variety of anatomical factors that contribute an increase in the collapsibility of the upper airways. Several craniofacial characteristics related to the morphology of the facial skeleton or the pharyngeal soft tissues can predispose to the collapse of the upper airways. The size of the mandible, the height of the upper maxillary bone and the position of the hyoid bone have been associated with a risk of obstructive sleep apnea-hypopnea syndrome (OSAHS)13,14. The decrease of the soft palate zone and both adenoid and tonsil hypertrophy are characteristics of the soft tissues that have been associated with an increase upper airway collapsibility15. In general, it is believed that these anatomical variants will increase the PCRIT by restricting the size of the bony enclosure around the pharynx and by increasing the amount of soft tissue contained in this area16. Obesity is also an anatomical risk factor of special importance for the upper airways and their obstruction during sleep. The upper airways of obese individuals are more susceptible to collapse17 because the PCRIT increases 1.0 and 1.7 cmH2O for every 10kg/m2 increase in body mass index (BMI) in women and men, respectively. The increase in the fatty deposits around the pharynx and the upper airways16 can increase the pressure exerted by the extraluminal tissue and, therefore, the collapsibility of the upper airway18. Moreover, lung volume decrease in the obese, which leads to a decrease in the flow in the upper airway through a positivization of the PCRIT19. These flow reductions are more pronounced in patients with adipose abdomen, in which lung volumes can decrease up to nearly the residual volume20. Inversely, improvement in OSAHS by weight loss are due probably to a reduction in the pressure of the surrounding tissues and the increase in air flow by generating a negativization of the PCRIT17.
4Pathophysiology associated with the development of the midfaceNasal breathing is healthy because the air is treated in many ways in the different structures of the nose, paranasal sinuses and the nasal mucosa. Nasal physiological functions, such as heating and humidification, are essential to the functionality of the airway. It is estimated that an adult inhales an average of 10,000 liters of air in about 30,000 breaths a day21.
- •
Filtration. The first filter of particles from the ambient is the nasal cavity. The nasal mucous and nose hair, or vibrissae, oversee trapping of the entering particles
- •
Humidification. Humidification is another important process of nasal physiology. The abundant vascularization of the nasal mucosa and the turbinates moisten the entering air, increasing its humidity up to 80% before it reaches the nasopharynx21
- •
Heating. Inhaled air must have a temperature of at least 33-35°C for it not to originate pathological reactions to the alveoli. Once again, due to the turbulence, cold air is forced to be in contact with water vapor that emanates from the mucosa and submucosa of the turbinates, increasing its temperature up to 10°C. A series of neurovascular reflexes are also produced so that, if necessary, capillaries are dilated and warm the overlying mucosa providing more heat to the air21
- •
Smell. Nasal aerodynamics also contributes to the olfactory system by letting ambient particles reach the olfactory striae located at the base of the skull21
- •
The nasal cavity as a resonance chamber. The nose and paranasal sinuses act as factors that contribute to modifying voice intensity
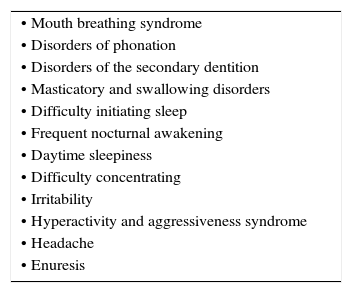
According to the previously stated, any disorder that generates permanent difficulties to nasal airflow during breathing will cause alterations that are secondary to the absence of the described functions as well as underdevelopment of the required amplitude of the nasal airway, since it will diminish the stimulation of growth of the sinus cavities and alter the development of the midface1. The different clinical characteristics (Table 1) are dependent on the affected region of the airway as well as the moment of diagnosis since children have growth spurts of the midface. Sixty percent of this growth occurs during the first four years of life. Therefore, an adequate clinical history by intentionally interrogating about associated signs will allow for an early suspicion of this disorder (Table 2).
Clinical characteristics commonly associated with nasal obstruction.
| • Mouth breathing syndrome |
| • Disorders of phonation |
| • Disorders of the secondary dentition |
| • Masticatory and swallowing disorders |
| • Difficulty initiating sleep |
| • Frequent nocturnal awakening |
| • Daytime sleepiness |
| • Difficulty concentrating |
| • Irritability |
| • Hyperactivity and aggressiveness syndrome |
| • Headache |
| • Enuresis |
The fundamental condition for respiratory sleep disorders to exist resides in the obstruction of the airway; flow decrease through its upstream segment, which is the nose, is the definitive factor. If the obstruction is prolonged until the end of the second childhood, this segment will be permanently insufficient in adolescence and adulthood, triggering the factors that generate a positive PCRIT and leading to the obstruction of the flexible segment of the airway during sleep, which could be denominated as obstructive syndrome in pediatrics. The proposed hypothesis establishes that if there is a moment when the definitive narrowing of the upstream segment can be prevented, it is during the development of the midface, which is fundamental, finite, and unrepeatable. Up to 60% of this development occurs in the first four years of life; thus, an early diagnosis and definitive unblocking is required to achieve an adequate development that will allow free transit of the necessary airflow to favor the negativization of the PCRIT, and avert the possibility of developing any respiratory disorder of sleep, particularly the development of OSAHS.
FundingAuthors’ own resources.
Conflict of interestThe authors declare no conflict of interest of any nature.
To the medical and directive personnel of the Hospital Médicas de la Salud and the Hospital Lomas de San Luis for their support to the care of our patients.
Please cite this article as: Rangel Chávez JJ, Espinosa Martínez C, Medina Serpa AU. Alteraciones del tercio medio facial en la infancia como patogénesis del síndrome de apnea obstructiva del sueño. Bol Med Hosp Infant Mex. 2016;73:278–282.



 PUS, PDS, the airway is closed. C. When both, PUS and PDS are maintained above the PCRIT, the airway is permeable. D. When the PUS is greater than the PCRIT, but the PDS is lower than the PCRIT, the airway presents a limited flow during inspiration and it can rapidly change between an open and closed state9,10.' title='Starling's upper airway resistance model. A. A flexible segment is connected to an upstream rigid segment (nasal fossa) and a downstream rigid segment (trachea). These rigid segments are characterized by the intraluminal pressures in the upstream and downstream segments (PUS and PDS), respectively, and airflow resistance in the upstream and downstream segments (RUS and RDS), respectively. B. When the PCRIT>PUS, PDS, the airway is closed. C. When both, PUS and PDS are maintained above the PCRIT, the airway is permeable. D. When the PUS is greater than the PCRIT, but the PDS is lower than the PCRIT, the airway presents a limited flow during inspiration and it can rapidly change between an open and closed state9,10.'/>
PUS, PDS, the airway is closed. C. When both, PUS and PDS are maintained above the PCRIT, the airway is permeable. D. When the PUS is greater than the PCRIT, but the PDS is lower than the PCRIT, the airway presents a limited flow during inspiration and it can rapidly change between an open and closed state9,10.' title='Starling's upper airway resistance model. A. A flexible segment is connected to an upstream rigid segment (nasal fossa) and a downstream rigid segment (trachea). These rigid segments are characterized by the intraluminal pressures in the upstream and downstream segments (PUS and PDS), respectively, and airflow resistance in the upstream and downstream segments (RUS and RDS), respectively. B. When the PCRIT>PUS, PDS, the airway is closed. C. When both, PUS and PDS are maintained above the PCRIT, the airway is permeable. D. When the PUS is greater than the PCRIT, but the PDS is lower than the PCRIT, the airway presents a limited flow during inspiration and it can rapidly change between an open and closed state9,10.'/>